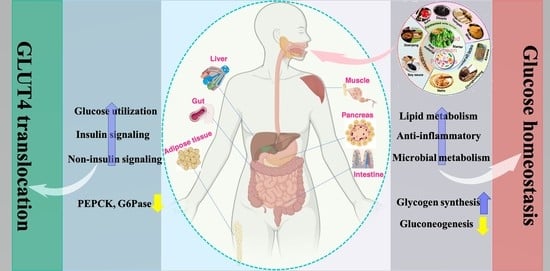
Glucoregulatory Properties of Fermented Soybean Products
Abstract
:1. Introduction
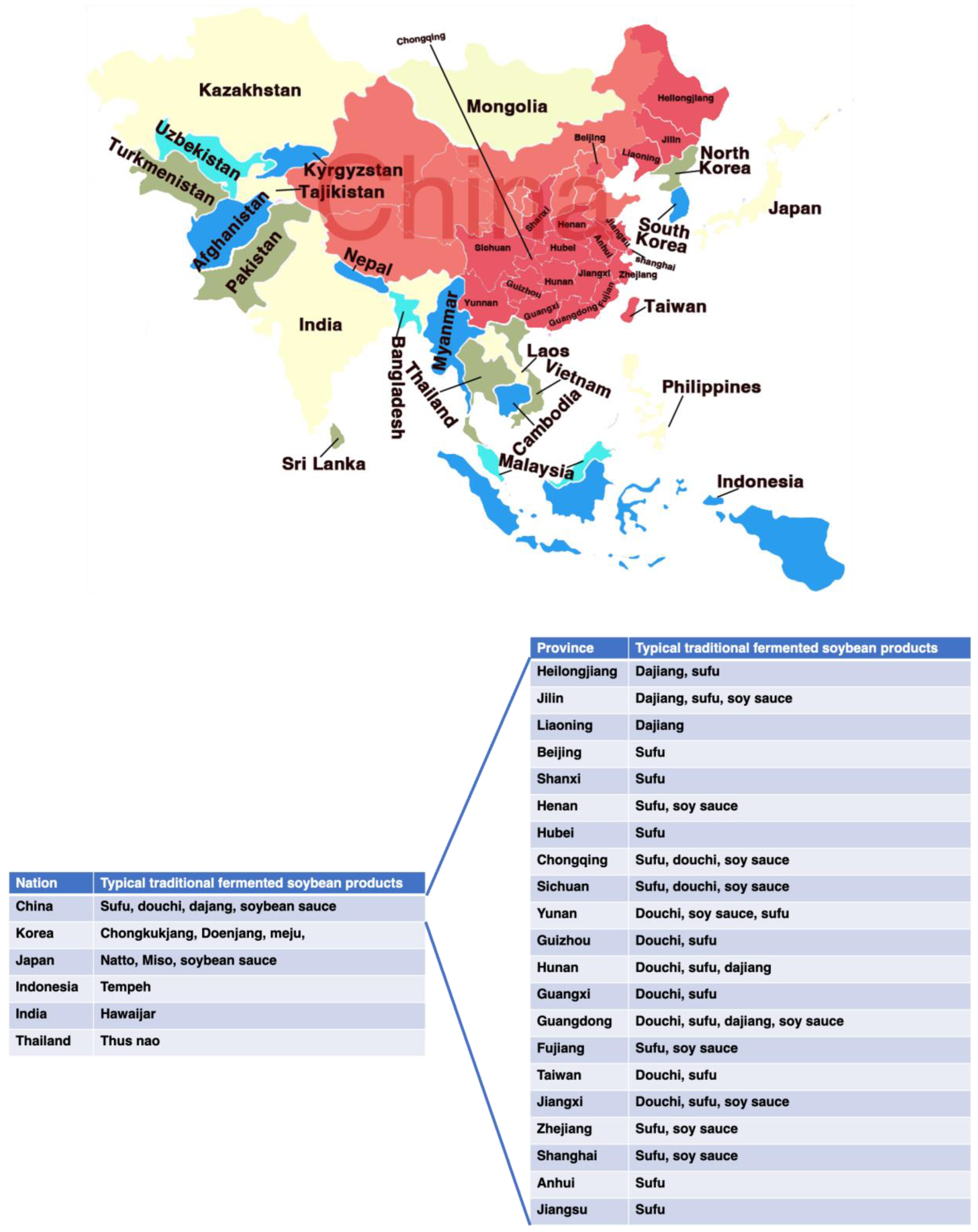
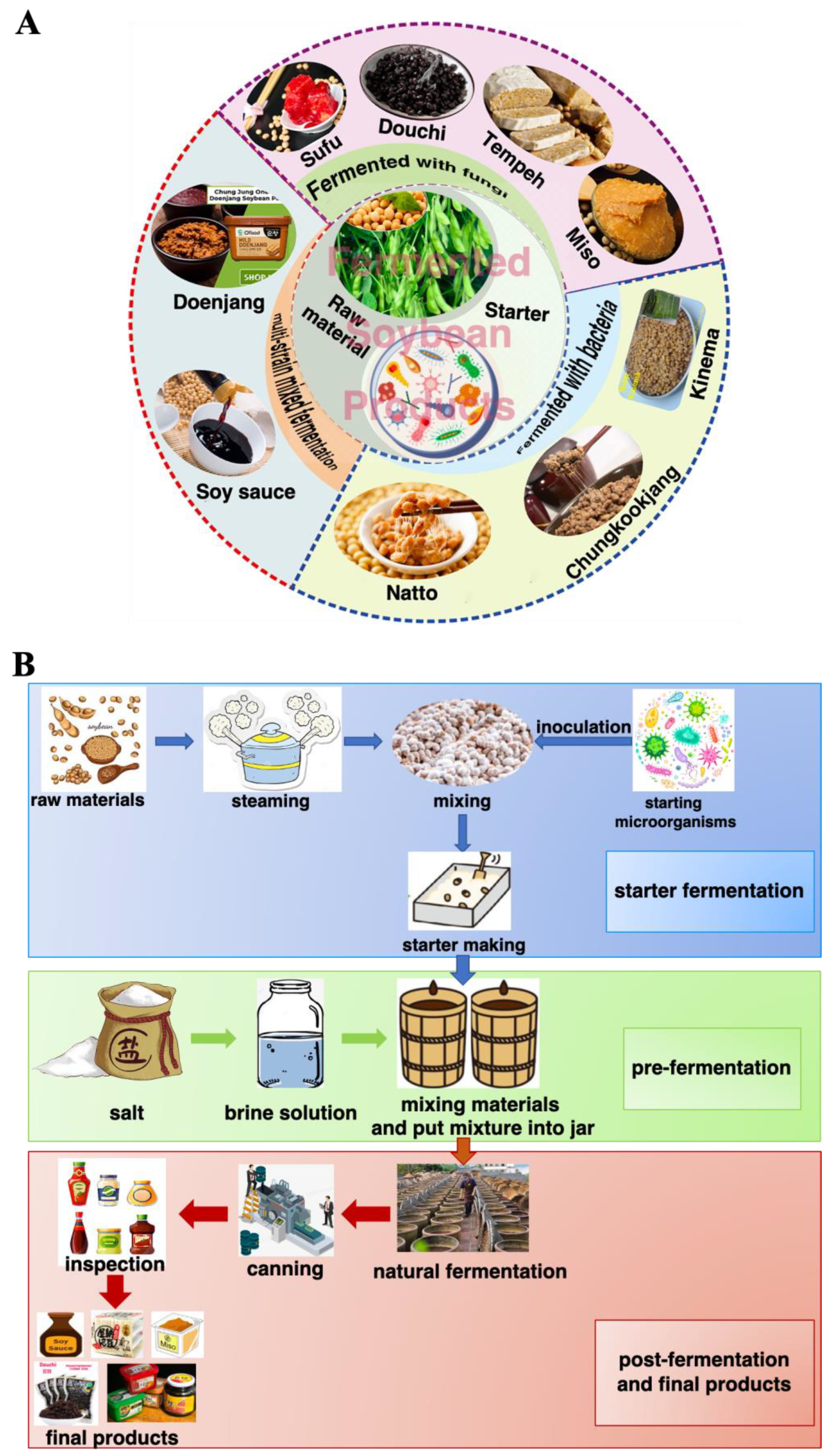
2. Traditional Fermented Soybean Foods: Processing and Products
3. T2DM and Its Pathogenesis
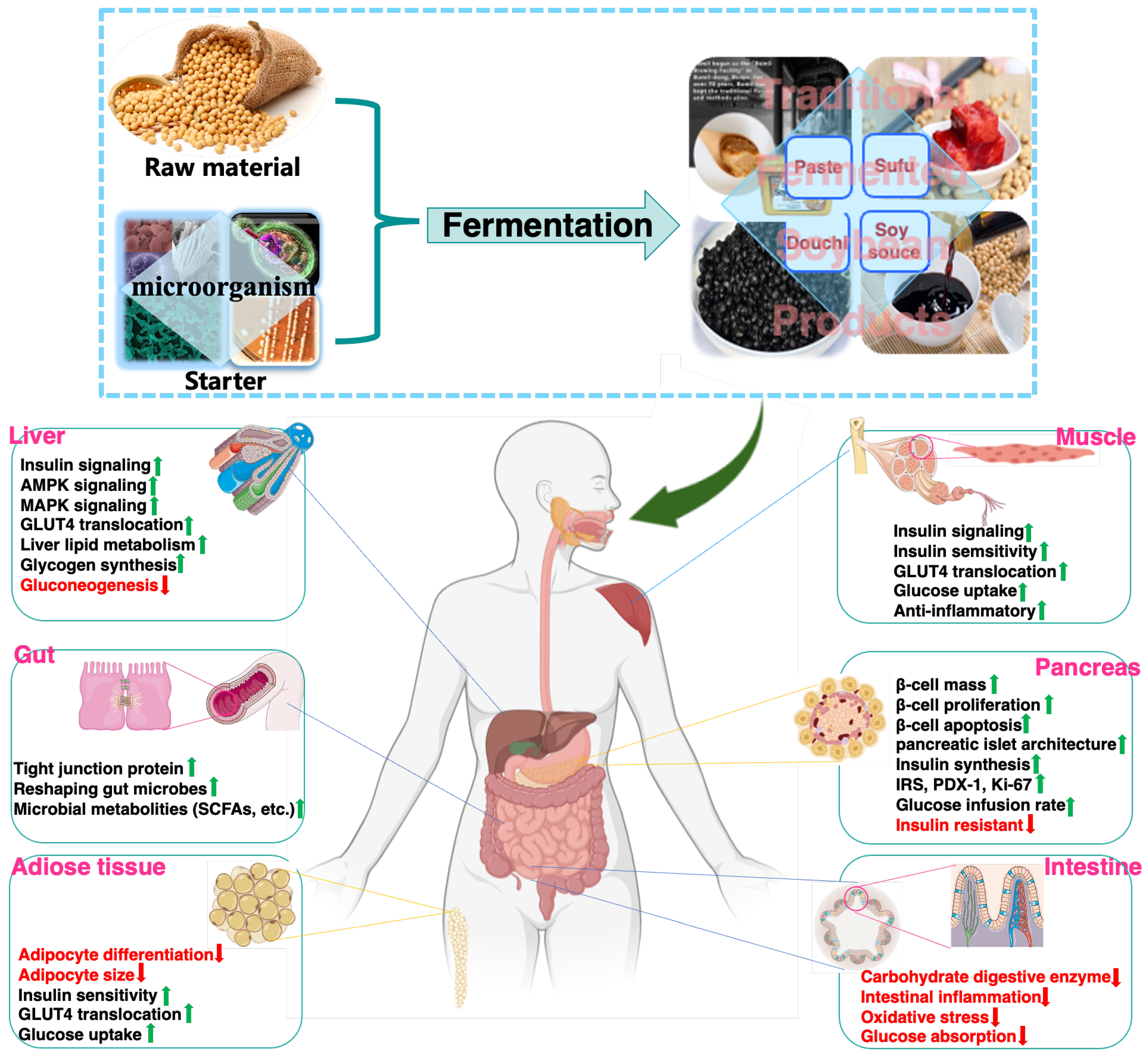
4. Major Targets and Related Signaling Pathways of Glucose Metabolism and Homeostasis
4.1. Effect of FSP on Carbohydrate Digestive Enzymes
4.2. Effect of FSP on Glucose Transporter-4 (GLUT4) Translocation and Glucose Utilization
4.3. Effect of FSP on Muscle Glucose Homeostasis
4.4. Effect of FSP on Hepatic Glucose Homeostasis
4.5. Effect of FSP on Adipose Tissue Glucose Homeostasis
4.6. Effect of FSP on Pancreatic Morphology and Function
4.7. Effect of FSP on Glucose Homeostasis through Gut Microbiota
5. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of islet amyloid polypeptide: A molecular perspective of risk factors and protective strategies for type II diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P.; Karuranga, S.; James, S.; Simmons, D.; Basit, A.; Shaw, J.E.; Wild, S.H.; Ogurtsova, K.; Saeedi, P. The International Diabetes Federation’s guide for diabetes epidemiological studies. Diabetes Res. Clin. Pract. 2021, 172, 108630. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. 2022, 62, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Synd. 2017, 11, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jia, X.; Wang, N.; Kang, J.; Hu, X.; Goff, H.D.; Cui, S.W.; Ding, H.H.; Guo, Q. Therapeutic potential of non-starch polysaccharides on type 2 diabetes: From hypoglycemic mechanism to clinical trials. Crit. Rev. Food Sci. 2022, 1–34. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Hasan, M.; Garcia-Gutierrez, E.; Kumari, S.; Prakash, O.; Nain, L.; Sachdev, A.; Dahuja, A. Current trends in the development of soy-based foods containing probiotics and paving the path for soy-synbiotics. Crit. Rev. Food Sci. 2022, 1–19. [Google Scholar] [CrossRef]
- He, F.; Chen, J. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Well. 2013, 2, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, S.; Borsingh Wann, S.; Kalita, J.; Manna, P. Current perspectives on the anti-inflammatory potential of fermented soy foods. Food Res. Int. 2022, 152, 110922. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Fermented foods, feeds, and beverages. Biotechnol. Adv. 1986, 4, 219–243. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; De Mejia, E.G. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food Sci. F 2005, 4, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.L. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kwon, D.Y.; Moon, N.R.; Kim, M.J.; Kang, H.J.; Park, S. Soybean fermentation with Bacillus licheniformis increases insulin sensitizing and insulinotropic activity. Food Funct. 2013, 4, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Daily, J.W.; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition 2011, 27, 244–252. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Daily, J.W.; Lee, G.H.; Ryu, M.S.; Yang, H.; Jeong, S.Y.; Qiu, J.Y.; Zhang, T.; Park, S. Short-term fermented soybeans with Bacillus amyloliquefaciens potentiated insulin secretion capacity and improved gut microbiome diversity and intestinal integrity to alleviate Asian type 2 diabetic symptoms. J. Agri. Food Chem. 2020, 68, 13168–13178. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional fermented soybean products: Processing, flavor formation, nutritional and biological activities. Crit. Rev. Food Sci. 2022, 62, 1971–1989. [Google Scholar] [CrossRef]
- Mah, J. Fermented soybean foods: Significance of biogenic amines. Austin J. Nutr. Food Sci. 2015, 3, 1058. [Google Scholar]
- do Prado, F.; Pagnoncelli, M.; de Melo Pereira, G.; Karp, S.; Soccol, C. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Xu, Y. Effects of Tetragenococcus halophilus and Candida versatilis on the production of aroma-active and umami-taste compounds during soy sauce fermentation. J. Sci. Food Agr. 2020, 100, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C.D. Flavor compounds of traditional fermented bean condiments: Classes, synthesis, and factors involved in flavor formation. Trends Food Sci. Technol. 2023, 133, 160–175. [Google Scholar] [CrossRef]
- Dai, S.; Pan, M.; El-Nezami, H.; Wan, J.; Wang, M.F.; Habimana, O.; Lee, J.; Louie, J.; Shah, N. Effects of lactic acid bacteria-fermented soymilk on isoflavone metabolites and short-chain fatty acids excretion and their modulating effects on gut microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef]
- Cho, K.; Lee, J.; Yun, H.; Ahn, B.; Kim, H.; Seo, W. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Teng, H.; Yuan, B.; Gothai, S.; Arulselvan, P.; Song, X.; Chen, L. Dietary triterpenes in the treatment of type 2 diabetes: To date. Trends Food Sci. Technol. 2018, 72, 34–44. [Google Scholar] [CrossRef]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The economic costs of type 2 diabetes: A global systematic review. Pharmacoeconomics 2015, 33, 811–831. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Xirouchaki, C.; Mangiafico, S.; Bate, K.; Ruan, Z.; Huang, A.; Tedjosiswoyo, B.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A. Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef]
- Das, D.; Kabir, M.E.; Sarkar, S.; Wann, S.B.; Kalita, J.; Manna, P. Antidiabetic potential of soy protein/peptide: A therapeutic insight. Int. J. Biol. Macromol. 2022, 194, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.; Scheen, A. Weight management in type 2 diabetes: Current and emerging approaches to treatment. Diabetes Care 2015, 38, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Keith Campbell, R. Fate of the beta-cell in the pathophysiology of type 2 diabetes. J. Am. Pharm. Assoc. 2009, 49, S10–S15. [Google Scholar] [CrossRef]
- Mithieux, G. The new functions of the gut in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Yamanaka-Okumura, H.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese style meal suppress postprandial glucose and insulin responses. Asia Pac. J. Clin. Nutr. 2008, 17, 663–668. [Google Scholar]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, Y.S.; Shin, D.W.; Park, S. Kochujang, a Korean fermented red pepper plus soybean paste, improves glucose homeostasis in 90% pancreatectomized diabetic rats. Nutrition 2009, 25, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yamagami, T. Fermented soybean-derived Touchi-extract with anti-diabetic effect via α-glucosidase inhibitory action in a long-term administration study with KKAy mice. Life Sci. 2001, 70, 219–227. [Google Scholar] [CrossRef]
- Yu, S.; Liu, L.; Bu, T.; Zheng, J.; Wang, W.; Wu, J.; Liu, D. Purification and characterization of hypoglycemic peptides from traditional Chinese soy-fermented douchi. Food Funct. 2022, 13, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit. Rev. Food Sci. 2017, 57, 3438–3448. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Si, X.; Wang, Y.; Gong, E.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L. Effect of fermented soybean condiment supplemented diet on α-amylase and α-glucosidase activities in Streptozotocin-induced diabetic rats. J. Funct. Foods 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, R.; Chen, G.; Wang, S.; Li, C.; Xu, Y.; Kan, J. Effect of different starter cultures on the control of biogenic amines and quality change of douchi by rapid fermentation. LWT 2019, 109, 395–405. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Y.; Yamaki, K.; Li, L. Anti-α-glucosidase activity of Chinese traditionally fermented soybean (douchi). Food Chem. 2007, 103, 1091–1096. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagami, T.; Ohshima, K. Fermented soybean derived touchi extract with anti-glycaemic effect via α-glucosidase inhibitory action in rats and humans. J. Nutr. 2001, 131, 1211–1213. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Park, H.; Lee, J.; Kim, J.; Kim, M. Reduction of biogenic amines and aflatoxins in Doenjang samples fermented with various Meju as starter cultures. Food Control 2014, 42, 181–187. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.A.; Kim, D.; Hong, S.M.; Lee, J.; Kim, M.A. Total phenolic content, antioxidant, tyrosinase and α-glucosidase inhibitory activities of water soluble extracts of noble starter culture Doenjang, a Korean fermented soybean sauce variety. Food Control 2016, 59, 854–861. [Google Scholar] [CrossRef]
- Chung, S.T.; Rico, C.W.; Kang, M. Comparative study on the hypoglycemic and antioxidative Effects of fermented paste (Doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients 2014, 6, 4610–4624. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Oh, Y.; Lim, J.; Park, J.; Kim, M.; Yoon, H.; Lim, S. Physiological properties of Jeju traditional Doenjang. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1656–1663. [Google Scholar] [CrossRef]
- Yang, H.; Kim, M.; Kim, K.; Lee, J.; Hong, S. In vitro antidiabetic and antiobesity activities of traditional Kochujang and Doenjang and their components. Prev. Nutr. Food Sci. 2019, 24, 274–282. [Google Scholar] [CrossRef]
- Kusumoto, K.; Yamagata, Y.; Tazawa, R.; Kitagawa, M.; Kato, T.; Isobe, K.; Kashiwagi, Y. Japanese traditional Miso and Koji making. J. Fungi 2021, 7, 579. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- Momose, A.; Goto, N.; Hayase, H.; Gomyo, T.; Miura, M. Effects of miso (soybean paste) on postprandial blood sugar levels. J. Jpn. Soc. Food Sci. 2010, 57, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Ci, Z.; Kojima, M. α-Glucosidase inhibitory activity in rice miso supplementary with black soybean. Am. J. Food Sci. Technol. 2019, 7, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Astawan, M.; Nurwitri, C.; Rochim, D.; Wresdiyati, T.; Widowati, S.; Bintari, S.; Ichsani, N.; Dingin, S.; Berbeda, W.; In, D. Application of vacuum packaging to extend the shelf life of fresh-seasoned tempe. Int. Food Res. J. 2016, 23, 2571–2580. [Google Scholar]
- Astawan, M.; Rahmawati, I.; Cahyani, A.; Wresdiyati, T.; Putri, S.; Fukusaki, E. Comparison between the potential of tempe flour made from germinated and nongerminated soybeans in preventing Diabetes mellitus. HAYATI J. Biosci. 2020, 27, 16. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef]
- Sayem, A.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadler, J.; Bryant, N.; Gould, G.; Welburn, C. Posttranslational modifications of GLUT4 affect its subcellular localization and translocation. Int. J. Mol. Sci. 2013, 14, 9963–9978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brereton, M.; Rohm, M.; Shimomura, K.; Holland, C.; Tornovsky-Babeay, S.; Dadon, D.; Iberl, M.; Chibalina, M.; Lee, S.; Glaser, B. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat. Commun. 2016, 7, 13496. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.L.; Taylor, C.G.; Zahradka, P. Rebelling against the (Insulin) Resistance: A review of the proposed insulin-sensitizing actions of soybeans, chickpeas, and their bioactive compounds. Nutrients 2018, 10, 434. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Sarkar, S.; Dihingia, A.; Afzal, N.; Wann, S.; Kalita, J.; Dewanjee, S.; Manna, P. A popular fermented soybean food of Northeast India exerted promising antihyperglycemic potential via stimulating PI3K/AKT/AMPK/GLUT4 signaling pathways and regulating muscle glucose metabolism in type 2 diabetes. J. Food Biochem. 2022, 46, e14385. [Google Scholar] [CrossRef]
- Huang, C.; Huang, W.; Hou, C.; Chi, Y.; Huang, H. Effect of black soybean koji extract on glucose utilization and adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Sci. 2014, 15, 8280–8292. [Google Scholar] [CrossRef]
- Hwang, J.; Do, H.; Kim, O.; Chung, J.; Lee, J.; Park, Y.; Hwang, K.; Seong, S.; Shin, M. Fermented soy bean extract suppresses differentiation of 3T3-L1 preadipocytes and facilitates its glucose utilization. J. Funct. Foods 2015, 15, 516–524. [Google Scholar] [CrossRef]
- Nizamutdinova, I.; Jin, Y.; Chung, J.; Shin, S.; Lee, S.; Seo, H.; Lee, J.; Chang, K.; Kim, H. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef]
- Kwon, D.; Jang, J.; Lee, J.; Kim, Y.; Shin, D.; Park, S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-γ activity in vitro. BioFactors 2006, 26, 245–258. [Google Scholar] [CrossRef]
- Das, D.; Afzal, N.; Wann, S.; Kalita, J.; Manna, P. A ~24 kDa protein isolated from protein isolates of Hawaijar, popular fermented soy food of North-East India exhibited promising antidiabetic potential via stimulating PI3K/AKT/GLUT4 signaling pathway of muscle glucose metabolism. Int. J. Biol. Macromol. 2023, 224, 1025–1039. [Google Scholar] [CrossRef]
- Sinacore, D.R.; Gulve, E.A. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: Implications for physical therapy. Phys. Ther. 1993, 73, 878–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.; Hong, S.; Lee, J.; Sung, S.; Park, S. Long-term consumption of fermented soybean-derived Chungkookjang attenuates hepatic insulin resistance in 90% pancreatectomized diabetic rats. Horm. Metab. Res. 2007, 39, 752–757. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.; Park, H.; Ji, Y.; Holzapfel, W.; Kim, D.; Hyun, C. Long-term fermented soybean paste improves metabolic parameters associated with non-alcoholic fatty liver disease and insulin resistance in high-fat diet-induced obese mice. Biochem. Biophys. Res. Commun. 2018, 495, 1744–1751. [Google Scholar] [CrossRef]
- Malardé, L.; Vincent, S.; Lefeuvre-Orfila, L.; Efstathiou, T.; Groussard, C.; Gratas-Delamarche, A. A fermented soy permeate improves the skeletal muscle glucose level without restoring the glycogen content in streptozotocin-induced diabetic rats. J. Med. Food 2013, 16, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, S.; Hwang, J.; Yi, S.; Nam, Y.; Lim, S. Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-Ay diabetic mice. Evid-Based Compl. Alt. 2012, 2012, 163280. [Google Scholar] [CrossRef]
- Mokashi, P.; Khanna, A.; Pandita, N. Flavonoids from Enicostema littorale blume enhances glucose uptake of cells in insulin resistant human liver cancer (HepG2) cell line via IRS-1/PI3K/Akt pathway. Biomed. Pharmacother. 2017, 90, 268–277. [Google Scholar] [CrossRef]
- Mathur, S.; Mehta, D.; Kapoor, S.; Yadav, S. Liver function in type-2 diabetes mellitus patients. Int. J. Sci. Study 2016, 3, 43–47. [Google Scholar]
- Yang, H.; Kwon, D.; Kim, M.; Kang, S.; Park, S. Meju, unsalted soybeans fermented with Bacillus subtilis and Aspergilus oryzae, potentiates insulinotropic actions and improves hepatic insulin sensitivity in diabetic rats. Nutr. Metab. 2012, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Jeong, Y.; Kwon, J.; Moon, K.; Kim, H.; Jeon, S.; Lee, M.; Park, Y.; Choi, M. Beneficial effect of chungkukjang on regulating blood glucose and pancreatic β-cell functions in C75BL/KsJ-db/db mice. J. Med. Food 2008, 11, 215–223. [Google Scholar] [CrossRef]
- Yang, H.; Park, S.; Pak, V.; Chung, K.; Kwon, D. Fermented soybean products and their bioactive compounds. In Soybean and Health; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Fujita, H.; Yamagami, T.; Ohshima, K. Long-term ingestion of a fermented soybean-derived Touchi-extract with α-glucosidase inhibitory activity is safe and effective in humans with borderline and mild type-2 diabetes. J. Nutr. 2001, 131, 2105–2108. [Google Scholar] [CrossRef] [Green Version]
- Hariyanto, I.; Hsieh, C.; Hsu, Y.; Chen, L.; Chu, C.; Weng, B. In vitro and in vivo assessments of anti-hyperglycemic properties of soybean residue fermented with Rhizopus oligosporus and Lactiplantibacillus plantarum. Life 2022, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.; Kim, Y. The role of estrogen in adipose tissue metabolism: Insights into glucose homeostasis regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef] [Green Version]
- Guilherme, A.; Virbasius, J.; Puri, V.; Czech, M. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, A.; Razavi, S. Therapeutic effects of polyphenols in fermented soybean and black soybean products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.; Hah, N.; Liddle, C.; Atkins, A.; Downes, M.; Evans, R. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Bourque, S.; Wu, J. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. X 2022, 13, 100222. [Google Scholar] [CrossRef]
- Ahn, I.; Do, M.; Kim, S.; Jung, H.; Kim, Y.; Kim, H.; Park, K. Antiobesity effect of Kochujang (Korean fermented red pepper paste) extract in 3T3-L1 adipocytes. J. Med. Food 2006, 9, 15–21. [Google Scholar] [CrossRef]
- Ko, J.; Chung, Y.; Kwak, C.; Kwon, Y. Doenjang, a Korean traditional fermented soybean paste, ameliorates neuroinflammation and neurodegeneration in mice fed a high-fat diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Park, Y. Food components with anti-obesity effect. Annu. Rev. Food Sci. Technol. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Andersen, D.; Korc, M.; Petersen, G.; Eibl, G.; Li, D.; Rickels, M.; Chari, S.; Abbruzzese, J. Diabetes, pancreatogenic piabetes, and pancreatic cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Jang, J.; Hong, S.; Lee, J.; Sung, S.; Park, H.; Park, S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Jung, H.; Karuppasamy, S.; Park, Y.; Cho, Y.; Lee, J.; Seong, S.; Suh, J. Anti-diabetic effect of the soybean extract fermented by Bacillus subtilis MORI in db/db mice. Food Sci. Biotechnol. 2012, 21, 1669–1676. [Google Scholar] [CrossRef]
- Masdar, H.; Satriyasumatri, T.; Hakiki, M.; Rafisyahputra, M.; Juananda, D. Histological apperarance of diabetes-rat pancreas administrated by soybean compared to tempeh. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; p. 020012. [Google Scholar]
- Lyon, L. ‘All disease begins in the gut’: Was Hippocrates right? Brain 2018, 141, e20. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Chang, H.; Yan, D.; Lee, K.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Lyu, W.; Xie, M.; Yuan, Q.; Ye, H.; Hu, B.; Zhou, L.; Zeng, X. Effects of α-galactooligosaccharides from chickpeas on high-fat-diet-induced metabolic syndrome in mice. J. Agric. Food Chem. 2017, 65, 3160–3166. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhänel, M.; Woting, A.; Petzke, K.; Gohlke, S.; Schulz, T.; Blaut, M. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Soka, S.; Suwanto, A.; Sajuthi, D.; Rusmana, I. Impact of tempeh supplementation on gut microbiota composition in Sprague-Dawley rats. Res. J. Microbiol. 2014, 9, 189. [Google Scholar]
- Jeong, D.; Ryu, M.; Yang, H.; Park, S. γ-PGA-rich Chungkookjang, short-term fermented soybeans: Prevents memory impairment by modulating brain insulin sensitivity, neuro-inflammation, and the gut–microbiome–brain axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, B.; Chu, Y.; Chang, W.; Wu, M. Effects of tempeh fermentation with Lactobacillus plantarum and Rhizopus oligosporus on streptozotocin-induced type II diabetes mellitus in rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Ylönen, K.; Saloranta, C.; Kronberg-Kippilä, C.; Groop, L.; Aro, A.; Virtanen, S. Associations of dietary fiber with glucose metabolism in nondiabetic relatives of subjects with type 2 diabetes: The botnia dietary study. Diabetes Care 2003, 26, 1979–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weickert, M.; Pfeiffer, A. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W. Demystification of fermented foods by omics technologies. Curr. Opin. Food Sci. 2022, 46, 100845. [Google Scholar] [CrossRef]
- Mohan, S.; Campbell, N. Salt and high blood pressure. Clin. Sci. 2009, 117, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Jousilahti, P.; Peltonen, M.; Lindström, J.; Tuomilehto, J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: A prospective study in Finland. Diabetologia 2005, 48, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.; Kenny, S.; Kerry, J.; Leenhardt, F.; Arendt, E. ‘Low-salt’ bread as an important component of a pragmatic reduced-salt diet for lowering blood pressure in adults with elevated blood pressure. Nutrients 2019, 11, 1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| FSP | Origin | Functional Fractions or Components | Targets | Models/Methods | IC50 Value/Anti-α-Glucosidase Activities a | Main Results | Ref c |
|---|---|---|---|---|---|---|---|
| Douchi Hunan Sichuan Jiangxi | China | Douchi aqueous extract | α-glucosidase | Fluorescence | 13.063 a 13.963 a 12.230 a | douchi samples from Hunan, Sichuan and Jiangxi province, respectively, showed a significantly higher anti-α-glucosidase activities than other samples (p < 0.05). | [47] |
| Douchi | China | Douchi aqueous extract | α-glucosidase | Fluorescence | 6.85 a | The anti-α-glucosidase activity of douchi qu fermented with Aspergillus oryzae were higher than those of Actinomucor elegans and Rhizopus arrhizus and the highest anti-α-glucosidase activities were observed in douchi qu fermented with A. orzyzae at 5.0% and 7.5% salt levels. | [47] |
| Douchi | Japan | Water-soluble douchi extract | α-glucosidase | Normal male rats Diabetic patients | - b | Douchi extract inhibited α-glucosidase and efficiently regulated postprandial in rats and diabetic patients | [48] |
| Douchi | China | Douchi aqueous extract | α-glucosidase | L6 cells | 0.35 mg/mL | Significantly improved glucose uptake in L6 cells | [41] |
| Doenjang | Korean | Doenjang aqueous extract | α-glucosidase | Fluorescence | 27.40–40.98 mg/mL | Doenjang samples demonstrated considerable antioxidant, α-glucosidase inhibitory, and tyrosinase inhibitory effects | [49] |
| Doenjang | Korean | Brown rice fermented paste | α-glucosidase | High fat-fed mice | - b | Significantly inhibited the high fat-induced hyperglycemia | [50] |
| Doenjang | Korean | betaine | α-glucosidase | Fluorescence | - b | Inhibited α-glucosidase activity and pre-adipocyte differentiation | [51] |
| Kochujang | Korean | p-coumaric acid | α-glucosidase | Fluorescence | - b | Inhibited α-glucosidase activity and pre-adipocyte differentiation | [51] |
| Miso | Japan | miso | α-amylase and α-glucosidase | Fluorescence Human intervention trial | - b | Inhibited the activities of various digestive enzymes (α-amylase, α-glucosidase and trypsin) in vitro, and improved the postprandial blood sugar | [52] |
| Rice miso | Japan | Melanoidins | α-glucosidase | Fluorescence | - b | α-glucosidase inhibitory activity in rice miso were increased by prolonging the fermentation periods (3, 6, 24, 36 months) | [53] |
| Tempeh | Indonesia | Isoflavone | α-amylase and α-glucosidase | Fluorescence | 74.8 mg/mL 85.3 mg/mL | Prevented diabetes due to the isoflavone (daidzein, genistein, and total isoflavone) in tempeh | [54] |
| FSP | Origin | Functional Fractions or Components | Targets | Models | Main Results | Ref c |
|---|---|---|---|---|---|---|
| BTD-1 a | Korea | - b | C/EBPα, PPAR-γ, GLUT4, ACC | 3T3-L1 cells | Decreased expression of C/EBPα, increased the expression of GLUT4, ACC, and PPAR-γ, facilitated glucose uptake, suppressed the adipocytes differentiation | [69] |
| Black soybean koji | Taiwan | isoflavone aglycones (daidzein and genistein) | GLUT1, GLUT4, AKT, PPAR-γ, Acrp30 | 3T3-L1 cells | Increased GLUT1, GLUT4 and AKT protein expression, downregulated PPAR-γ level, upregulated Acrp30 protein expression, and improved glucose uptake | [68,70] |
| Chungkookjang | Korea | isoflavone aglycones (daidzein) | IRS-1, AKT, GLUT4, PPAR-γ | 3T3-L1 cells | Increased IRS-1, AKT, and GLUT4 protein expression, stimulated PPAR-γ activity, enhanced glucose utilization | [71] |
| Douchi | China | Peptides VY and SFLLR | AKT, AMPK, p38 MAPK, p44/42 MAPK, GLUT4 | L6 cells | Increased AKT, AMPK, p38 MAPK, p44/42 MAPK and GLUT4 protein expression, improved glucose uptake | [41] |
| Hawaijar | India | isoflavone | PI3K, AKT, AMPK, GLUT4, G6P | L6 myotubes High-fat diet-fed mice | Upregulated glucose uptake, G6P level, and AKT/AMPK/GLUT4 protein expression, reduced body weight, FBG, glycated hemoglobin, insulin resistance, and glucose intolerance | [67] |
| Hawaijar | India | ~24 kDa protein | PI3K, AKT, AMPK, GLUT4, G6P | C2C12 cells High fructose high fat diet-fed animals | Upregulated glucose uptake, and PI3K/AKT/GLUT4 protein expression, reduced BW, FBG, IR, GHb levels, and glucose intolerance | [72] |
| FSP | Model | Target | Treatment | Effects | Ref b |
|---|---|---|---|---|---|
| Douchi | L6 myotubes cells | Skeletal muscle | 100 μM VY/SFLLR |
| [41] |
| Hawaijar | C2C12 myotubes cells High-fat diet-fed mice | Skeletal muscle | 2.5, 5, and 10 mg/mL in C2C12 cells 100 mg/kg BW/d for 16 weeks |
| [72] |
| Chungkookjang | 90% pancreatectomized diabetic rats | Skeletal muscle | 40% fat diet with Chungkookjang for 8 weeks |
| [74] |
| Fermented soybean paste | High-fat diet-induced obese mice | Skeletal muscle | 100 mg/kg BW/d for 14 weeks |
| [75] |
| Fermented soy permeate | STZ-induced diabetic rats | Skeletal muscle | FSP dose equivalent to 1 mg of soy isoflavones and 70 mg of alpha- galactooligosaccharides/kg of BW for 3 weeks. |
| [76] |
| FMCE a | C2C12 cells | Skeletal muscle | 50–400 μg/mL in C2C12 cells |
| [77] |
| FSP | Model | Target | Treatment | Effects | Ref a |
|---|---|---|---|---|---|
| Meju | 90% pancreatectomized diabetic rats | Liver | 10% meju for 8 weeks |
| [80] |
| Chungkookjang | 90% pancreatectomized diabetic rats | Liver | 40% fat diet with Chungkookjang for 8 weeks |
| [74] |
| Chungkukjang | C57BL/KsJ-db/db mice | Liver | 5% Chungkukjang for 6 weeks |
| [81] |
| Kochujang | 90% pancreatectomized diabetic rats | Liver | 5% Kochujang for 8 weeks |
| [39] |
| Douchi | KKAy mice | Liver | 0.4% douchi extract for 8 weeks |
| [40,83] |
| Doenjang | High fat diet-fed C57BL/6N mice | Liver | 10% Doenjang for 8 weeks |
| [50] |
| of increased the activities of SOD, GR, and PON enzymes in hepatic tissue |
| FSP | Model | Target | Treatment | Effects | Ref b |
|---|---|---|---|---|---|
| Fermented soybean paste | High-fat diet-induced obese mice | Adipocytes | 100 mg/kg BW/d for 14 weeks |
| [75] |
| BTD-1 a | 3T3-L1 cells | Adipocytes | 10–100 μg/mL in 3T3-L1 cells |
| [69] |
| Chungkookjang | 3T3-L1 cells | Adipocytes | 50 μg/mL in 3T3-L1 cells |
| [71] |
| Kochujang | 3T3-L1 cells | Adipocytes | 100 μg/mL in 3T3-L1 cells |
| [90] |
| FSP | Model | Target | Treatment | Effects | Ref b |
|---|---|---|---|---|---|
| Chungkukjang | C57BL/KsJ-db/db mice | Pancreas | 5% Chungkukjang for 6 weeks |
| [81] |
| Chungkookjang | 90% pancreatectomized diabetic rats | Pancreas | 40% Chungkookjang for 8 weeks |
| [95] |
| BTD-1 a | db/db mice | Pancreas | 500 mg/kg BW for 8 weeks |
| [96] |
| Tempeh | STZ-induced diabetic rats | Pancreas | 200 mg/kg BW for 30 days |
| [97] |
| FSP | Model | Target | Treatment | Effects | Ref a |
|---|---|---|---|---|---|
| Tempeh | SD rats | Gut microbiota | 10% tempeh for 28 days |
| [103] |
| Chungkookjang | 90% pancreatectomized diabetic rats | Gut microbiota | 4.5% diet for 8 weeks |
| [21,104] |
| Tempeh | STZ-induced diabetic rats | Gut microbiota | 40 mg/kg BW for 4 weeks |
| [105] |
| FSP a | Bioactive Compounds | Chemical Structures b | Ref c |
|---|---|---|---|
| Kochujang | p-coumaric acid |  | [54] |
| Tempeh | Daidzein | 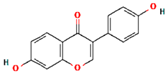 | [60] |
| Tempeh | Genistein |  | [60] |
| Douchi | Val-Tyr | 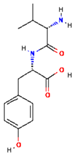 | [41] |
| Douchi | Ser-Phe-Leu-Leu-Arg | 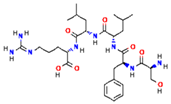 | [41] |
| Doenjang | Betaine | 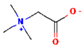 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Wang, W.; Li, S.; Li, J.; Zhao, R.; Liu, D.; Wu, J. Glucoregulatory Properties of Fermented Soybean Products. Fermentation 2023, 9, 254. https://doi.org/10.3390/fermentation9030254
Yu S, Wang W, Li S, Li J, Zhao R, Liu D, Wu J. Glucoregulatory Properties of Fermented Soybean Products. Fermentation. 2023; 9(3):254. https://doi.org/10.3390/fermentation9030254
Chicago/Turabian StyleYu, Songfeng, Wenjun Wang, Shanshan Li, Jiaheng Li, Runan Zhao, Donghong Liu, and Jianping Wu. 2023. "Glucoregulatory Properties of Fermented Soybean Products" Fermentation 9, no. 3: 254. https://doi.org/10.3390/fermentation9030254