Enhancing Productivity and Improving Nutritional Quality of Subtropical and Temperate Leafy Vegetables in Tropical Greenhouses and Indoor Farming Systems
Abstract
:1. Introduction
2. Enhancing Productivity of Aeroponically Grown Leafy Vegetables in a Tropical Greenhouse through Manipulation of Root-Zone Temperature (RZT)
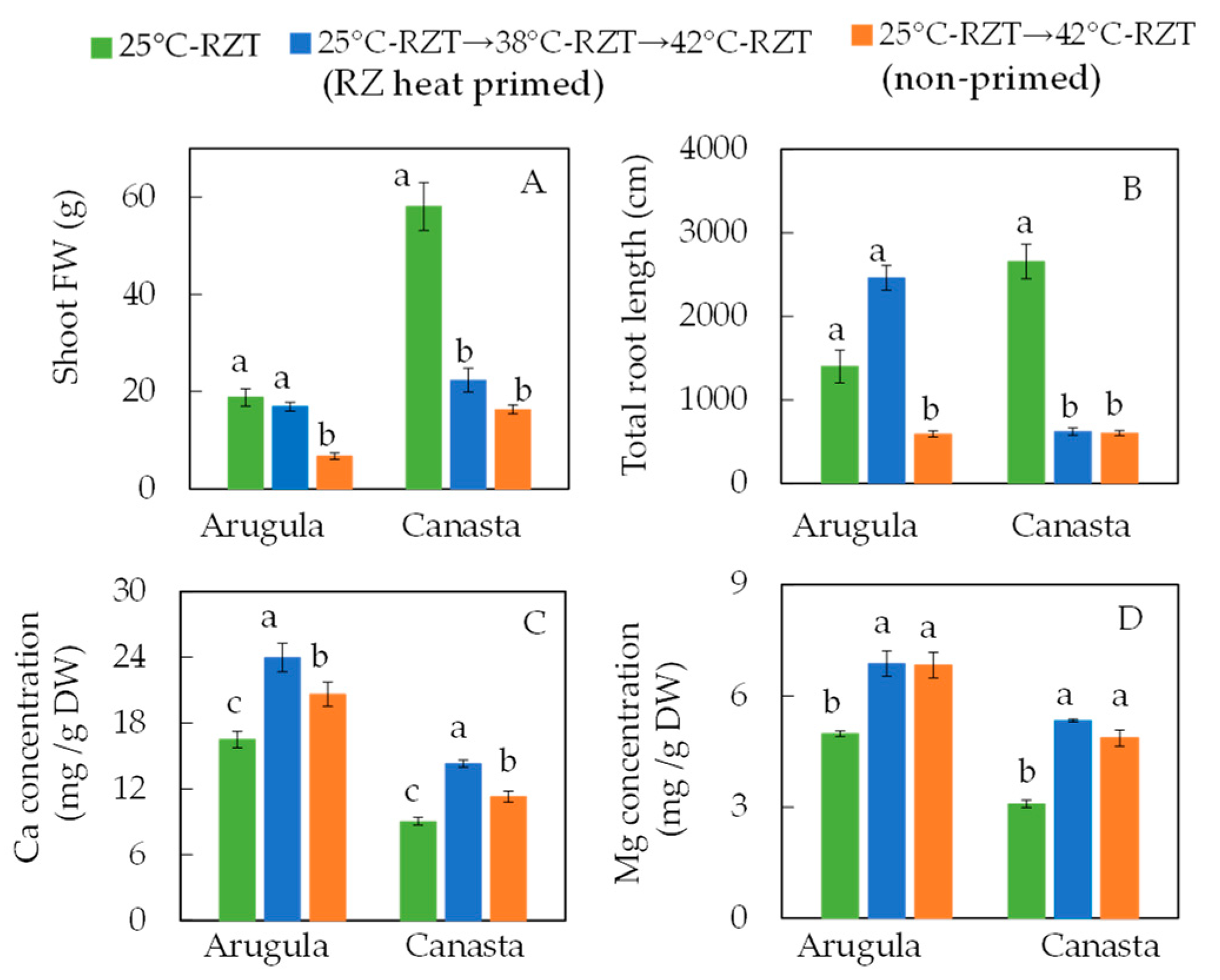
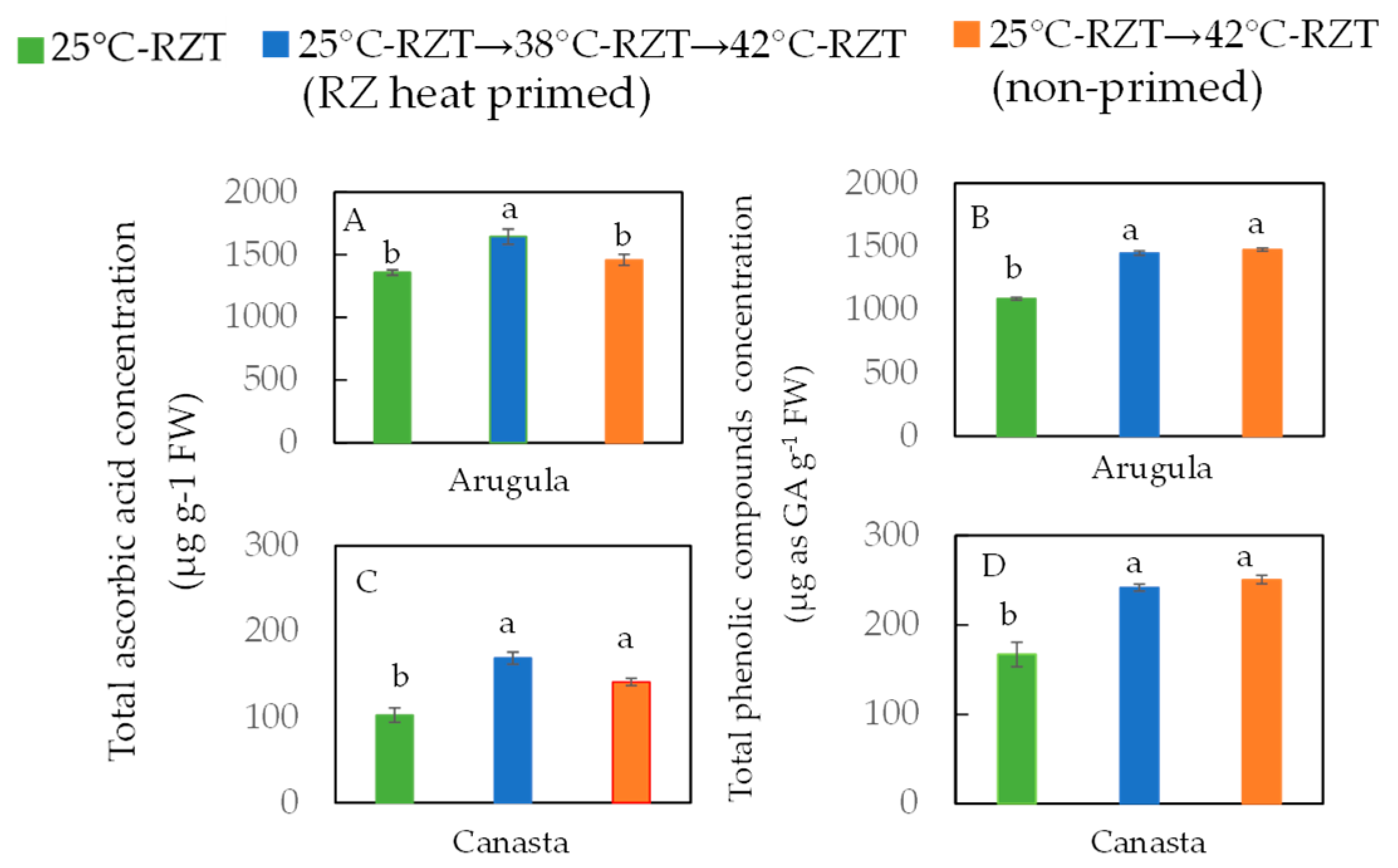
3. Root-Zone (RZ) Heat Priming Effects on Growth, Productivity, Physiology, and Nutritional Quality
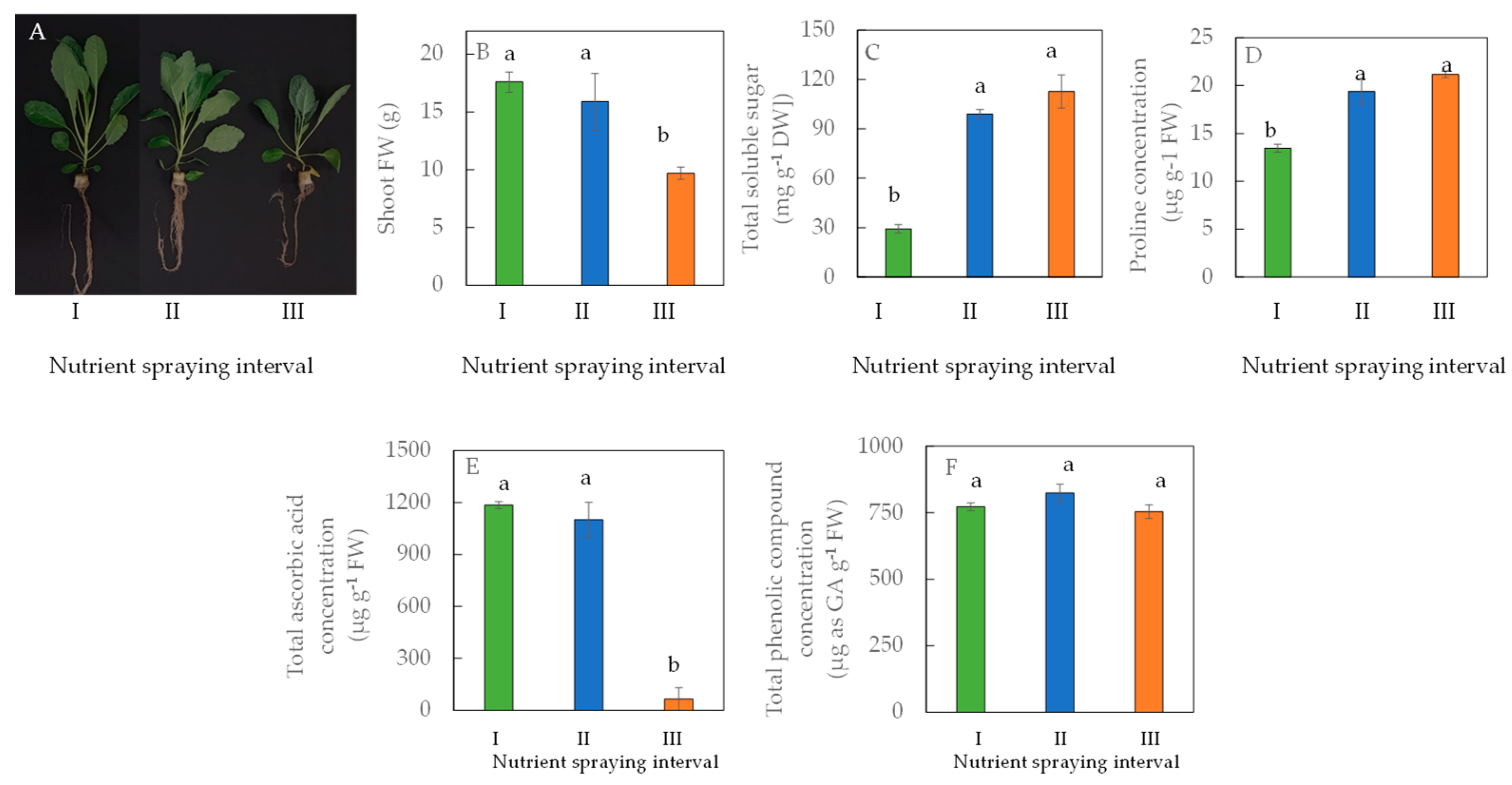
4. Deficit Irrigation Effects on Productivity and Nutritional Quality of Aeroponically Grown Leafy Vegetables Indoors and in a Tropical Greenhouse
5. Impacts of LED Spectral Quality, Intensity, and Photoperiod on Productivity and Nutritional Quality of Leafy Vegetables Grown Indoors and in the Tropical Greenhouse
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diehl, J.A.; Sweeney, E.; Wong, E.; Sia, C.S.; Yao, H.; Prabhudesai, M. Feeding cities: Singapore’s approach to land use planning for urban agriculture. Glob. Food Secur. 2020, 26, 100377. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- He, J. Impact of root-zone temperature on photosynthetic efficiency of aeroponically grown temperate and subtropical vegetable crops in the tropics. In Theory and Applications in Energy, Biotechnology and Nanotechnology; Buchner, T.B., Ewingen, N.H., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 111–144. [Google Scholar]
- Ludher, E.K. Singapore’s smart governance of food. In The Governance of City Food Systems: Case Studies from Around the World; Deakin, M.M., Davide, D., Nunzia, B., Eds.; Fondazione Giangiacomo Feltrinelli: Milan, Italy, 2016; pp. 131–154. [Google Scholar]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant factories are heating up: Hunting for the best combination of light intensity, air temperature and root-zone temperature in lettuce production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Klerkx, L.; Rose, D. Dealing with the game-changing technologies of Agriculture 4.0: How do we manage diversity and responsibility in food system transition pathways? Glob. Food Secur. 2020, 24, 100347. [Google Scholar] [CrossRef]
- Ritzel, C.; Ammann, J.; Mack, G.; El Benni, N. Determinants of the decision to build up excessive food stocks in the COVID-19 crisis. Appetite 2022, 176, 106089. [Google Scholar] [CrossRef]
- Tortajada, C.; Lim, N.S.W. Food Security and COVID-19: Impacts and resilience in Singapore. Front. Sustain. Food Syst. 2021, 5, 740780. [Google Scholar] [CrossRef]
- Singapore Parliament. Impact of COVID-19 Restrictions on Singapore’s Economy and Robustness of National Stockpile of Essential Items. 2020. Available online: https://sprs.parl.gov.sg/search/sprs3topic?reportid=oral-answer-2191 (accessed on 23 October 2023).
- Teng, P. Assuring food security in Singapore, a small island state facing COVID-19. Food Sec. 2020, 12, 801–804. [Google Scholar] [CrossRef]
- Singapore Food Agency. 30 by 30—Our Food Future. 2019. Available online: https://www.ourfoodfuture.gov.sg/30by30/ (accessed on 23 October 2023).
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant responses to heat stress: Physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.T. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- He, J.; Lee, S.K. Growth and photosynthetic characteristics of lettuce (Lactuca sativa L.) grown under fluctuating hot ambient temperatures with the manipulation of cool rootzone temperature. J. Plant Physiol. 1998, 152, 387–391. [Google Scholar]
- He, J.; Lee, S.K.; Dodd, I.C. Limitations to photosynthesis of lettuce grown under tropical conditions: Alleviation by root-zone cooling. J. Exp. Bot. 2001, 52, 1323–1330. [Google Scholar] [CrossRef]
- He, J.; Lee, S.K. Relationship among photosynthesis, ribulose-1,5-bisphosphate carboxylase (Rubisco) and water relations of subtropical vegetable Chinese broccoli grown in the tropics by manipulation of root-zone temperature. Environ. Exp. Bot. 2001, 46, 119–128. [Google Scholar] [CrossRef]
- He, J.; Lai, C.-H.; Lim, Y.J.; Qin, L. Heat priming impacts on root morphology, productivity and photosynthesis of temperate vegetable crops grown in the tropics. J. Adv. Agric. Technol. (JOAAT) 2019, 6, 14–19. [Google Scholar] [CrossRef]
- He, J.; Tan, C.; Qin, L. Root-zone heat priming effects on maximum quantum efficiency of PSII, productivity, root Morphology and nutritional quality of two aeroponically grown leafy greens in a tropical greenhouse. Plants 2022, 11, 1684. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The effect of LED light spectra on the growth, yield and nutritional value of red and green lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, R.; Guo, S. Effects of LED spectra on growth, gas exchange, antioxidant activity and nutritional quality of vegetable species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.; Li, J.; Zhang, Y.; Yang, Y.; Zheng, W.; Xue, X. Effects of light-emitting diode spectral combinations on growth and quality of pea sprouts under long photoperiod. Front. Plant Sci. 2022, 13, 978462. [Google Scholar] [CrossRef]
- He, J.; Chua, E.L.; Qin, L. Drought does not induce crassulacean acid metabolism (CAM) but regulates photosynthesis and enhances nutritional quality of Mesembryanthemum crystallinum. PLoS ONE 2020, 15, e0229897. [Google Scholar] [CrossRef]
- He, J.; Chang, C.; Qin, L.; Lai, C.H. Impacts of deficit irrigation on photosynthetic performance, productivity and nutritional quality of aeroponically grown Tuscan Kale (Brassica oleracea L.) in a tropical greenhouse. Int. J. Mol. Sci. 2023, 24, 2014. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, H.; Mozafari, V.; Roosta, H.R. Optimizing growth conditions in vertical farming: Enhancing lettuce and basil cultivation through the application of the Taguchi method. Sci. Rep. 2023, 13, 6717. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Chong, E.L.C.; Choong, T.W.; Lee, S.K. Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs. Front. Plant Sci. 2017, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, L.; Chow, W.S. Impacts of LED spectral quality on leafy vegetables: Productivity closely linked to photosynthetic performance or associated with leaf traits? Int. J. Agric. Biol. Eng. 2019, 12, 16–25. [Google Scholar] [CrossRef]
- Niu, Y.; Lyu, H.; Zhang, L.M.; Li, H. Photosynthesis prediction and light spectra optimization of greenhouse tomato based on response of red–blue ratio. Sci. Hortic. 2023, 318, 112065. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of spectral quality of monochromatic LED lights on the growth of artichoke seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef]
- Cavallaro, V.; Muleo, R. The effects of LED light spectra and intensities on plant growth. Plants 2022, 11, 1911. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Aljafer, N.; Jbara, M.; McCallum, L.; Lengger, S.; Fuller, M.P. The Impact of LED lighting spectra in a plant factory on the growth, physiological traits and essential oil content of lemon balm (Melissa officinalis). Plants 2022, 11, 342. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Microclimate control to increase productivity and nutritional quality of leafy vegetables in a cost-effective manner. Int. J. Agric. Biol. Eng. 2022, 15, 55–61. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Boon, N.; Geelen, D. Vertical Farming: The only way is up? Agronomy 2022, 12, 2. [Google Scholar] [CrossRef]
- He, J.; Bte Jawahir, N.K.; Qin, L. Quantity of supplementary LED lightings regulates photosynthetic apparatus, improves photosynthetic capacity and enhances productivity of Cos lettuce grown in a tropical greenhouse. Photosyn. Res. 2021, 149, 187–199. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. J. Plant Physiol. 2020, 252, 153–239. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Chaudhry, U.K.; Raza, A.; Charagh, S.; Bakhsh, A.; Bohra, A.; Ali, S.; Chitikineni, A.; Saeed, Y.; Visser, R.G.F.; et al. Developing future heat-resilient vegetable crops. Funct. Integr. Genom. 2023, 23, 47. [Google Scholar] [CrossRef]
- Dumitru, E.A.; Sterie, C.M.; Rodino, S.; Butu, M. Consumer preferences in the purchase of agri-food products: Implications for the development of family farms. Agriculture 2023, 13, 1478. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Wien, H.C. The Physiology of Vegetable Crops; Cab International: Oxon, NY, USA, 1997. [Google Scholar]
- Dumitru, E.A.; Berevoianu, R.L.; Tudor, V.C.; Teodorescu, F.-R.; Stoica, D.; Giucă, A.; Ilie, D.; Sterie, C.M. Climate change impacts on vegetable crops: A systematic review. Agriculture 2023, 13, 1891. [Google Scholar] [CrossRef]
- Porter, J.R.; Challinor, A.J.; Henriksen, C.B.; Howden, S.M.; Martre, P.; Smith, P. Invited review: Intergovernmental panel on climate change, agriculture, and food—A case of shifting cultivation and history. Global Chang. Biol. 2019, 25, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Scheelbeek, P.F.D.; Birda, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Nat. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef]
- Mylonas, I.; Stavrakoudis, D.; Katsantonis, D.; Korpetis, E. Chapter 1—Better farming practices to combat climate change. In Climate Change and Food Security with Emphasis on Wheat; Ozturk, M., Gul, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–29. [Google Scholar]
- Kozai, T. Plant factory in Japan—Current situation and perspectives. Chron. Hortic. 2013, 53, 8–11. [Google Scholar]
- Wang, R.; Isozaki, M.; Iwasaki, Y.; Muramatsu, Y. Root-zone temperature effects on spinach biomass production using a nutrient film technique system. HortSci. 2022, 57, 532–540. [Google Scholar] [CrossRef]
- Yamori, N.; Levine, C.P.; Mattson, N.S.; Yamori, W. Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants. Plant Mol. Biol. 2022, 110, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, B.; McCormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, Q.; Michelsen, A.; Lu, M.; Huang, L.; Zhao, R. The effect of experimental warming on fine root functional traits of woody plants: Data synthesis. Sci. Total Environ. 2023, 894, 165003. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.C.; Tachibana, S. Effect of supraoptimal root temperature on the growth, root respiration and sugar content of cucumber plants. Sci. Hortic. 1994, 58, 289–301. [Google Scholar] [CrossRef]
- He, J. Mineral nutrition of aeroponically grown subtropical and temperate crops in the tropics with manipulation of root-zone temperature at different growth irradiances. Plant Stress 2010, 4, 14–30. [Google Scholar]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Stokes, A. Root traits as drivers of plant and ecosystem functioning: Current understanding, pifalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Luo, H.; Xu, H.; Chu, C.; He, F.; Fang, S. High temperature can change root system architecture and intensify root interactions of plant seedlings. Front. Plant Sci. 2020, 11, 160. [Google Scholar]
- Monje, O.; Anderson, S.; Stutte, G.W. The effects of elevated root zone temperature on the development and carbon partitioning of spring wheat. J. Amer. Soc. Hort. Sci. 2007, 132, 178–184. [Google Scholar] [CrossRef]
- Aldous, D.E.; Kaufmann, J.E. Role of root temperature on shoot growth of two Kentucky bluegrass cultivars. Agron. J. 1979, 71, 545–547. [Google Scholar] [CrossRef]
- Kuroyanagi, T.; Paulsen, G.M. Mediation of high-temperature injury by roots and shoots during reproductive growth of wheat. Plant Cell Environ. 1998, 11, 517–523. [Google Scholar] [CrossRef]
- Paulsen, G.M. High temperature responses of crop plants. In Physiology and Determination of Crop Yield; Boote, K.J., Bennett, J.M., Sinclair, T.R., Paulsen, G.M., Eds.; American Society of Agronomy, Crop Science Society of America and Soil Science Society of America: Madison, WI, USA, 1994; pp. 365–389. [Google Scholar]
- Sakamoto, M.; Suzuki, T. Effect of rootzone temperature on growth and quality of hydroponically grown red leaf lettuce (Lactuca sativa L. cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350–2360. [Google Scholar] [CrossRef]
- Levine, C.P.; Hayashi, S.; Ohmori, Y.; Kusano, M.; Kobayashi, M.; Nishizawa, T.; Kurimoto, I.; Kawabata, S.; Yamori, W. Controlling root zone temperature improves plant growth and pigments in hydroponic lettuce. Ann. Bot. 2023, 132, 455–469. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chua, N.Y.A.; Qin, L. Interaction between iron stress and root-zone temperature on physiological aspects of aeroponically grown Chinese broccoli (Brassica alboglabra). J. Plant Nutri. 2008, 31, 1–20. [Google Scholar]
- Tan, L.P.; He, J.; Lee, S.K. Effects of root-zone temperature on the root development and nutrient uptake of Lactuca sativa L. cv ‘Panama’ grown in an aeroponic system in the tropics. J. Plant Nutr. 2002, 25, 297–314. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef] [PubMed]
- Nada, K.; He, L.; Tachibana, S. Impaired photosynthesis in cucumber (Cucumis sativus L.) by high root-zone temperature involves ABA-induced stomatal closure and reduction in ribulose-1,5-biphosphate carboxylase/oxygenase activity. J. Jpn. Soc. Hortic. Sci. 2003, 72, 504–510. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Qiao, Y.X.; Zhang, Y.L.; Zhou, Y.H.; Yu, J.Q. Effects of root temperature on leaf gas exchange and xylem sap abscisic acid concentrations in six Cucurbitaceae species. Photosynthetica 2008, 46, 356–362. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Lee, K.; Dodd, I.C. An assessment of ethylene mediation of lettuce (Lactuca sativa) root growth at high temperatures. J. Exp. Bot. 2007, 58, 3017–3024. [Google Scholar] [CrossRef]
- Choong, T.W.; He, J.; Lee, S.K.; Dodd, I.C. Growing different Lactuca genotypes aeroponically within a tropical greenhouse—Cool rootzone temperatures decreased rootzone ethylene concentrations and increased shoot growth. Front. Physiol. 2016, 7, 405. [Google Scholar] [CrossRef]
- Dodd, I.C.; He, J.; Turnbull, C.G.N.; Lee, S.K.; Critchley, C. Influence of supra-optimal root temperatures on growth and stomatal conductance in Capsicum annuum L. J. Exp. Bot. 2000, 51, 239–248. [Google Scholar] [CrossRef]
- Udagawa, Y.; Ito, T.; Gomi, K. Effects of root temperature on the absorption of water and mineral nutrients by strawberry plants ‘Reiko’ grown hydroponically. J. Jpn. Soc. Hortic. Sci. 1991, 59, 711–717. [Google Scholar] [CrossRef]
- Klock, K.A.; Graves, W.R.; Taber, H.G. Growth and phosphorus, zinc, and manganese content of tomato, muskmelon, and honey locust at high root-zone temperatures. J. Plant Nutr. 1996, 19, 795–806. [Google Scholar] [CrossRef]
- He, J.; Tan, L.P.; Lee, S.K. Root-zone temperature effects on photosynthesis, 14C-photoassimilate partitioning and growth of temperate lettuce (Lactuca sativa cv. Panama) grown in the tropics. Photosynthetica 2009, 47, 95–103. [Google Scholar] [CrossRef]
- Ruter, J.M.; Ingram, D.L. High root-zone temperatures influence rubisco activity and pigment accumulation in leaves of ‘Rotundifolia’ holly. J. Am. Soc. Hortic. Sci. 1992, 117, 154–157. [Google Scholar] [CrossRef]
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef]
- Andriy, B.; Ilnytskyy, Y.; Wóycicki, R.; Kepeshchuk, N.; Fogen, D.; Kovalchuk, I. The elucidation of stress memory inheritance in Brassica rapa plants. Front. Plant Sci. 2015, 6, 5. [Google Scholar]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Yeh, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. Int. J. Exp. Plant Biol. 2012, 195, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in Achillea millefolium. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; McLoughlin, F.; Eman Basha, E.; Vierling, E. Assessing plant tolerance to acute heat stress. Bio-Protocol 2017, 7, e2405. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, M.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Phil. Soc. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Sulaiman, H.Y.; Liu, B.; Abiola, Y.O.; Kaurilind, E.; Niinemets, Ü. Impact of heat priming on heat shock responses in Origanum vulgare: Enhanced foliage photosynthetic tolerance and biphasic emissions of volatiles. Plant Physiol. Biochem. 2023, 196, 567–579. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, S.; Prasad, P.V.V.; Jha, U.; Kumar, J.; Siddique, K.H.M.; Nayyar, H. Heat priming of lentil (Lens culinaris Medik.) seeds and foliar treatment with γ-aminobutyric acid (GABA), confers protection to reproductive function and yield traits under high-temperature stress environments. Int. J. Mol. Sci. 2021, 22, 5825. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, S.K. Photosynthetic utilization of radiant energy by temperate lettuce grown under natural tropical condition with manipulation of root-zone temperature. Photosynthetica 2004, 42, 457–463. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Agovino, M.; Casaccia, M.; Ciommi, M.; Ferrara, M.; Marchesano, K. Agriculture, climate change and sustainability: The case of EU-28. Ecol. Indic. 2019, 105, 525–543. [Google Scholar] [CrossRef]
- Carotti, L.; Pistillo, A.; Zauli, I.; Meneghello, D.; Martin, M.; Pennisi, G.; Gianquinto, G.; Orsini, F. Improving water use efficiency in vertical farming: Effects of growing systems, far-red radiation and planting density on lettuce cultivation. Agric. Water Manag. 2023, 285, 108365. [Google Scholar] [CrossRef]
- Incrocci, L.; Thompson, R.B.; Fernandez-Fernandez, M.D.; De Pascale, S.; Pardossi, A.; Stanghellini, C.; Rouphael, Y.Y.; Gallardo, M. Irrigation management of European greenhouse vegetable crops. Agric. Water Manag. 2020, 242, 106393. [Google Scholar] [CrossRef]
- Mahmoud, M.M.A.; Fayad, A.M. The effect of deficit irrigation, partial root drying and mulching on tomato yield, and water and energy saving. Irrig. Drain. 2022, 71, 295–309. [Google Scholar] [CrossRef]
- Asres, L.A. Alternative techniques of irrigation water management for improving crop water productivity. Rev. Agric. Sci. 2023, 11, 36–53. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef]
- Yazgan, S.; Ayas, S.; Demirtas, C.; Büyükcangaz, H.; Candogan, B.N. Deficit irrigation effects on lettuce (Lactuca sativa var. Olenka) yield in unheated greenhouse condition. J. Food Agric. Environ. 2008, 6, 357–361. [Google Scholar]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Yu, H.; Yang, X.; Jiang, W. Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot pepper grown in soilless culture. HortScience 2014, 49, 722–728. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Malejane, D.N.; Tinyani, P.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Deficit irrigation improves phenolic content and antioxidant activity in leafy lettuce varieties. Food Sci. Nutr. 2018, 6, 334–341. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Wongs-Aree, C.; Setha, S.; Komkhuntod, R.; Kondo, S.; Srilaong, V. Pre-harvest drought stress treatment improves antioxidant activity and sugar accumulation of sugar apple at harvest and during storage. Agric. Nat. Resour. 2018, 52, 146–154. [Google Scholar] [CrossRef]
- Tatum, M. Inside Singapore’s Huge Bet on Vertical Farming. 2020. Available online: https://www.technologyreview.com/2020/10/13/1009497/singapore-vertical-farming-food-security (accessed on 18 December 2023).
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Weaver, G.; van Iersel, M.W. Photochemical characterization of greenhouse-grown lettuce (Lactuca sativa L. ‘Green Towers’) with applications for supplemental lighting control. HortScience 2019, 54, 317–322. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Liu, Y.; Choong, T.W. Photosynthetic capacities and productivity of indoor hydroponically grown Brassica alboglabra Bailey under different light sources. Am. J. Plant Sci. 2015, 6, 554–563. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities. Acta Hortic. 2020, 1296, 219–226. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Koh, J.Q.D. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2022, 49, 483–495. [Google Scholar] [CrossRef]
- He, J.; Leng, S.Y.; Qin, L. Growth, physiology and nutritional quality of C4 halophyte Portulaca oleracea L. grown aeroponically in different percentages of artificial seawater under different light-emitting diode spectral qualities. Plants 2023, 12, 3214. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kong, S.M.; Choong, T.W.; Qin, L. Productivity and photosynthetic characteristics of heat-resistant and heat-sensitive recombinant inbred lines (RILs) of Lactuca sativa in response to different durations of LED lighting. Acta Hortic. 2016, 1134, 187–194. [Google Scholar] [CrossRef]
- Choong, T.W.; He, J.; Qin, L.; Lee, S.K. Quality of supplementary LED lighting effects on growth and photosynthesis of two different Lactuca recombinant inbred lines (RILs) grown in a tropical greenhouse. Photosynthetica 2018, 54, 1278–1286. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Alahakoon, P.K.D.T.; Chua, B.L.J.; Choong, T.W.; Lee, S.K. LED-integrated vertical aeroponic farming system for vegetable production in Singapore. Acta Hortic. 2018, 1227, 599–606. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoying, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Bai, Z.; Mao, S.; Han, Y.; Feng, L.; Wang, G.; Yang, B.; Zhi, X.; Fan, Z.; Lei, Y.; Du, W. Study on light interception and biomass production of different cotton cultivars. PLoS ONE 2016, 11, e0156335. [Google Scholar] [CrossRef]
- Koester, R.P.; Skoneczka, J.A.; Cary, T.R.; Diers, B.W.; Ainsworth, E.A. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J. Exp. Bot. 2014, 65, 3311–3321. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, A.; Cheng, Z.-M. (Max). Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Nájera, C.; Urrestarazu, M. Effect of the intensity and spectral quality of LED light on yield and nitrate accumulation in vegetables. HortScience 2019, 54, 1745–1750. [Google Scholar] [CrossRef]
- Keller, B.; Zimmermann, L.; Rascher, U.; Matsubara, S.; Steier, A.; Muller, O. Toward predicting photosynthetic efficiency and biomass gain in crop genotypes over a field season. Plant Physiol. 2022, 188, 301–317. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Hanan, J.J. Greenhouses: Advanced Technology for Protected Horticulture; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Tewolde, F.T.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLoS ONE 2018, 13, e0206592. [Google Scholar] [CrossRef]
- Brito, C.; Ferreira, H.; Dinis, L.-T.; Trindade, H.; Marques, D.; Correia, C.M.; Moutinho-Pereira, J. Different LED light intensity and quality change perennial ryegrass (Lolium perenne L.) physiological and growth responses and water and energy consumption. Front. Plant Sci. 2023, 14, 1160100. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A meta-analysisof plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Baumbauer, D.A.; Schmidt, C.B.; Burgess, M.H. Leaf lettuce yield is more sensitive to low daily light integral than kale and spinach. HortScience 2019, 54, 2159–2162. [Google Scholar] [CrossRef]
- Cui, J.; Song, S.; Yu, J.; Liu, H. Effect of daily light integral on cucumber plug seedlings in artificial light plant factory. Horticulturae 2021, 7, 139. [Google Scholar] [CrossRef]
- He, J.; Gan, J.H.S.; Qin, L. Productivity, photosynthetic light-use efficiency, nitrogen metabolism and nutritional quality of C4 halophyte Portulaca oleracea L. grown indoors under different light intensities and durations. Front. Plant Sci. 2023, 14, 106394. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Rosenqvist, E.; van Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Gavhane, K.P.; Hasan, M.; Singh, D.K. Determination of optimal daily light integral (DLI) for indoor cultivation of iceberg lettuce in an indigenous vertical hydroponic system. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef]

| Vegetable Species | LED Spectral Quality/ Intensity/Photoperiod | Parameters Studied | Reference (Year & Reference) |
|---|---|---|---|
| Chinese Broccoli (Brassica alboglabra Bailey) | LED spectral quality (indoors and greenhouse) | Leaf growth, shoot and root productivity, photosynthetic gas exchanges, stomatal conductance, photosynthetic pigments, photosynthetic light use efficiency | 2015 [108] 2018 [114] 2019 [30] |
| Nai bai (Brassica chinensis L.) and Mizuna (B. juncea var. japonica) | LED spectral quality (greenhouse) | Leaf growth, shoot and root productivity, photosynthetic gas exchanges, stomatal conductance, photosynthetic pigments | 2018 [114] |
Lettuce (Lactuca sativa L.)
| LED spectral quality and intensity and photoperiod; supplemental LED lighting to natural sunlight (indoors and greenhouse) | Leaf growth, shoot and root productivity, photosynthetic gas exchanges, stomatal conductance, photosynthetic pigments photosynthetic light use efficiency, light interception area, light absorption, photosynthetic capacity, photosynthetic characteristics | 2016 [112] 2018 [113] 2019 [30] 2020 [37] |
| Common ice plants (Mesembryanthemum crystallinum) | LED spectral quality (indoors) | Leaf growth, leaf water status, shoot and root productivity, photosynthetic pigment photosynthetic gas exchanges, stomatal conductance, light use efficiency, nitrogen metabolism, nutritional quality | 2017 [29] 2019 [30] 2020 [109] 2022 [110] |
| Sweet potato | LED intensity, supplemental LED lighting to natural sunlight (greenhouse) | Leaf growth, photosynthetic pigments photosynthetic gas exchanges, stomatal conductance, light use efficiency | 2020 [38] |
| Purslane (Portulaca oleracea L.) | LED spectral quality, intensity, photoperiod, DLI (indoors) | Root morphology, leaf growth, leaf water status, shoot and root productivity, photosynthetic pigments, light use efficiency, nitrogen metabolism, nutritional quality | 2023 [111] 2023 [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J. Enhancing Productivity and Improving Nutritional Quality of Subtropical and Temperate Leafy Vegetables in Tropical Greenhouses and Indoor Farming Systems. Horticulturae 2024, 10, 306. https://doi.org/10.3390/horticulturae10030306
He J. Enhancing Productivity and Improving Nutritional Quality of Subtropical and Temperate Leafy Vegetables in Tropical Greenhouses and Indoor Farming Systems. Horticulturae. 2024; 10(3):306. https://doi.org/10.3390/horticulturae10030306
Chicago/Turabian StyleHe, Jie. 2024. "Enhancing Productivity and Improving Nutritional Quality of Subtropical and Temperate Leafy Vegetables in Tropical Greenhouses and Indoor Farming Systems" Horticulturae 10, no. 3: 306. https://doi.org/10.3390/horticulturae10030306





