Effect of Complex Prebiotics on the Intestinal Colonization Ability of Limosilactobacillus fermentum DALI02
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Cell Strains
2.2. Preparation of Whey Hydrolysate and Prebiotics
2.3. The Effects of Probiotics on L. fermentum DALI02
2.4. Experimental Design, Modelling, and Optimization
2.5. Adhesion Ability to Caco-2 Cell Lines
2.6. Adhesion Parameters in Rat Model
2.6.1. Experimental Design Based on the Animal Model
2.6.2. Colonization of L. fermentum DALI02
2.6.3. Residence Time of L. fermentum DALI02 in the Intestine
2.7. Determination of the Integrity of the Intestinal Barrier in Rats
2.8. Statistical Methods
3. Results
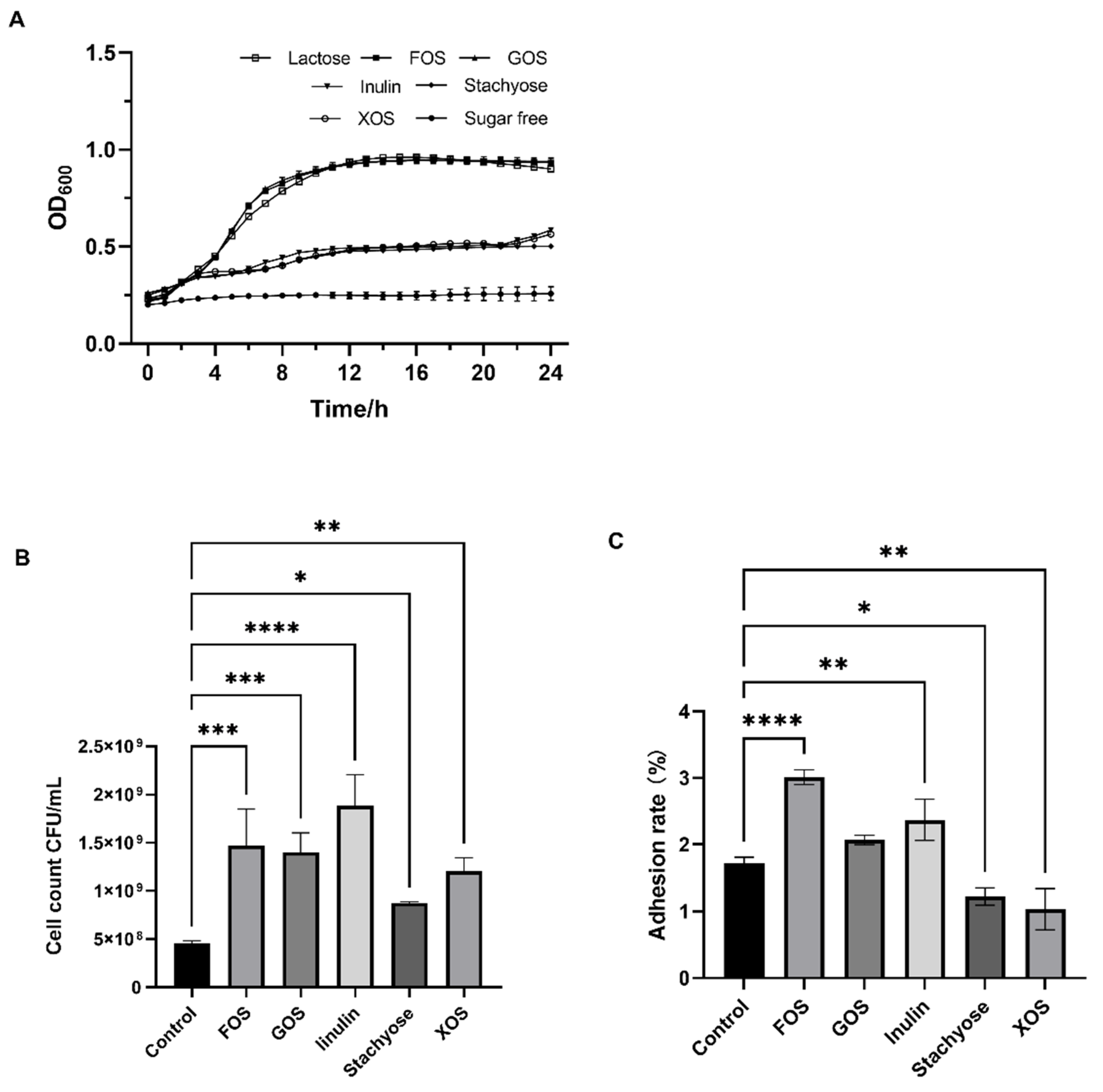
3.1. Effects of Probiotics on the Growth and Adhesion of L. fermentum DALI02
3.2. Response Criteria for the RSM Analysis
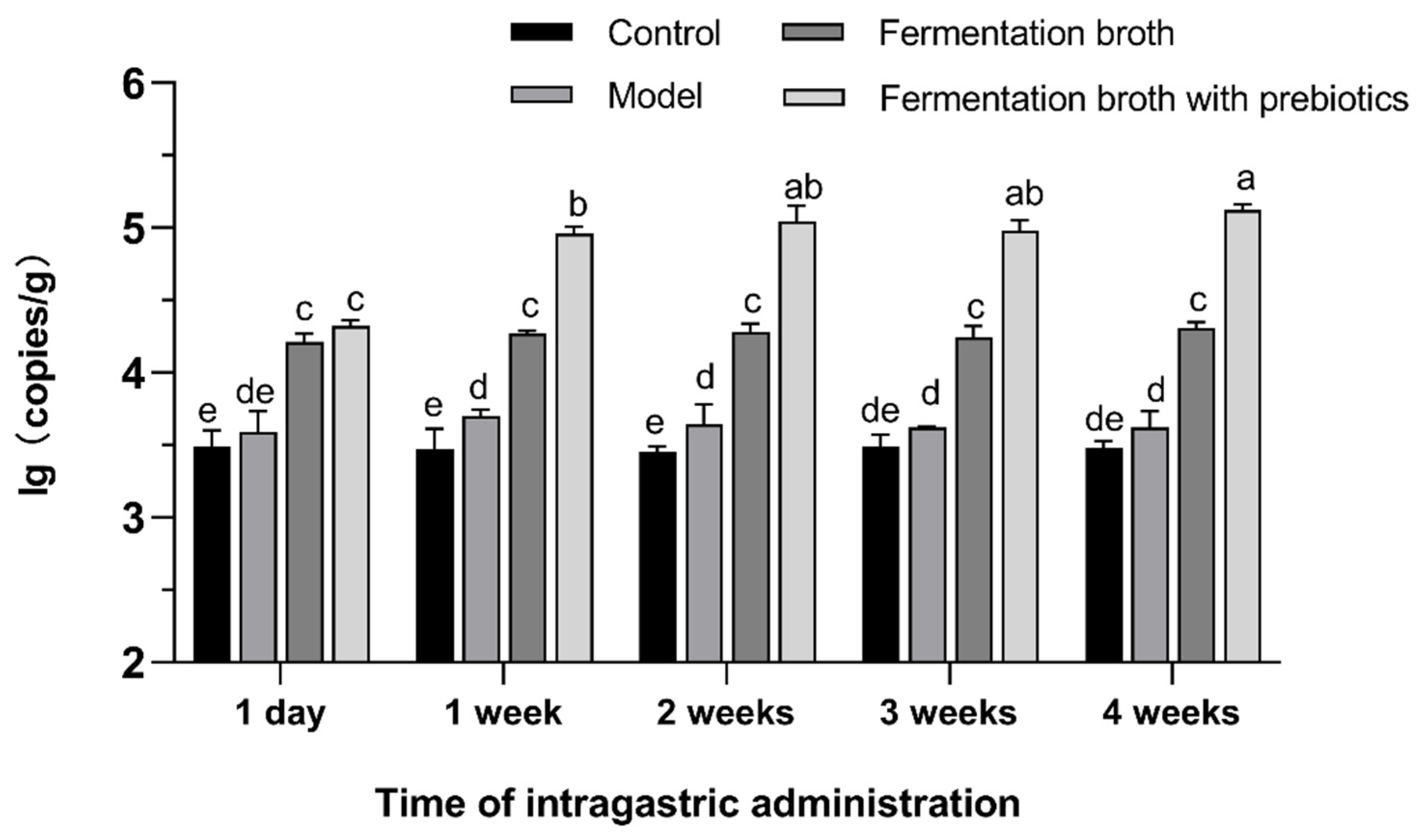
3.3. Effect of Intragastric Administration on Colonization Quantity
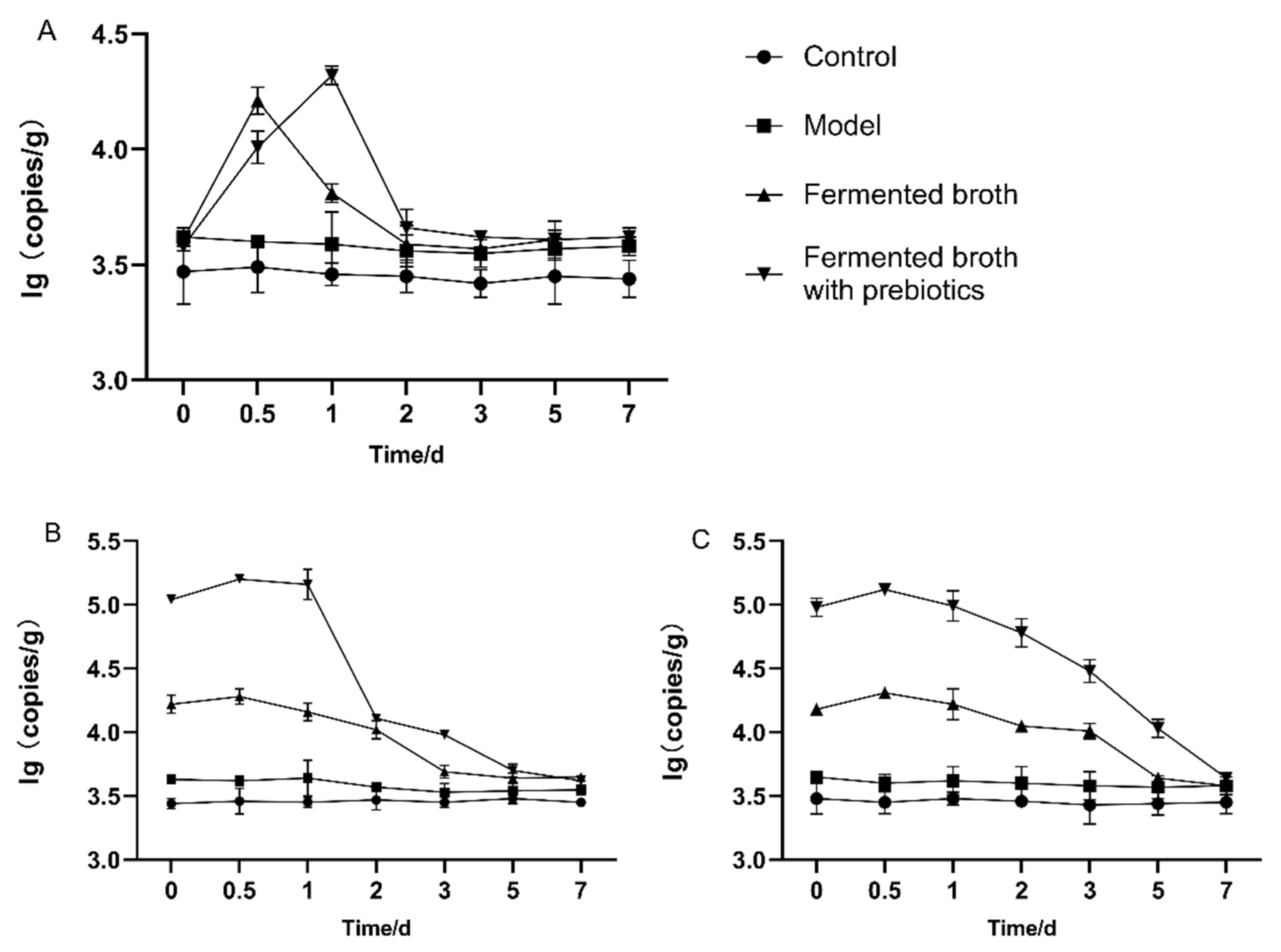
3.4. Effect of Intragastric Administration Time on Intestinal Residence Time
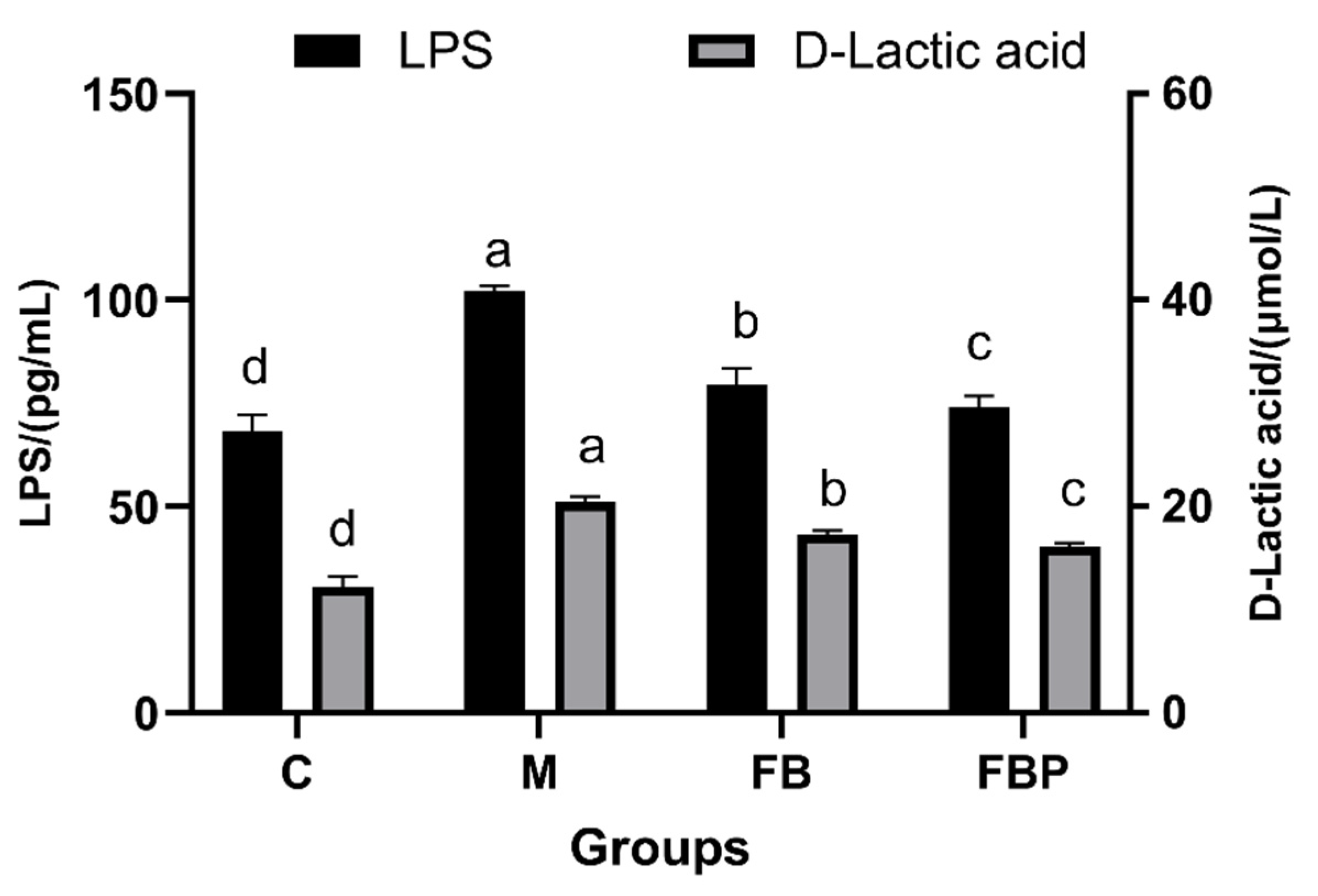
3.5. Intestinal Barrier Integrity in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol.-Mysore 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, H.U.; Svensson, B. Dietary Nutrients, Proteomes, and Adhesion of Probiotic Lactobacilli to Mucin and Host Epithelial Cells. Microorganisms 2018, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiotain vitroand short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Brink, M.; Todorov, S.D.; Martin, J.H.; Senekal, M.; DicksL, M.T. The effect of prebiotics on production of antimicrobial compounds, resistance to growth at low pH and in the presence of bile, and adhesion of probiotic cells to intestinal mucus. J. Appl. Microbiol. 2006, 100, 813–820. [Google Scholar] [CrossRef]
- Nagpal, R.; Kaur, A. Synbiotic Effect of Various Prebiotics on In Vitro Activities of Probiotic Lactobacilli. Ecol. Food Nutr. 2011, 50, 63–68. [Google Scholar] [CrossRef]
- Russo, P.; López, P.; Capozzi, V.; De Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-Glucans Improve Growth, Viability and Colonization of Probiotic Microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Kumaree, K.K.; Shah, N.P. Physiological Changes of Surface Membrane in Lactobacillus with Prebiotics. J. Food Sci. 2017, 82, 744–750. [Google Scholar] [CrossRef]
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus amyloliquefaciens JN4 acts as a prebiotic for enhancing the intestinal adhesion capacity of Lactobacillus reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Wen, R.; Zhu, X.; Liu, L.; Li, C. 2′-Fucosyllactose promotes Bifidobacterium bifidum DNG6 adhesion to Caco-2 cells. J. Dairy Sci. 2020, 103, 9825–9834. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Z.; Shi, C.; Wang, J.; Wang, T.; Dziugan, P.; Zhang, B.; Zhao, H.; Jia, G. How do Lycium barbarum polysaccharides promote the adhesion of Lactobacillus to Caco-2 cells? J. Funct. Foods 2022, 89, 104929. [Google Scholar] [CrossRef]
- Anand, S.; Mandal, S.; Singh, K.S.; Patil, P.; Tomar, S.K. Synbiotic combination of Lactobacillus rhamnosus NCDC 298 and short chain fructooligosaccharides prevents enterotoxigenic Escherichia coli infection. LWT-Food Sci. Technol. 2018, 98, 329–334. [Google Scholar] [CrossRef]
- Krausova, G.; Hynstova, I.; Svejstil, R.; Mrvikova, I.; Kadlec, R. Identification of Synbiotics Conducive to Probiotics Adherence to Intestinal Mucosa Using an In Vitro Caco-2 and HT29-MTX Cell Model. Processes 2021, 9, 569. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Liu, X.; Li, Q.; Liu, Y.; Wang, W.; Chen, D.; Xiao, L.; Gu, R. Comparison and selection of probiotic Lactobacillus from human intestinal tract and traditional fermented food in vitro via PCA, unsupervised clustering algorithm, and heat-map analysis. Food Sci. Nutr. 2022, 10, 4247–4257. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Lim, B.; Park, S.H.; Rackerby, B.; Kim, H.Y. Design of PCR assays to specifically detect and identify 37 Lactobacillus species in a single 96 well plate. BMC Microbiol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Li, X.; Song, L.; Huang, Y.; Xiao, Y.; Chu, Q.; Kang, Y.; Duan, S.; Wu, D.; Ren, Z. Stachyose inhibits vancomycin-resistant Enterococcus colonization and affects gut microbiota in mice. Microb. Pathog. 2021, 159, 105094. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior AI, M.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Liu, X.; Xue, M.; Jiang, Q.; Lei, H. Effect of alpha-linolenic acid (ALA) on proliferation of probiotics and its adhesion to colonic epithelial cells. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Murtini, D.; Aryantini NP, D.; Sujaya, I.N.; Urashima, T.; Fukuda, K. Effects of prebiotic oligosaccharides consumption on the growth and expression profile of cell surface-associated proteins of a potential probiotic Lactobacillus rhamnosus FSMM15. Biosci. Microbiota Food Health 2016, 35, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunova, G.; Rada, V.; Lisova, I.; Ročková, Š.; Vlkova, E. In vitro Fermentability of Prebiotic Oligosaccharides by Lactobacilli. Czech J. Food Sci. 2011, 29, S49–S54. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebola, O.O.; Corcoran, O.; Morgan, W.A. Synbiotics: The impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J. Funct. Foods 2014, 10, 75–84. [Google Scholar] [CrossRef]
- Abouloifa, H.; Khodaei, N.; Rokni, Y.; Karboune, S.; Brasca, M.; D’Hallewin, G.; Salah, R.B.; Saalaoui, E.; Asehraou, A. The prebiotics (Fructo-oligosaccharides and Xylo-oligosaccharides) modulate the probiotic properties of Lactiplantibacillus and Levilactobacillus strains isolated from traditional fermented olive. World J. Microbiol. Biotechnol. 2020, 36, 185. [Google Scholar] [CrossRef]
- Arnold, J.W.; Simpson, J.B.; Roach, J.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Prebiotics for Lactose Intolerance: Variability in Galacto-Oligosaccharide Utilization by Intestinal Lactobacillus rhamnosus. Nutrients 2018, 10, 1517. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.W.; Chen, C.M.; Qu, H.X.; Ren, C.Y.; Yan, X.T.; Huang, Y.J.; Guan, C.R.; Zhang, C.C.; Li, Q.M.; Gu, R.X. Screening of Lactobacillus strains that enhance SCFA uptake in intestinal epithelial cells. Eur. Food Res. Technol. 2021, 247, 1049–1060. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, N.; Kong, L.; Wang, M.; Lei, H. Promoting effects of 6-Gingerol on probiotic adhesion to colonic epithelial cells. Food Sci. Technol. 2021, 41, 678–686. [Google Scholar] [CrossRef]
- Minelli, E.B.; Benini, A. Relationship between number of bacteria and their probiotic effects. Microb. Ecol. Health Dis. 2008, 20, 180–183. [Google Scholar] [CrossRef]
- Larsen, C.N.; Nielsen, S.; Kaestel, P.; Brockmann, E.; Bennedsen, M.; Christensen, H.R.; Eskesen, D.C.; Jacobsen, B.L.; Michaelsen, K.F. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur. J. Clin. Nutr. 2006, 60, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef] [PubMed]
- Saxami, G.; Ypsilantis, P.; Sidira, M.; Simopoulos, C.; Kourkoutas, Y.; Galanis, A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe 2012, 18, 417–420. [Google Scholar] [CrossRef] [PubMed]
| Code X | Extrusion Condition | ||||
|---|---|---|---|---|---|
| X1 (%) | X2 (%) | X3 (%) | X4 (%) | X5 (%) | |
| −2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| −1 | 0.05 | 0.15 | 0.10 | 0.10 | 0.30 |
| 0 | 0.10 | 0.30 | 0.20 | 0.20 | 0.60 |
| 1 | 0.15 | 0.45 | 0.30 | 0.30 | 0.90 |
| 2 | 0.20 | 0.60 | 0.40 | 0.40 | 1.20 |
| Sum of Squares | df | Mean Square | F Value | p Value | Significance | |
|---|---|---|---|---|---|---|
| Model | 28.98 | 20 | 1.45 | 2.65 | 0.0296 | * |
| X1 | 5.39 | 1 | 5.39 | 9.85 | 0.0068 | ** |
| X2 | 0.0070 | 1 | 0.0070 | 0.0128 | 0.9114 | |
| X3 | 2.99 | 1 | 2.99 | 5.46 | 0.0337 | * |
| X4 | 0.0570 | 1 | 0.0570 | 0.1043 | 0.7512 | |
| X5 | 2.66 | 1 | 2.66 | 4.86 | 0.0435 | * |
| X1X2 | 0.4456 | 1 | 0.4456 | 0.8146 | 0.3810 | |
| X1X3 | 3.14 | 1 | 3.14 | 5.74 | 0.0300 | * |
| X1X4 | 0.2730 | 1 | 0.2730 | 0.4991 | 0.4907 | |
| X1X5 | 2.49 | 1 | 2.49 | 4.55 | 0.0499 | * |
| X2X3 | 0.2525 | 1 | 0.2525 | 0.4616 | 0.5072 | |
| X2X4 | 2.90 | 1 | 2.90 | 5.30 | 0.0361 | * |
| X2X5 | 0.1871 | 1 | 0.1871 | 0.3420 | 0.5674 | |
| X3X4 | 0.8327 | 1 | 0.8327 | 1.52 | 0.2363 | |
| X3X5 | 0.6281 | 1 | 0.6281 | 1.15 | 0.3009 | |
| X4X5 | 0.0297 | 1 | 0.0298 | 0.0544 | 0.8187 | |
| X12 | 0.0966 | 1 | 0.0966 | 0.1766 | 0.6803 | |
| X22 | 1.38 | 1 | 1.38 | 2.52 | 0.1334 | |
| X32 | 1.41 | 1 | 1.41 | 2.58 | 0.1290 | |
| X42 | 2.14 | 1 | 2.14 | 3.92 | 0.0665 | |
| X52 | 1.67 | 1 | 1.67 | 3.06 | 0.1007 | |
| Residual | 8.20 | 15 | 0.5470 | |||
| Lack of fit | 1.13 | 6 | 0.1884 | 0.2398 | 0.9519 | |
| Pure error | 7.07 | 9 | 0.7860 | |||
| Cor total | 37.18 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, D.; Li, Q.; Zhang, C.; Zhang, L.; Qu, H.; Wang, W.; Zhou, Y.; Huang, Y.; Xiao, L.; et al. Effect of Complex Prebiotics on the Intestinal Colonization Ability of Limosilactobacillus fermentum DALI02. Fermentation 2023, 9, 25. https://doi.org/10.3390/fermentation9010025
Liu X, Chen D, Li Q, Zhang C, Zhang L, Qu H, Wang W, Zhou Y, Huang Y, Xiao L, et al. Effect of Complex Prebiotics on the Intestinal Colonization Ability of Limosilactobacillus fermentum DALI02. Fermentation. 2023; 9(1):25. https://doi.org/10.3390/fermentation9010025
Chicago/Turabian StyleLiu, Xiaoxiao, Dawei Chen, Qiming Li, Chenchen Zhang, Longfei Zhang, Hengxian Qu, Wenqiong Wang, Yuanyuan Zhou, Yujun Huang, Lixia Xiao, and et al. 2023. "Effect of Complex Prebiotics on the Intestinal Colonization Ability of Limosilactobacillus fermentum DALI02" Fermentation 9, no. 1: 25. https://doi.org/10.3390/fermentation9010025





