Effect of Electronic Cold-PasteurizationTM (ECPTM) on Fruit Quality and Postharvest Diseases during Blueberry Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Collection and Irradiation
2.2. Evaluation of Fruit Quality Attributes
2.3. Evaluation of Fruit Surface Contaminants
2.4. Assessment of Postharvest Disease
3. Results
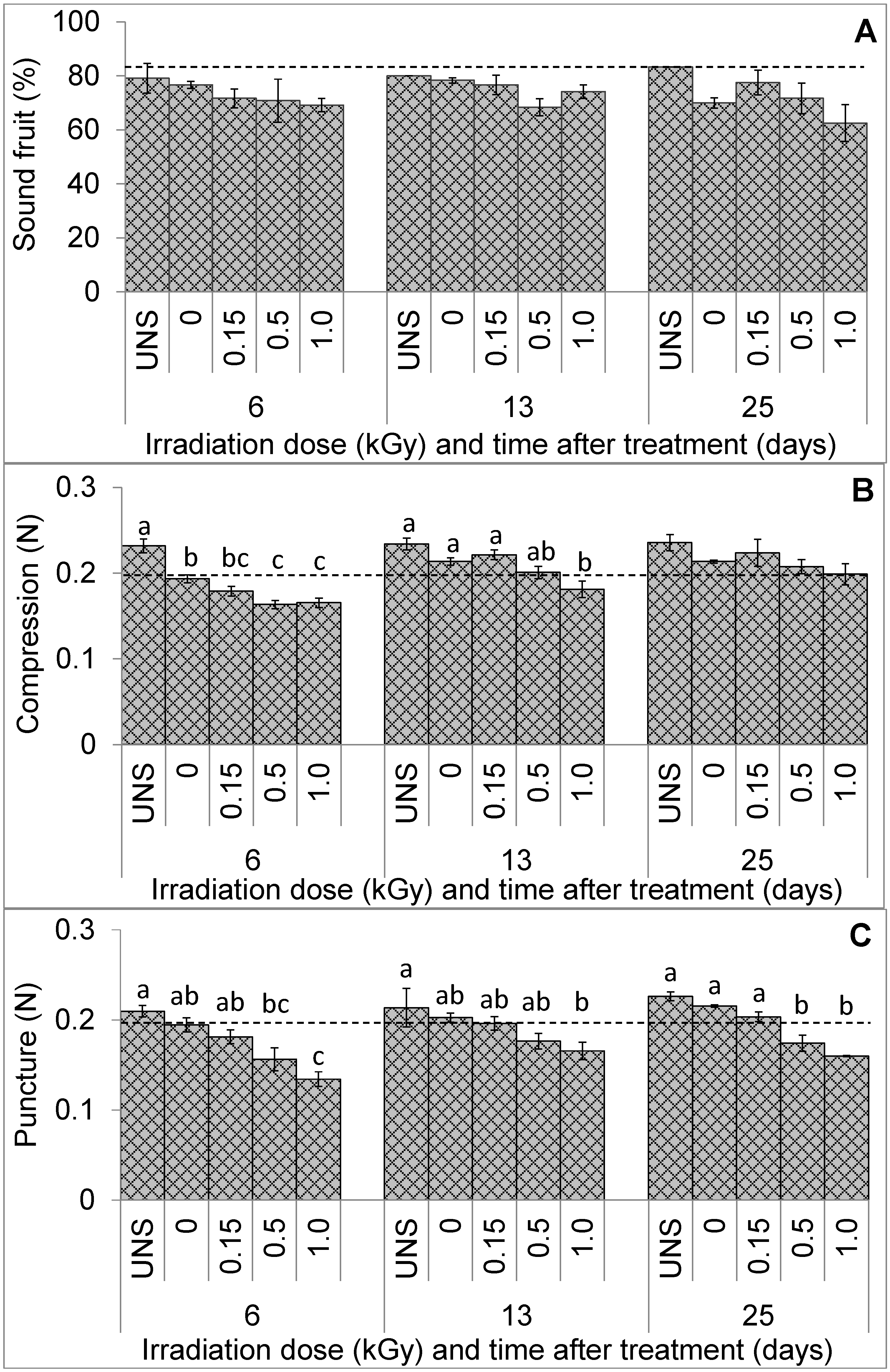
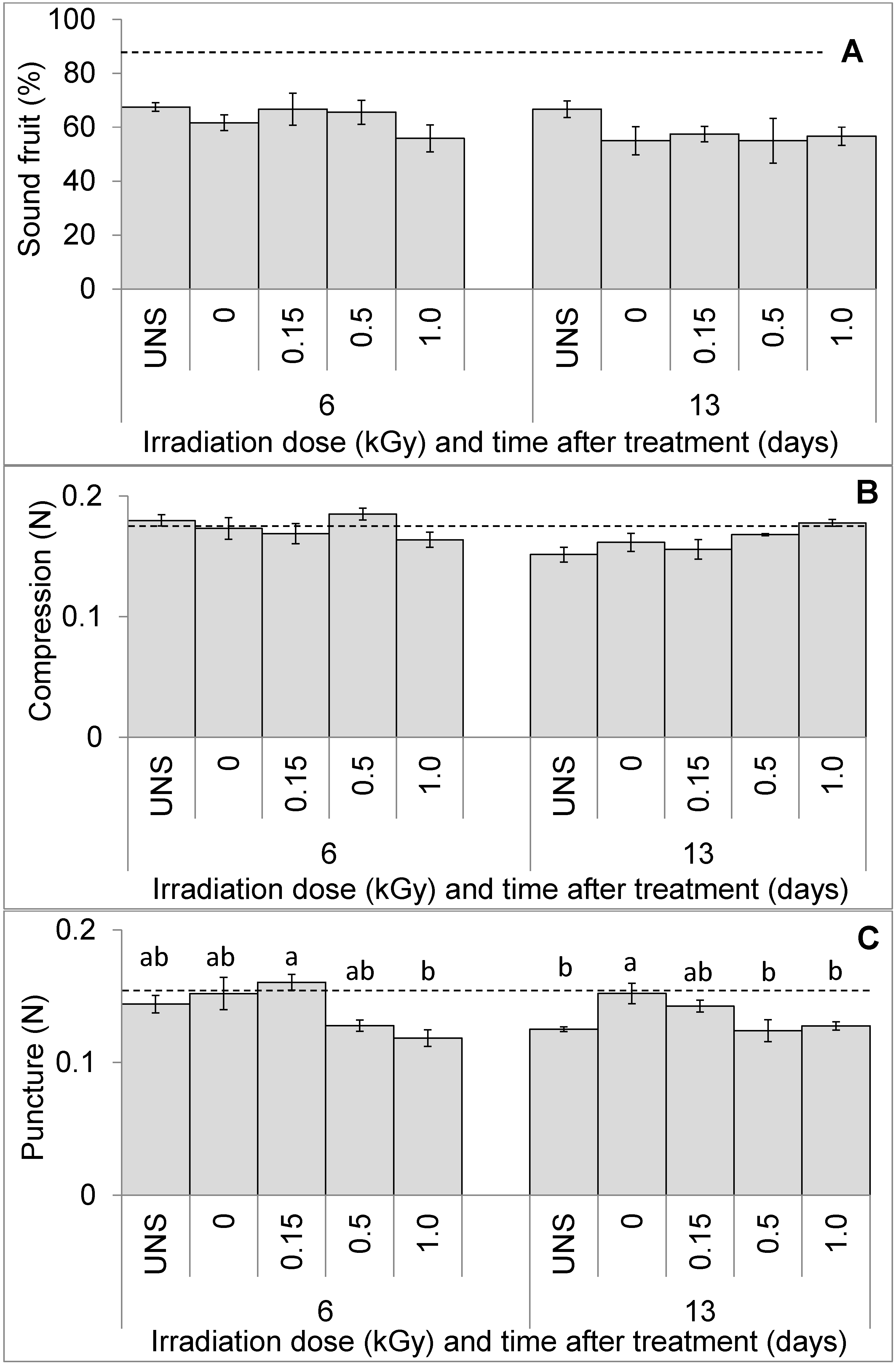
3.1. Fruit Visual Quality and Texture
3.2. Total Soluble Solids Content, Titratable Acidity, and Weight
3.3. Microbial Load on Fruit after Treatment
3.4. Postharvest Disease Incidence on Fruit after Treatment
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food. Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.A. Southern highbush blueberries: Physiological and cultural factors important for optimal cropping of these complex hybrids. Acta Hortic. 1993, 346, 72–80. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Bassil, N.; Buck, E.J.; McCallum, S.; Graham, J.; Brown, A.; Wiedow, C.; Campbell, A.M.; Haynes, K.G.; et al. Construction of a genetic linkage map of an interspecific diploid blueberry population and identification of QTL for chilling requirement and cold hardiness. Mol. Breed. 2014, 34, 2033–2048. [Google Scholar] [CrossRef]
- Evans, E.A.; Ballen, F.H. An Overview of US Blueberry Production, Trade, and Consumption, with Special Reference to Florida; Publication FE952; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2014; Available online: http://edis.ifas.ufl.edu/fe952 (accessed on 28 August 2018).
- United States Department of Agriculture-National Agricultural Statistics Service (USDA-NASS). Noncitrus Fruits and Nuts, 2016 Summary; USDA-NASS: Washington, DC, USA, 2017. Available online: http://www.clientadvisoryservices.com/Downloads/NoncFruiNu-01-23-2015.pdf (accessed on 28 August 2018).
- Marzolo, G. Ag Marketing Resource Center; Iowa State University: Ames, IA, USA, 2015; Available online: http://www.agmrc.org/commodities-products/fruits/blueberries/ (accessed on 28 August 2018).
- United States Department of Agriculture Economic Research Service (USDA-ERS). Fruit and Tree Nut Data; USDA-ERS: Washington, DC, USA, 2017. Available online: http://www.ers.usda.gov/data-products/fruit-and-tree-nut-data/data-by-commodity.aspx (accessed on 28 August 2018).
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of chitosan-essential oil coatings on safety and quality of fresh blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Cappellini, R.A.; Ceponis, M.J.; Koslow, G. Nature and extent of losses in consumer-grade samples of blueberries in greater New York. HortScience 1982, 17, 55–56. [Google Scholar]
- Daykin, M.E.; Milholland, R.D. Infection of blueberry fruit by Colletotrichum gloeosporioides. Plant Dis. 1984, 68, 948–950. [Google Scholar] [CrossRef]
- Smith, B.J.; Magee, J.B.; Gupton, C.L. Susceptibility of rabbiteye blueberry cultivars to postharvest diseases. Plant Dis. 1996, 80, 215–218. [Google Scholar] [CrossRef]
- Schilder, A.M.C.; Gillett, J.M.; Woodworth, J.A. The kaleidoscopic nature of blueberry fruit rots. Acta Hortic. 2002, 574, 81–83. [Google Scholar] [CrossRef]
- Fan, X.; Niemira, B.; Prakash, A. Irradiation of fruits and vegetables. Food Technol. 2008, 3, 37–43. [Google Scholar]
- Palumbo, M.; Harris, L.J.; Danyluk, M.D. Survival of Foodborne Pathogens on Berries; Publication FSHN13-12; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2013; Available online: https://edis.ifas.ufl.edu/pdffiles/FS/FS23600.pdf (accessed on 28 August 2018).
- United States Department of Health and Human Services, Centers for Disease Control and Prevention (US CDC). Foodborne Outbreaks; CDC: Atlanta, GA, USA, 2016. Available online: http://wwwn.cdc.gov/foodborneoutbreaks/Default.aspx (accessed on 28 August 2018).
- Popa, I.; Hanson, E.J.; Todd, E.C.D.; Schilder, A.C.; Ryser, E.T. Efficacy of chlorine dioxide gas sachets for enhancing the microbiological quality and safety of blueberries. J. Food Prot. 2007, 70, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rodas, A.; Bourquin, L.; Garcia-Salazar, C.; Valera-Gomez, A.; Wise, J. Good Agricultural Practices for Food Safety in Blueberry Production: Basic Principles; Michigan State University Extension: East Lansing, MI, USA, 2009; Available online: http://caff.org/wp-content/uploads/2012/06/MI-Blueberry-GAPs-manual.pdf (accessed on 28 August 2018).
- Miller, W.R.; Mitcham, E.J.; McDonald, R.E.; King, J.R. Postharvest storage quality of gamma-irradiated ‘Climax’ rabbiteye blueberries. HortScience 1994, 29, 98–101. [Google Scholar]
- United States Department of Agriculture Animal and Plant Health Inspection Service (USDA APHIS). Plant Protection and Quarantine: Treatment Manual; USDA-APHIS: Washington, DC, USA, 2015. Available online: http://www.aphis.usda.gov/import_export/plants/manuals/ports/downloads/treatment.pdf (accessed on 28 August 2018).
- Drake, S.R.; Neven, L.G. Irradiation as an alternative to methyl bromide for quarantine treatment of stone fruits. J. Food Qual. 1998, 22, 529–538. [Google Scholar] [CrossRef]
- Aegerter, A.F.; Folwell, R.J. Economic aspects of alternatives to methyl bromide in the postharvest and quarantine treatment of selected fresh fruits. Crop Prot. 2000, 19, 161–168. [Google Scholar] [CrossRef]
- Kitinoja, L. Use of Cold Chains for Reducing Food Losses in Developing Countries; White Paper 13-03; The Postharvest Education Foundation (PEF): La Pine, OR, USA, 2013; Available online: http://www.postharvest.org/use%20of%20cold%20chains%20pef%20white%20paper%2013-03%20final.pdf (accessed on 28 August 2018).
- Morehouse, K.M.; Komolprasert, V. Irradiation of Food and Packaging: An Overview. In Irradiation of Food and Packaging; ACS Symposium Series 875; American Chemical Society: Washington, DC, USA, 2004. Available online: https://www.fda.gov/food/ingredientspackaginglabeling/irradiatedfoodpackaging/ucm081050.htm (accessed on 28 August 2018).
- Miller, W.R.; McDonald, R.E.; Smittle, B.J. Quality of ‘Sharpblue’ blueberries after electron beam irradiation. HortScience 1995, 30, 306–308. [Google Scholar]
- Moreno, M.A.; Castell-Perez, M.E.; Gomes, C.; Da Silva, P.F.; Moreira, R.G. Quality of electron beam irradiation of blueberries (Vaccinium corymbosum L.) at medium dose levels (1.0–3.2 kGy). Food Sci. Technol. 2007, 40, 1123–1132. [Google Scholar]
- Golding, J.B.; Blades, B.L.; Satyan, S.; Jessup, A.J.; Spohr, L.J.; Harris, A.M.; Banos, C.; Davies, J.B. Low dose gamma irradiation does not affect the quality, proximate or nutritional profile of ‘Brigitta’ blueberry and ‘Maravilla’ raspberry fruit. Postharvest Biol. Technol. 2014, 96, 49–52. [Google Scholar] [CrossRef]
- Lires, C.M.L.; Docters, A.; Horak, C.I. Evaluation of the quality and shelf life of gamma irradiated blueberries by quarantine purposes. Radiat. Phys. Chem. 2018, 143, 79–84. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (US FDA). Code of Federal Regulations Title 21; US FDA: Washington, DC, USA, 2016. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=179.26 (accessed on 28 August 2018).
- Niemira, B.A.; Sommers, C.H. New applications in food irradiation. In Encyclopedia of Agricultural, Food and Biological Engineering, 1st ed.; Heldman, D.R., Ed.; Taylor & Francis: New York, NY, USA, 2006; pp. 1–6. [Google Scholar]
- Niemira, B.A.; Fan, X. Irradiation enhances quality and safety of fresh and fresh-cut fruits and vegetables. In Microbial Safety of Fresh Produce; Niemira, B.A., Doona, C.J., Feeherry, F.E., Gravani, R.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 191–204. ISBN 9781444319347. [Google Scholar]
- Thang, K.; Au, K.; Rakovski, C.; Prakash, A. Effect of phytosanitary irradiation and methyl bromide fumigation on the physical, sensory, and microbiological quality of blueberries and sweet cherries. J. Sci. Food Agric. 2016, 96, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Mehra, L.K.; MacLean, D.D.; Savelle, A.T.; Scherm, H. Postharvest disease development on southern highbush blueberry fruit in relation to berry flesh type and harvest method. Plant Dis. 2013, 97, 213–221. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; Macmillan: New York, NY, USA, 1987; ISBN 9780890541920. [Google Scholar]
- Wharton, P.; Schilder, A. Blueberry Fruit Rot Identification Guide; E-2847; Michigan State University: East Lansing, MI, USA, 2003; Available online: http://msue.anr.msu.edu/uploads/resources/pdfs/Michigan_Blueberry_Facts_-_Blueberry_Fruit_Rot_Identification_Guide_(E2847).pdf (accessed on 28 August 2018).
- Eaton, G.W.; Meehan, C.; Turner, N. Some physical effects of postharvest gamma radiation on the fruit of sweet cherry, blueberry, and cranberry. J. Can. Inst. Food Sci. Technol. 1970, 3, 152–156. [Google Scholar] [CrossRef]
- Trigo, M.J.; Sousa, M.B.; Sapata, M.M.; Ferreira, A.; Curado, T.; Andrada, L.; Ferreira, E.S.; Antunes, C.; Horta, M.P.; Pereira, A.R.; et al. Quality of gamma irradiated blueberries. Acta Hortic. 2006, 715, 573–578. [Google Scholar] [CrossRef]
- Guimarães, C.; Menezes, E.G.T.; de Abreu, P.S.; Rodrigues, A.C.; Borges, P.R.S.; Batista, L.R.; Cirilo, M.A.; Lima, L.C.O. Physicochemical and microbiological quality of raspberries (Rubus idaeus) treated with different doses of gamma irradiation. Food Sci. Technol. 2013, 33, 316–322. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H.; Yook, H.S. The effects of gamma irradiation on the microbiological, physicochemical and sensory quality of peach (Prunus persica L. Batsch Cv. Dangeumdo). J. Korean Soc. Food Sci. Nutr. 2009, 38, 364–371. [Google Scholar] [CrossRef]
- McDonald, H.; McCulloch, M.; Caporaso, F.; Winborne, I.; Oubichon, M.; Rakovski, C.; Prakash, A. Commercial scale irradiation for insect disinfestations preserves peach quality. Radiat. Phys. Chem. 2012, 81, 697–704. [Google Scholar] [CrossRef]
- Kim, C.G.; Rakowski, C.; Caporaso, F.; Prakash, A. Low-dose irradiation can be used as a phytosanitary treatment for fresh table grapes. J. Food Sci. 2014, 79, S81–S91. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Harris, L.J.; Danyluk, M.D. Outbreaks of Foodborne Illness Associated with Common Berries, 1983 through May 2013; Publication FSHN13-08; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2013; Available online: http://ucfoodsafety.ucdavis.edu/files/223896.pdf (accessed on 28 August 2018).
- Ceponis, M.J.; Capellini, R.A. Control of postharvest decays of blueberry fruits by precooling, fungicide, and modified atmospheres. Plant Dis. 1979, 63, 1049–1053. [Google Scholar]
- Hardenburg, R.E.; Watada, A.E.; Wang, C.Y. The commercial storage of fruits, vegetables, and florist and nursery stocks. USDA Agric. Handb. 1986, 66, 240–242. [Google Scholar]
- Jeong, R.D.; Shin, E.J.; Chu, E.H.; Park, H.J. Effects of ionizing radiation on postharvest fungal pathogens. Plant Pathol. J. 2015, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Spalding, D.H.; Reeder, W.F. Decay and acceptability of mangoes treated with combinations of hot water, imazalil, and γ-radiation. Plant Dis. 1986, 70, 1149–1151. [Google Scholar] [CrossRef]
- Johnson, G.I.; Boag, T.S.; Cooke, A.W.; Izard, M.; Panitz, M.; Sangchote, S. Interaction of post harvest disease control treatments and gamma irradiation on mangoes. Ann. Appl. Biol. 1990, 116, 245–257. [Google Scholar] [CrossRef]
- Lacroix, M.; Lafortune, R. Combined effects of gamma irradiation and modified atmosphere packaging on bacterial resistance in grated carrots (Daucus carota). Radiat. Phys. Chem. 2004, 71, 79–82. [Google Scholar] [CrossRef]
- Lafortune, R.; Caillet, S.; Lacroix, M. Combined effects of coating, modified atmosphere packaging and gamma irradiation on quality maintenance of ready-to-use carrots (Daucus carota). J. Food Prot. 2005, 68, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int. J. Food Sci. Technol. 2012, 47, 2318–2324. [Google Scholar] [CrossRef]

| Days after Treatment | Treatment a | Farthing Trial 1 | Farthing Trial 2 | Rebel Trial 2 | |||
|---|---|---|---|---|---|---|---|
| TSS | TA | TSS | TA | TSS | TA | ||
| (kGy) | (% Brix) | (% CA) | (% Brix) | (% CA) | (% Brix) | (% CA) | |
| 0 | UNS | 13.0 | 0.64 | 13.0 | 0.68 | 8.3 | 0.21 |
| 6 | UNS | 12.4 | 0.51 | 12.2 | 0.56 | 7.9 | 0.20 |
| 0 | 12.6 | 0.59 | 12.0 | 0.56 | 8.2 | 0.20 | |
| 0.15 | 12.7 | 0.54 | 13.0 | 0.45 | 8.2 | 0.23 | |
| 0.5 | 12.9 | 0.51 | 12.2 | 0.47 | 8.4 | 0.20 | |
| 1.0 | 13.0 | 0.51 | 12.1 | 0.53 | 8.0 | 0.21 | |
| 13 | UNS | 12.6 | 0.51 | 12.8 | 0.53 | 8.0 | 0.20 |
| 0 | 12.7 | 0.57 | 12.9 | 0.53 | 8.0 | 0.21 | |
| 0.15 | 12.8 | 0.54 | 12.0 | 0.51 | 8.1 | 0.21 | |
| 0.5 | 12.7 | 0.52 | 12.3 | 0.53 | 8.1 | 0.20 | |
| 1.0 | 12.9 | 0.51 | - | - | 8.1 | 0.21 | |
| 25 | UNS | 12.8 | 0.45 | 13.0 | 0.38 | - | - |
| 0 | 12.8 | 0.47 | 12.4 | 0.41 | - | - | |
| 0.15 | 12.7 | 0.47 | 12.8 | 0.42 | - | - | |
| 0.5 | 12.6 | 0.48 | 12.4 | 0.33 | - | - | |
| 1.0 | 12.9 | 0.44 | 12.6 | 0.30 | - | - | |
| Days after Treatment | Treatment a (kGy) | Farthing Trial 1 | Farthing Trial 2 | Rebel Trial 2 |
|---|---|---|---|---|
| Weight (g) | Weight (g) | Weight (g) | ||
| 0 | UNS | 1.8 | 2.1 | 1.6 |
| 6 | UNS | 1.8 | 1.8 b | 1.7 |
| 0 | 1.9 | 2.1 ab | 1.6 | |
| 0.15 | 2.0 | 2.3 a | 1.7 | |
| 0.5 | 1.9 | 2.1 ab | 1.7 | |
| 1.0 | 2.0 | 2.1 ab | 1.7 | |
| Prob > F | ns | 0.0484 | Ns | |
| 13 | UNS | 2.1 a | 2.0 | 1.6 |
| 0 | 1.9 ab | 2.0 | 1.6 | |
| 0.15 | 1.9 ab | 2.0 | 1.6 | |
| 0.5 | 1.9 ab | 2.0 | 1.6 | |
| 1.0 | 1.8 b | - | 1.6 | |
| Prob > F | 0.0694 | ns | Ns | |
| 25 | UNS | 1.7 | 2.1 | - |
| 0 | 1.8 | 1.9 | - | |
| 0.15 | 1.8 | 2.1 | - | |
| 0.5 | 1.7 | 1.9 | - | |
| 1.0 | 1.8 | 2.2 | - | |
| Prob > F | ns | ns |
| Treatment a (kGy) | Aerobic Bacteria | Yeasts | Molds | Coliforms |
|---|---|---|---|---|
| Farthing Trial 1 | ||||
| UNS | 3.83 a | 4.09 a | 1.82 | 1.15 |
| 0 | 3.94 a | 3.99 a | 1.07 | 0.89 |
| 0.15 | 3.83 a | 4.00 a | 1.11 | 0.88 |
| 0.5 | 3.59 ab | 3.77 ab | 1.26 | 0.52 |
| 1.0 | 3.14 b | 3.48 b | 1.41 | 0.19 |
| Prob > F | 0.0226 | 0.0119 | ns | ns |
| Farthing Trial 2 | ||||
| UNS | 3.15 a | 3.15 a | 2.31 | 0.73 |
| 0 | 3.00 a | 2.98 a | 1.43 | 0.41 |
| 0.15 | 3.25 a | 3.21 a | 1.78 | 0.47 |
| 0.5 | 3.06 a | 3.03 a | 1.44 | 0.34 |
| 1.0 | 2.34 b | 2.47 b | 1.94 | 0.06 |
| Prob > F | 0.0182 | 0.0169 | ns | ns |
| Rebel Trial 2 | ||||
| UNS | 5.03 a | 4.75 a | 4.28 | 2.90 a |
| 0 | 4.82 a | 4.74 a | 3.98 | 3.05 a |
| 0.15 | 4.28 b | 4.10 b | 3.85 | 2.43 ab |
| 0.5 | 4.10 b | 4.20 ab | 3.70 | 1.40 bc |
| 1.0 | 3.93 b | 3.96 b | 3.38 | 0.85 c |
| Prob > F | 0.0003 | 0.0295 | ns | 0.0106 |
| Days after Harvest | Treatment a (kGy) | Farthing Trial 1 | Farthing Trial 2 | Rebel Trial 2 |
|---|---|---|---|---|
| 0 | UNS | 0.75 | 1.4 | 17.5 |
| 6 | UNS | 0.83 | 0.42 | 28.3 |
| 0 | 4.2 | 1.3 | 17.1 | |
| 0.15 | 4.6 | 0.63 | 15.4 | |
| 0.5 | 4.6 | 0.42 | 16.7 | |
| 1 | 4.2 | 0 | 14.2 | |
| Prob > F | ns | ns | ns | |
| 13 | UNS | 0 b | - | - |
| 0 | 4.2 a | - | - | |
| 150 | 3.8 a | - | - | |
| 500 | 5.4 a | - | - | |
| 1000 | 2.8 a | - | - | |
| Prob > F | 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nambeesan, S.U.; Doyle, J.W.; Capps, H.D.; Starns, C.; Scherm, H. Effect of Electronic Cold-PasteurizationTM (ECPTM) on Fruit Quality and Postharvest Diseases during Blueberry Storage. Horticulturae 2018, 4, 25. https://doi.org/10.3390/horticulturae4030025
Nambeesan SU, Doyle JW, Capps HD, Starns C, Scherm H. Effect of Electronic Cold-PasteurizationTM (ECPTM) on Fruit Quality and Postharvest Diseases during Blueberry Storage. Horticulturae. 2018; 4(3):25. https://doi.org/10.3390/horticulturae4030025
Chicago/Turabian StyleNambeesan, Savithri U., John W. Doyle, Helaina D. Capps, Chip Starns, and Harald Scherm. 2018. "Effect of Electronic Cold-PasteurizationTM (ECPTM) on Fruit Quality and Postharvest Diseases during Blueberry Storage" Horticulturae 4, no. 3: 25. https://doi.org/10.3390/horticulturae4030025
APA StyleNambeesan, S. U., Doyle, J. W., Capps, H. D., Starns, C., & Scherm, H. (2018). Effect of Electronic Cold-PasteurizationTM (ECPTM) on Fruit Quality and Postharvest Diseases during Blueberry Storage. Horticulturae, 4(3), 25. https://doi.org/10.3390/horticulturae4030025






