Autochthonous Microbes to Produce Ligurian Taggiasca Olives (Imperia, Liguria, NW Italy) in Brine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olives Sampling
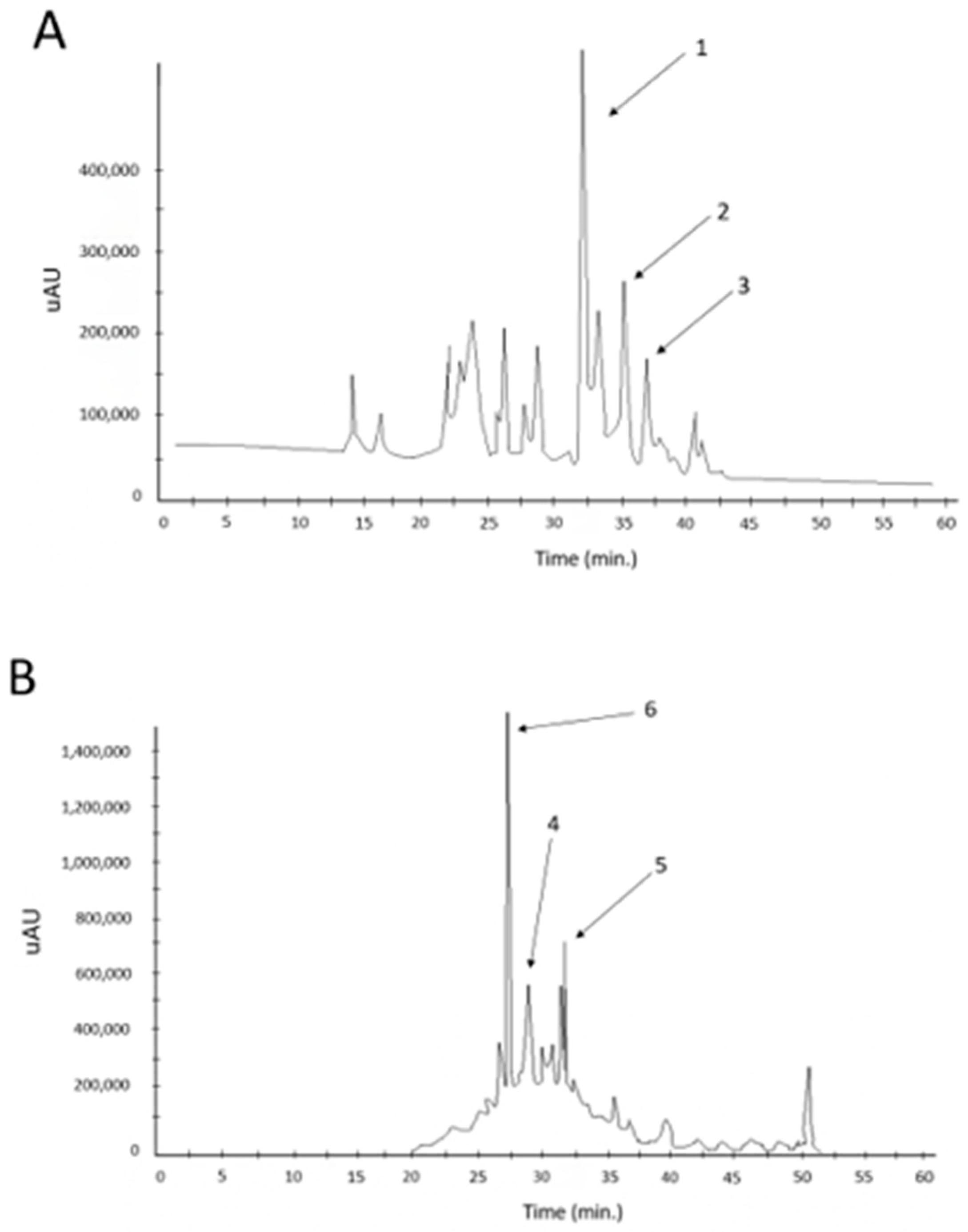
2.2. Fungal Characterisation of Drupes and Brines
2.3. Bacteria Characterisation of the Brines
- Thermophilic lactobacilli: 42 °C for 48 h;
- Mesophilic lactobacilli: 35 °C for 48 h;
- Psychrophilic lactobacilli: 25 °C for 5 days;
- Mesophiles and psychrophiles: 30 °C for 48 h + 22 °C for 24 h.
2.4. Evaluation of the Autochthonous Microbial Strains’ Properties of Oleuropein Degradation
3. Results
3.1. Fungal Characterisation
3.2. Bacteria
3.3. Evaluation of the Autochthonous Microbial Strains’ Properties of Oleuropein Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pistarino, E.; Aliakbarian, B.; Casazza, A.A.; Paini, M.; Cosulich, M.E.; Perego, P. Combined effect of starter culture and temperature on phenolic compounds during fermentation of Taggiasca black olives. Food Chem. 2013, 138, 2043–2049. [Google Scholar] [CrossRef]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A preliminary study on the authentication and traceability of extra virgin olive oil made from Taggiasca olives by means of trace and ultra-trace elements distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Senizza, B.; Ganugi, P.; Trevisan, M.; Lucini, L. Combining untargeted profiling of phenolics and sterols, supervised multivariate class modelling and artificial neural networks for the origin and authenticity of extra-virgin olive oil: A case study on Taggiasca Ligure. Food Chem. 2023, 404, 134543. [Google Scholar] [CrossRef]
- Rellini, I.; Demasi, M.; Scopesi, C.; Ghislandi, S.; Salvidio, S.; Pini, S.; Stagno, A. Evaluation of the environmental components of the taggiasca “terroir” olive (imperia, Italy). BELS-Bull. Environ. Life Sci. 2022, 4, 1. [Google Scholar]
- Penland, M.; Pawtowski, A.; Pioli, A.; Maillard, M.B.; Debaets, S.; Deutsch, S.M.; Coton, M. Brine salt concentration reduction and inoculation with autochthonous consortia: Impact on Protected Designation of Origin Nyons black table olive fermentations. Food Res. Int. 2022, 155, 111069. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Use of air-protected headspace to prevent yeast film formation on the brine of Leccino and Taggiasca black table olives processed in industrial-scale plastic barrels. Foods 2020, 9, 941. [Google Scholar] [CrossRef]
- Ciafardini, G.; Venditti, G.; Zullo, B.A. Yeast dynamics in the black table olives processing using fermented brine as starter. Food Res. 2021, 5, 92–106. [Google Scholar] [CrossRef]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of starter cultures to table olive fermentation: An overview on the experimental studies. Front. Microbiol. 2012, 3, 248. [Google Scholar] [CrossRef] [Green Version]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table olives more than a fermented food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, R.; Di Vaio, C.; Cirillo, C. Endophytic fungi of olive tree. Microorganisms 2020, 8, 1321. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue (No. RESEARCH). Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Fryer, T.F.; Smith, D.G. Identification of the lactic acid bacteria. In Identification Methods for Microbiologists Part.A. The Society for Applied Bacteriology; Gibbs, B.M., Skinner, F.A., Eds.; Technical Series, 1; Academic Press: London, UK, 1966; pp. 65–79. [Google Scholar]
- Kneifel, W.; Pacher, B. An X-glu based agar medium for the selective enumeration of Lactobacillus acidophilus in yogurt-related milk products. Int. Dairy J. 1993, 3, 277–291. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A.; Montedoro, G.F. HPLC evaluation of phenols in olive fruit, virgin olive oil, vegetation waters and pomace and 1D and 2DNMR characterization. J. Am. Oil Chem. Soc. 1999, 76, 873882. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Occurrence and infection of Cladosporium, Fusarium, Epicoccum and Aureobasidium in withered rotten grapes during post-harvest dehydration. Antonie van Leeuwenhoek 2015, 108, 1171–1180. [Google Scholar] [CrossRef]
- Almeida, M.; Hébert, A.; Abraham, A.L.; Rasmussen, S.; Monnet, C.; Pons, N.; Delbès, C.; Loux, V.; Batto, J.M.; Leonard, P.; et al. Construction of a dairy microbial genome catalog opens new perspectives for the metagenomic analysis of dairy fermented products. BMC Genom. 2014, 15, 1101. [Google Scholar] [CrossRef] [Green Version]
- Demirci, H.; Kurt-Gur, G.; Ordu, E. Microbiota profiling and screening of the lipase active halotolerant yeasts of the olive brine. World J. Microbiol. Biotechnol. 2021, 37, 23. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Esteve-Zarzoso, B.; Bordons, A.; Rozès, N. Microbial population dynamics during the processing of Arbequina table olives. Food Res. Int. 2008, 41, 738–744. [Google Scholar] [CrossRef]
- Botta, C.; Cocolin, L. Microbial dynamics and biodiversity in table olive fermentation: Culture-dependent and-independent approaches. Front. Microbiol. 2012, 3, 245. [Google Scholar] [CrossRef] [Green Version]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Frost, B.; Liu, J. Oleuropein, unexpected benefits! Oncotarget 2017, 8, 17409. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Guven, E.; Ozturk, A. Understanding the characteristics of oleuropein for table olive processing. J. Food Process. Technol. 2014, 5, 1. [Google Scholar]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.E. Table Olive Fermentation Using Starter Cultures with Multifunctional Potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A Review on Adventitious Lactic Acid Bacteria from Table Olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Bozoudi, D.; Tsaltas, D. Enterococci Isolated from Cypriot Green Table Olives as a New Source of Technological and Probiotic Properties. Fermentation 2018, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to Wickerhamomyces anomalus. Antonie van Leeuwenhoek 2011, 99, 13–23. [Google Scholar] [CrossRef]
- Cosmai, L.; Campanella, D.; De Angelis, M.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Use of starter cultures for table olives fermentation as possibility to improve the quality of thermally stabilized olive-based paste. LWT 2018, 90, 381–388. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Todaro, A.; Pino, A.; Corona, O.; Caggia, C. Microbiota and metabolome during controlled and spontaneous fermentation of Nocellara Etnea table olives. Food Microbiol. 2017, 65, 136–148. [Google Scholar] [CrossRef]
- Medina, E.; Ruiz-Bellido, M.A.; Romero-Gil, V.; Rodríguez-Gómez, F.; Montes-Borrego, M.; Landa, B.B.; Arroyo-López, F.N. Assessment of the bacterial community in directly brined Aloreña de Málaga table olive fermentations by metagenetic analysis. Int. J. Food Microbiol. 2016, 236, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Campus, M.; Degirmencioglu, N.; Comunian, R. Technologies and Trends to Improve Table Olive Quality and Safety. Front. Microbiol. 2018, 9, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tıraş, Z.E.; Yıldırım, H.K. Application of mixed starter culture for table olive production. Grasas Y Aceites 2021, 72, e405. [Google Scholar] [CrossRef]



| Species | Year 2020 | Year 2021 | Farm 1 (DB) | Farm 2 (CS) | Farm 3 (RF) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wash | Not Wash | Brine | Wash | Not Wash | Brine | Wash | Not Wash | Brine | |||
| Acrodontium crateriforme (J.F.H. Beyma) de Hoog | X | X | |||||||||
| Alternaria alternata (Fr.) Keissl. | X | X | X | X | X | X | |||||
| Alternaria infectoria E.G. Simmons | X | X | |||||||||
| Alternaria longipes (Ellis and Everh.) E.W. Mason | X | X | X | ||||||||
| Apiospora sacchari (Speg.) Pintos and P. Alvarado | X | X | X | X | |||||||
| Ascochyta rabiei (Pass.) Labr. | X | X | |||||||||
| Aspergillus heyangensis Z.T. Qi, Z.M. Sun and Yu X. Wang | X | X | |||||||||
| Aspergillus niger Tiegh. | X | X | X | X | |||||||
| Aspergillus pseudoustus Frisvad, Varga and Samson | X | X | |||||||||
| Aureobasidium microstictum (Bubák) W.B. Cooke | X | X | X | X | |||||||
| Aureobasidium pullulans (de Bary) G. Arnaud | X | X | X | X | X | ||||||
| Chaetomium sp. | X | X | |||||||||
| Cladosporium cladosporioides (Fresen.) G.A. de Vries | X | X | X | ||||||||
| Cladosporium perangustum Bensch, Crous and U. Braun | X | X | X | ||||||||
| Cladosporium sp. | X | X | X | X | X | ||||||
| Didymella pinodella (L.K. Jones) Qian Chen and L. Cai | X | X | |||||||||
| Epicoccum nigrum Link | X | X | X | X | |||||||
| Fusarium acuminatum Ellis and Everh. | X | X | |||||||||
| Fusarium brachygibbosum Padwick | X | X | |||||||||
| Fusarium oxysporum Schltdl. | X | X | X | ||||||||
| Fusarium sp. | X | X | X | X | X | X | |||||
| Mucor racemosus Bull. | X | X | X | X | |||||||
| Neocucurbitaria juglandicola Jaklitsch and Voglmayr | X | X | |||||||||
| Nigrospora sp. | X | X | |||||||||
| Penicillium brevicompactum Dierckx | X | X | |||||||||
| Penicillium carneum (Frisvad) Frisvad | X | X | X | ||||||||
| Penicillium sp. | X | X | X | X | |||||||
| Pyrenophora avenicola Y. Marín and Crous | X | X | |||||||||
| Rhodotorula sp. | X | X | |||||||||
| Trichoderma gamsii Samuels and Druzhin. | X | X | |||||||||
| Trichoderma sp. | X | X | X | X | X | ||||||
| Wickerhamomyces anomalus (E.C. Hansen) Kurtzman, Robnett and Basehoar-Powers | X | X | X | X | X | ||||||
| Jar-DB22-Wash | Jar-DB2-Not Wash | Jar-CS05-Wash | Jar-CS02-Not Wash | Jar-RF05-Wash | Jar-RF02-Not Wash |
|---|---|---|---|---|---|
| Presence of fermentation and microbial film | Presence of few bubbles and little microbial film | Few bubbles and microbial film | bubbles and microbial film on the surface | Presence of filaments on the surface: moulds | Presence of filaments on the surface: moulds |
| Growth in abundant culture after 7 days in MRS broth | Abundant growth in culture after 7 days in MRS broth, presence of strong fermentation | Growth in abundant culture after 7 days in MRS broth | NOT Growth in culture after 7 days in MRS broth | Growth in abundant culture after 24 h in MRS broth | Growth in abundant culture after 4 days in MRS broth |
| DB2-Not Wash | CS05-Wash | RF05-Wash | RF05-Wash |
|---|---|---|---|
| High growth in tubes after 4 days in MRS broth. | High growth in tubes after 4 days in MRS broth. Presence of filaments on the surface. | High growth in tubes after 48 h in MRS broth. Presence of filaments on the surface. | Weak growth in tubes after 7 days in MRS broth. Presence of filaments on the surface. |
| MRS plate: diffuse growth after 7 days. | MRS plate: diffuse growth after 7 days. | MRS plate: diffuse growth after 7 days. | MRS plate: no growth after 7 days. |
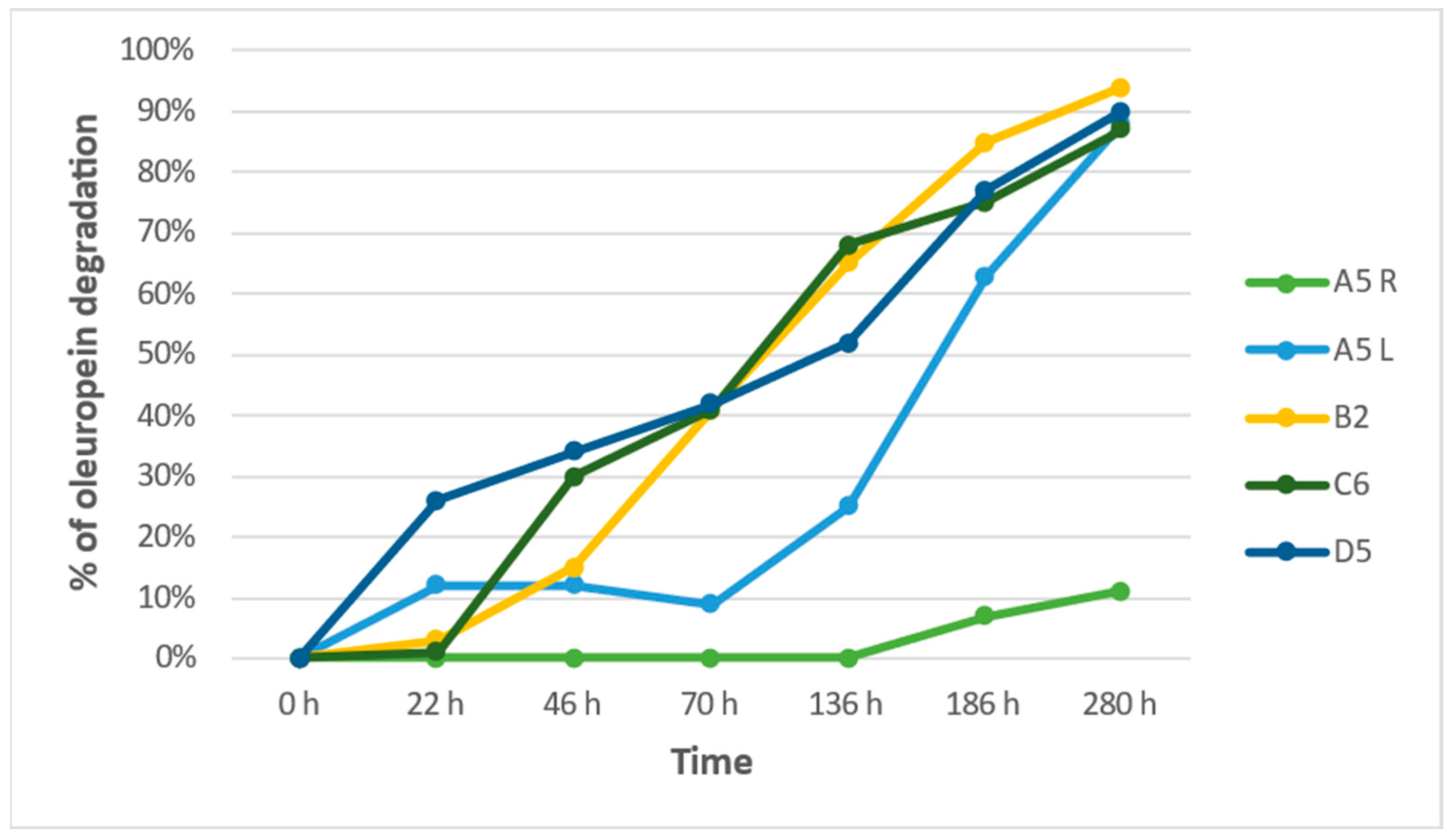
| % of Oleuropein Degradation | |||||
|---|---|---|---|---|---|
| Hours | A5 R | A5 L | B2 | C6 | D5 |
| 0 h | 0% | 0% | 0% | 0% | 0% |
| 22 h | 0% | 12% | 3% | 1% | 26% |
| 46 h | 0% | 12% | 15% | 30% | 34% |
| 70 h | 0% | 9% | 41% | 41% | 42% |
| 136 h | 0% | 25% | 65% | 68% | 52% |
| 186 h | 7% | 63% | 85% | 75% | 77% |
| 280 h | 11% | 88% | 94% | 87% | 90% |
| Total | ||||
|---|---|---|---|---|
| Hours | Probify g/L | Smooth g/L | Little Smooth g/L | Wrinkled g/L |
| 0 h | 1.14 | 1.14 | 1.14 | 1.14 |
| 18 h | 1.12 | 0.64 | 0.63 | 0.69 |
| 42 h | 0.58 | 0.69 | 0.67 | 0.56 |
| 114 h | 0.53 | 0.47 | 0.47 | 0.49 |
| First Peak | ||||
|---|---|---|---|---|
| Hours | Probify g/L | Smooth g/L | Little Smooth g/L | Wrinkled g/L |
| 0 h | 0 | 0 | 0 | 0 |
| 18 h | 0.35 | 0.23 | 0.25 | 0.24 |
| 42 h | 0.28 | 0.32 | 0.31 | 0.26 |
| 114 h | 0.24 | 0.23 | 0.23 | 0.22 |
| Second Peak | ||||
|---|---|---|---|---|
| Hours | Probify g/L | Smooth g/L | Little Smooth g/L | Wrinkled g/L |
| 0 h | 0 | 0 | 0 | 0 |
| 18 h | 0.77 | 0.41 | 0.38 | 0.45 |
| 42 h | 0.3 | 0.37 | 0.36 | 0.3 |
| 114 h | 0.31 | 0.3 | 0.27 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchi, G.; Di Piazza, S.; Rosa, E.; De Vecchis, F.; Silvagno, M.S.; Rombi, J.V.; Tiso, M.; Zotti, M. Autochthonous Microbes to Produce Ligurian Taggiasca Olives (Imperia, Liguria, NW Italy) in Brine. Fermentation 2023, 9, 680. https://doi.org/10.3390/fermentation9070680
Cecchi G, Di Piazza S, Rosa E, De Vecchis F, Silvagno MS, Rombi JV, Tiso M, Zotti M. Autochthonous Microbes to Produce Ligurian Taggiasca Olives (Imperia, Liguria, NW Italy) in Brine. Fermentation. 2023; 9(7):680. https://doi.org/10.3390/fermentation9070680
Chicago/Turabian StyleCecchi, Grazia, Simone Di Piazza, Ester Rosa, Furio De Vecchis, Milena Sara Silvagno, Junio Valerio Rombi, Micaela Tiso, and Mirca Zotti. 2023. "Autochthonous Microbes to Produce Ligurian Taggiasca Olives (Imperia, Liguria, NW Italy) in Brine" Fermentation 9, no. 7: 680. https://doi.org/10.3390/fermentation9070680





