Characterization of Saccharomyces Strains Isolated from “Kéknyelű” Grape Must and Their Potential for Wine Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains Used in This Study
2.2. Species Specific PCR and PCR-RFLP Analyses of CYR1, HIS4, YCL008c Genes
2.3. Inoculum Preparation and Physiological Assays
2.4. Laboratory-Scale Fermentation and Analysis of Final Products
2.5. Identification of Volatile Compounds by Gas Chromatography-Mass Spectrometry (GC-MS) Method
2.6. Measurement of the Glycerol Content
2.7. Statistical Analysis of Physiological Assays and GC-MS Data
3. Results
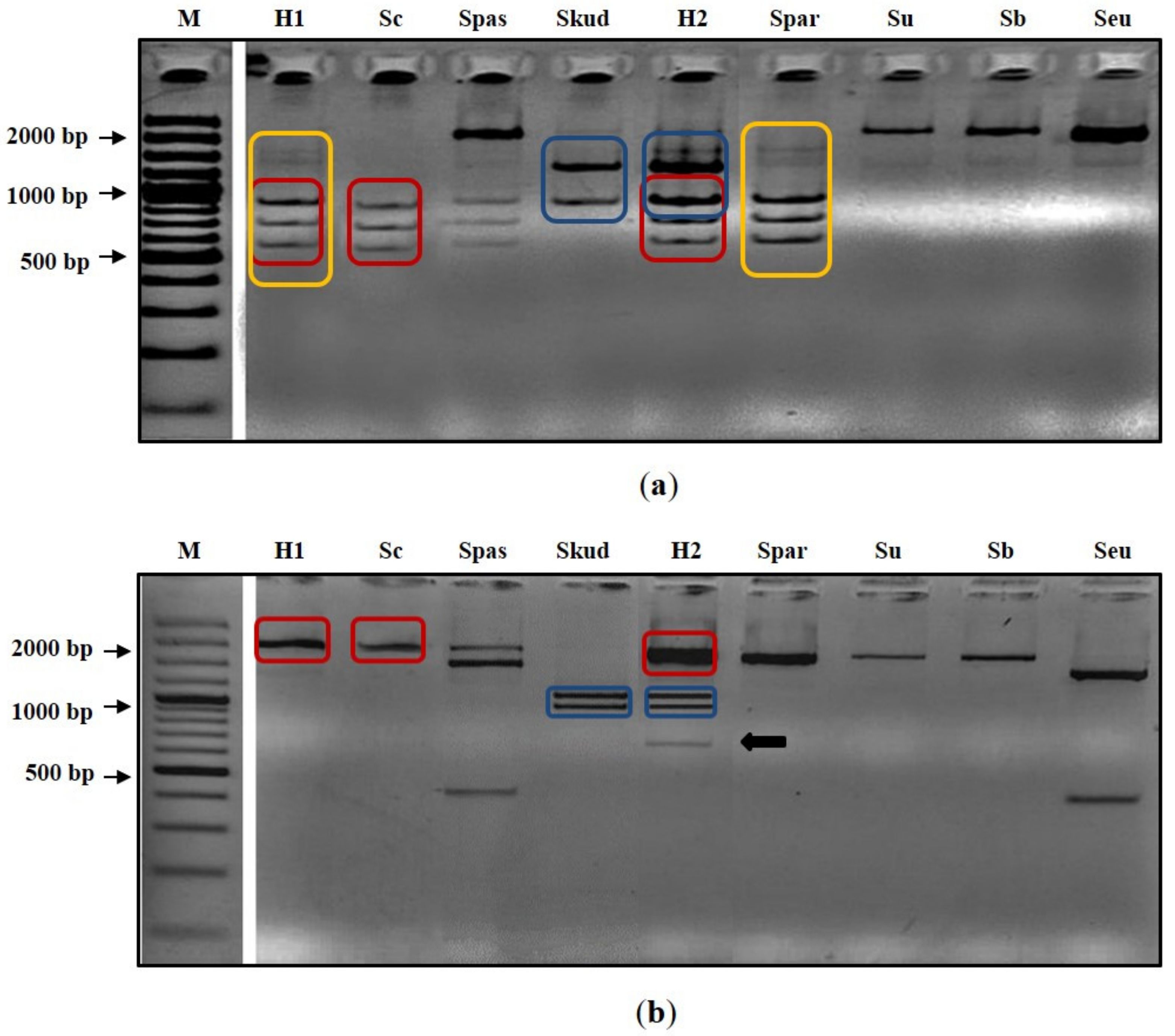
3.1. Molecular Identification and Confirmation of Hybrid Status
3.1.1. Species-Specific PCR
3.1.2. Restriction Pattern Analysis
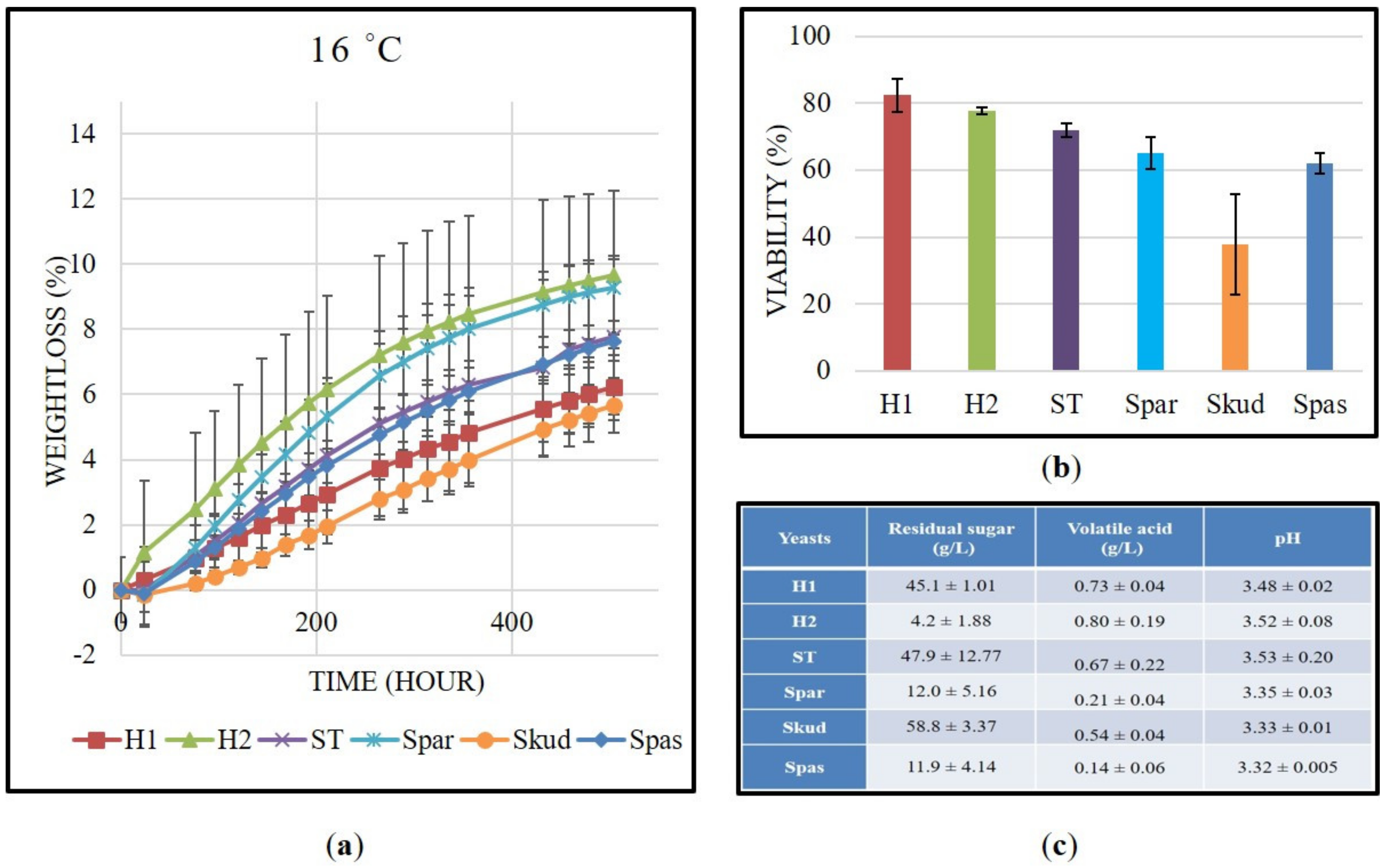
3.2. Oenological Features of Investigated Strains
3.3. Kinetics of “Kéknyelű” Fermentation and Analytical Parameters of Final Products
3.4. Volatile Compounds Analysis of the “Kéknyelű” Wine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korenika, A.M.J.; Tomaz, I.; Preiner, D.; Plichta, V.; Jeromel, A. Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia. Fermentation 2021, 7, 92. [Google Scholar] [CrossRef]
- Degree, R. Selection and Commercial Cultivation of Wine Yeast and Bacteria. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 421–448. [Google Scholar]
- Nikolaou, E.; Soufleros, E.H.; Bouloumpasi, E.; Tzanetakis, N. Selection of Indigenous Saccharomyces cerevisiae Strains According to Their Oenological Characteristics and Vinification Results. Food Microbiol. 2006, 23, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Villena, M.; Briones-Perez, A.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Biotechnological Application of Yeasts in Food Science: Starter Cultures, Probiotics and Enzyme Production. J. Appl. Microbiol. 2017, 123, 1360–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.J.V.; Silva, M.F. Microchip Electrophoresis for Wine Analysis. Anal. Bioanal. Chem. 2016, 408, 8643–8653. [Google Scholar] [CrossRef]
- González, S.S.; Gallo, L.; Climent, M.D.; Barrio, E.; Querol, A. Enological Characterization of Natural Hybrids from Saccharomyces cerevisiae and S. kudriavzevii. Int. J. Food Microbiol. 2007, 116, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Tronchoni, J.; Querol, A.; Belloch, C. Production of Aroma Compounds by Cryotolerant Saccharomyces Species and Hybrids at Low and Moderate Fermentation Temperatures. J. Appl. Microbiol. 2013, 114, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s Balancing Act between Ethanol and Glycerol Production in Low-Alcohol Wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Bisson, L.F. Yeast Hybrids in Winemaking. Catalyst 2017, 1, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Gamero, A.; Hernández-Orte, P.; Querol, A.; Ferreira, V. Effect of Aromatic Precursor Addition to Wine Fermentations Carried out with Different Saccharomyces Species and Their Hybrids. Int. J. Food Microbiol. 2011, 147, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. Novel Brewing Yeast Hybrids: Creation and Application. Appl. Microbiol. Biotechnol. 2017, 101, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ríos, E.; Guillén, A.; De La Cerda, R.; Pérez-Través, L.; Querol, A.; Guillamón, J.M. Improving the Cryotolerance of Wine Yeast by Interspecific Hybridization in the Genus Saccharomyces. Front. Microbiol. 2019, 9, 3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellon, J.R.; Eglinton, J.M.; Siebert, T.E.; Pollnitz, A.P.; Rose, L.; De Barros Lopes, M.; Chambers, P.J. Newly Generated Interspecific Wine Yeast Hybrids Introduce Flavour and Aroma Diversity to Wines. Appl. Microbiol. Biotechnol. 2011, 91, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Través, L.; Lopes, C.A.; Barrio, E.; Querol, A. Evaluation of Different Genetic Procedures for the Generation of Artificial Hybrids in Saccharomyces genus for Winemaking. Int. J. Food Microbiol. 2012, 156, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.; Krogerus, K.; Vidgren, V.; Sandell, M.; Gibson, B. Improved Cider Fermentation Performance and Quality with Newly Generated Saccharomyces cerevisiae × Saccharomyces eubayanus Hybrids. J. Ind. Microbiol. Biotechnol. 2017, 44, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Origone, A.C.; Rodríguez, M.E.; Oteiza, J.M.; Querol, A.; Ariel Lopes, C. Saccharomyces cerevisiae x Saccharomyces uvarum Hybrids Generated under Different Conditions Share Similar Winemaking Features. Yeast 2018, 35, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Gallone, B.; Steensels, J.; Mertens, S.; Dzialo, M.C.; Gordon, J.L.; Wauters, R.; Theßeling, F.A.; Bellinazzo, F.; Saels, V.; Herrera-Malaver, B.; et al. Interspecific Hybridization Facilitates Niche Adaptation in Beer Yeast. Nat. Ecol. Evol. 2019, 3, 1562–1575. [Google Scholar] [CrossRef]
- Masneuf, I.; Hansen, J.; Groth, C.; Piskur, J.; Dubourdieu, D. New Hybrids between Saccharomyces Sensu Stricto Yeast Species Found among Wine and Cider Production Strains. Appl. Environ. Microbiol. 1998, 64, 3887–3892. [Google Scholar] [CrossRef] [Green Version]
- González, S.S.; Barrio, E.; Gafner, J.; Querol, A. Natural Hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in Wine Fermentations. FEMS Yeast Res. 2006, 6, 1221–1234. [Google Scholar] [CrossRef] [Green Version]
- Langdon, Q.K.; Peris, D.; Kyle, B.; Hittinger, C.T. Sppider: A Species Identification Tool to Investigate Hybrid Genomes with High-Throughput Sequencing. Mol. Biol. Evol. 2018, 35, 2835–2849. [Google Scholar] [CrossRef] [Green Version]
- Monerawela, C.; Bond, U. The Hybrid Genomes of Saccharomyces pastorianus: A Current Perspective. Yeast 2018, 35, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barros Lopes, M.; Bellon, J.R.; Shirley, N.J.; Ganter, P.F. Evidence for Multiple Interspecific Hybridization in Saccharomyces Sensu Stricto Species. FEMS Yeast Res. 2002, 1, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumov, G.I.; James, S.A.; Naumova, E.S.; Louis, E.J.; Roberts, I.N. Three New Species in the Saccharomyces Sensu Stricto Complex: Saccharomyces Cariocanus, Saccharomyces Kudriavzevii and Saccharomyces Mikatae. Int. J. Syst. Evol. Microbiol. 2000, 50, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Hansen, J.; Piskur, J. A Natural Chimeric Yeast Containing Genetic Material from Three Species. Int. J. Syst. Bacteriol. 1999, 49, 1933–1938. [Google Scholar] [CrossRef]
- Gibson, B.; Liti, G. Saccharomyces Pastorianus: Genomic Insights Inspiring Innovation for Industry. Yeast 2015, 32, 17–27. [Google Scholar] [CrossRef]
- Jahnke, G.; Deák, T.; Szigeti, G.K.; Németh, C.; Molnár, A.; Kocsis, D.; Oláh, R.; Németh, E.K.; Szőke, B.Á.; Nyitrayné Sárdy, D.Á. Application of the „Double Maturation Raisonnée” (Dmr) Method for Quality Wine Production in Badacsony, Hungary. SSRN Electron. J. 2022, 1–31. [Google Scholar] [CrossRef]
- Gerőcs, A.; Nemes-Barnás, K.; Pál, S.; Szőke, B.; Májer, J.; Farkas, T.; Olasz, F. Isolation and Characterization of Yeast Strains from Badacsony, Hungary. Indian J. Exp. Biol. 2020, 58, 461–473. [Google Scholar]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. New Lager Yeast Strains Generated by Interspecific Hybridization. J. Ind. Microbiol. Biotechnol. 2015, 42, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Banno, I. Reexamination of Saccharomyces Bayanus Strains by DNA–DNA Hybridization and Electrophoretic Karyotyping. IFO Res. Commun. 1991, 15, 20–41. [Google Scholar]
- Liti, G.; Barton, D.B.H.; Louis, E.J. Sequence Diversity, Reproductive Isolation and Species Concepts in Saccharomyces. Genetics 2006, 174, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Ekberg, J.; Rautio, J.; Mattinen, L.; Vidgren, V.; Londesborough, J.; Gibson, B.R. Adaptive Evolution of the Lager Brewing Yeast Saccharomyces Pastorianus for Improved Growth under Hyperosmotic Conditions and Its Influence on Fermentation Performance. FEMS Yeast Res. 2013, 13, 335–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumov, G.I.; Naumova, E.S.; Masneuf-Pomarède, I. Genetic Identification of New Biological Species Saccharomyces Arboricolus Wang et Bai. Antonie Van Leeuwenhoek 2010, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Legras, J.L.; Neuvéglise, C.; Gaillardin, C. Deciphering the Hybridisation History Leading to the Lager Lineage Based on the Mosaic Genomes of Saccharomyces Bayanus Strains NBRC1948 and CBS380 T. PLoS ONE 2011, 6, e25821. [Google Scholar] [CrossRef]
- Muir, A.; Harrison, E.; Wheals, A. A Multiplex Set of Species-Specific Primers for Rapid Identification of Members of the Genus Saccharomyces. FEMS Yeast Res. 2011, 11, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Casaregola, S.; Nguyen, H.V.; Lapathitis, G.; Kotyk, A.; Gaillardin, C. Analysis of the Constitution of the Beer Yeast Genome by PCR, Sequencing and Subtelomeric Sequence Hybridization. Int. J. Syst. Evol. Microbiol. 2001, 51, 1607–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimoh, S.O.; Ado, S.A.; Ameh, J.B.; Whong, C.M.Z.; Anglais, A.E. Osmotolerance and Fermentative Pattern of Brewer’s Yeast. World J. Life Sci. Med. Res. 2012, 2, 59–64. [Google Scholar]
- Maturano, Y.P.; Nally, M.C.; Toro, M.E.; de Figueroa, L.I.C.; Combina, M.; Vazquez, F. Monitoring of Killer Yeast Populations in Mixed Cultures: Influence of Incubation Temperature of Microvinifications Samples. World J. Microbiol. Biotechnol. 2012, 28, 3135–3142. [Google Scholar] [CrossRef] [Green Version]
- Mattisek, R.; Steiner, G. Food Analysis: Basics, Methods, Applications, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 3-540-62513-5. [Google Scholar]
- Torrens, J.; Riu-Aumatell, M.; López-Tamames, E.; Buxaderas, S. Volatile Compounds of Red and White Wines by Headspace-Solid-Phase Microextraction Using Different Fibers. J. Chromatogr. Sci. 2004, 42, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Rice, S.; Koziel, J.A.; Dharmadhikari, M. Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Separations 2017, 4, 24. [Google Scholar] [CrossRef]
- López, E.F.; Gómez, E.F. Simultaneous Determination of the Major Organic Acids, Sugars, Glycerol, and Ethanol by HPLC in Grape Musts and White Wines. J. Chromatogr. Sci. 1996, 34, 254–257. [Google Scholar] [CrossRef] [Green Version]
- Lopandic, K.; Gangl, H.; Wallner, E.; Tscheik, G.; Leitner, G.; Querol, A.; Borth, N.; Breitenbach, M.; Prillinger, H.; Tiefenbrunner, W. Genetically Different Wine Yeasts Isolated from Austrian Vine-Growing Regions Influence Wine Aroma Differently and Contain Putative Hybrids between Saccharomyces cerevisiae and Saccharomyces kudriavzevii. FEMS Yeast Res. 2007, 7, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Malfeito-Ferreira, M. Yeasts and Wine Off-Flavours: A Technological Perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Gangl, H.; Batusic, M.; Tscheik, G.; Tiefenbrunner, W.; Hack, C.; Lopandic, K. Exceptional Fermentation Characteristics of Natural Hybrids from Saccharomyces Cerevisiae and S. Kudriavzevii. N. Biotechnol. 2009, 25, 244–251. [Google Scholar] [CrossRef]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A Large Set of Newly Created Interspecific Saccharomyces Hybrids Increases Aromatic Diversity in Lager Beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef] [Green Version]
- Torija, M.J.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Effects of Fermentation Temperature on the Strain Population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Belloch, C.; Orlic, S.; Barrio, E.; Querol, A. Fermentative Stress Adaptation of Hybrids within the Saccharomyces Sensu Stricto Complex. Int. J. Food Microbiol. 2008, 122, 188–195. [Google Scholar] [CrossRef]
- Divol, B.; Du Toit, M.; Duckitt, E. Surviving in the Presence of Sulphur Dioxide: Strategies Developed by Wine Yeasts. Appl. Microbiol. Biotechnol. 2012, 95, 601–613. [Google Scholar] [CrossRef]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective Effects of Sulfur Dioxide and Yeast Starter Culture Addition on Indigenous Yeast Populations and Sensory Characteristics of Wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Nardi, T.; Corich, V.; Giacomini, A.; Blondin, B. A Sulphite-Inducible Form of the Sulphite Efflux Gene SSU1 in a Saccharomyces cerevisiae Wine Yeast. Microbiology 2010, 156, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Alfonzo, A.; Prestianni, R.; Gaglio, R.; Matraxia, M.; Maggio, A.; Naselli, V.; Craparo, V.; Badalamenti, N.; Bruno, M.; Vagnoli, P.; et al. Effects of Different Yeast Strains, Nutrients and Glutathione-Rich Inactivated Yeast Addition on the Aroma Characteristics of Catarratto Wines. Int. J. Food Microbiol. 2021, 360, 109325. [Google Scholar] [CrossRef]
- Verzera, A.; Merlino, M.; Cincotta, F.; Prestia, O.; Sparacio, A.; Sparla, S.; Condurso, C. Varietal Aromas of Fortified Wines from Different Moscato Var. (Vitis Vinifera L.) under the Same Pedoclimatic Conditions. Foods 2021, 10, 2549. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.; Krogerus, K.; Castillo, S.; Ortiz-Julien, A.; Dequin, S.; Gibson, B. Exploring the Potential of Saccharomyces Eubayanus as a Parent for New Interspecies Hybrid Strains in Winemaking. FEMS Yeast Res. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, K.; Chen, S.; Xiong, S.; Tao, Y. Increase in Fruity Ester Production during Spine Grape Wine Fermentation by Goal-Directed Amino Acid Supplementation. Fermentation 2021, 7, 231. [Google Scholar] [CrossRef]



| Strains Designation | Species | Source | Reference |
|---|---|---|---|
| H1 | potential Saccharomyces cerevisiae | “Kéknyelű” must, Badacsonytomaj, Hungary | this work |
| H2 | potential S. cerevisiae × S. kudriavzevii natural hybrid | “Kéknyelű” must, Badacsonytomaj, Hungary | this work |
| ST | Fermol Elegance S. cerevisiae commercially available starter | AEB Hungária Kft. | AEB Hungária Kft. |
| Sb | Saccharomyces bayanus * DBVPG 8001 | University of Debrecen, Department of Genetics and Applied Microbiology Culture Collection | - |
| Sc | Saccharomyces cerevisiae CBS 1171 | National Collection of Agricultural and Industrial Microorganisms MATE | Meyen ex E. C. Hansen, 1883 |
| Seu | Saccharomyces eubayanus CBS 12357 | VTT Culture Collection | [29] |
| Skud | Saccharomyces kudriavzevii CBS 8840 | VTT Culture Collection | [30] |
| Spar | Saccharomyces paradoxus CBS 432 | VTT Culture Collection | [31] |
| Spas | Saccharomyces pastorianus VTT-A63015 | VTT Culture Collection | [32] |
| Su | Saccharomyces uvarum CBS 395 | University of Debrecen, Department of Genetics and Applied Microbiology Culture Collection | [33] |
| S6 | Saccharomyces cerevisiae K2 killer sensitive strain | National Collection of Agricultural and Industrial Microorganisms MATE | - |
| Analyzes | Genus/Species | Name of Primers | Primer Sequences | Tm (°C) | Fragment Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Species—specific PCR | S. paradoxus | Spar F7 (forward) | CTTTCTACCCCTTCTCCATGTTGG | 66 | 739 | [35] |
| Spar R7 (reverse) | CAATTTCAGGGCGTTGTCCAACAG | |||||
| S. cerevisiae | ScerF2 (forward) | GCGCTTTACATTCAGATCCCGAG | 63 | 149 | ||
| ScerR2 (reverse) | TAAGTTGGTTGTCAGCAAGATTG | |||||
| S. bayanus/S. uvarum | SbayF1 (forward) | GCTGACTGCTGCTGCTGCCCCCG | 62 | 275 | ||
| SbayR1 (reverse) | TGTTATGAGTACTTGGTTTGTCG | |||||
| S. kudriavzevii | SkudF2 (forward) | ATCTATAACAAACCGCCAAGGGAG | 66 | 660 | ||
| SkudR1 (reverse) | CGTAACCTACCTATATGAGGGCCT | |||||
| CYR1 gene amplification | Saccharomyces | CYR1-5 (forward) | CTACGAAGGAAAGTGTCCTCTTTRGTTCGTGG | 60 | 570 | [20] |
| CYR1-3 (reverse) | CCGTGTGTAGAATTTAGTGTAGAATTGACRGC | |||||
| HIS4 gene amplification | Saccharomyces | HIS4-U (forward) | ACTCTAATAGTGACTCCG | 46 | 2100 | [36] |
| HIS4-L (reverse) | AACTTGGGAGTCAATACC | |||||
| YCL008c gene amplification | Saccharomyces | YCL008c-U (forward) | TTCGTTGGATGTGCCATCG | 55 | 1600 | |
| YCL008c-L (reverse) | GGAGCCACCAAGGGATGG |
| Tolerance to | Glucose Fermentation | Killer Activity | Temperature Tolerance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Glucose 30% | Ethanol 30% | Sulphur Dioxide 30 °C (mg/L) | |||||||
| 30 °C | 30 °C | 48 h | 72 h | 16 °C | 24 °C | 24 °C | 16 °C | 24 °C | 37 °C | |
| H1 | +++ | +++ | 240 | 240 | 3 | 2 | +++ | ++ | +++ | +++ |
| H2 | ++ | +++ | 200 | 200 | 3 | 2 | - | ++ | +++ | ++ |
| ST | +++ | +++ | 240 | 260 | 3 | 2 | - | + | +++ | +++ |
| Sc | ++ | ++ | 60 | 80 | 4 | 3 | - | + | ++ | ++ |
| Spar | +++ | +++ | 160 | 160 | 3 | 2 | - | +++ | +++ | + |
| Skud | ++ | + | 40 | 60 | 3 | 2 | - | + | +++ | - |
| Spas | nd | ++ | 60 | 80 | 3 | 2 | - | ++ | +++ | - |
| Spas 1 | Skud 1 | Spar 1 | ST 1 | H1 1 | H2 1 | ||
|---|---|---|---|---|---|---|---|
| Means (mg/L) | |||||||
| acids | acetic acid | 33.06 (17.7) | 116.94 (6.5) | 44.24 (28.6) | 122.31 (4.9) | 134.91 (4.8) | 194.19 (12.0) |
| butanoic acid | bdl | bdl | bdl | bdl | bdl | bdl | |
| alcohols | 1-hexanol | 0.56 (28.7) | 0.42 (6.4) | 0.47 (2.7) | 0.46 (0.1) | 0.66 (39.3) | 0.43 (0.1) |
| 2-phenylethanol | 17.35 (29.4) | 12.58 (21.1) | 31.79 (20.1) | 6.14 (1.9) | 7.43 (15.9) | 15.64 (3.6) | |
| butanol | 2.54 (22.5) | 0.51 (42.5) | 4.41 (20.4) | 0.23 (0.1) | 0.26 (6.1) | 0.76 (0.2) | |
| isobutanol | 7.77 (43.7) | 56.38 (23.4) | 14.63 (6.4) | 6.34 (2.6) | 4.93 (28.3) | 16.61 (2.0) | |
| isopentyl alcohol | 59.66 (23.6) | 59.75 (21.3) | 102.57 (4.8) | 29.21 (7.9) | 25.37 (21.1) | 70.29 (15.1) | |
| glycerol | 4800.01 (6.9) | 10,319.64 (5.1) | 8072.51 (2.4) | 7218.24 (322.5) | 3482.6 (11.7) | 6521.74 (345.6) | |
| esters | 2-phenethyl acetate | 0.27 (27.2) | 0.1 (14.3) | 0.29 (13.8) | 0.07 (0.1) | 0.11 (12.5) | 0.23 (0.1) |
| diethyl-succinate | 0.06 (21.8) | 0.04 (16.4) | 0.09 (33.4) | 0.03 (0.1) | 0.04 (23.5) | 0.04 (0.1) | |
| ethyl-acetate | 13.73 (38.3) | 11.47 (18.8) | 20.58 (12.5) | 23.1 (1.8) | 10.86 (22.1) | 31.06 (5.5) | |
| ethyl-butyrate | 0.20 (17.0) | 0.06 (35.3) | 0.49 (11.3) | 0.24 (0.1) | 0.06 (6.63) | 0.36 (0.1) | |
| ethyl-hexanoate | 0.69 (34.6) | 0.15 (19.3) | 1.36 (11.1) | 0.65 (0.2) | 0.26 (28.1) | 1.47 (0.2) | |
| ethyl-lactate | 1.48 (26.9) | 0.65 (28.6) | 1.86 (26.7) | 0.27 (0.1) | 0.36 (12.8) | 1.67 (0.1) | |
| hexyl-acetate | 0.1 (29.7) | bdl | 0.08 (2.3) | 0.06 (0.1) | 0.06 (34.6) | 0.1 (0.1) | |
| isobutyl-acetate | 0.02 (24.2) | 0.07 (25.4) | 0.03 (3.5) | 0.04 (0.1) | 0.01 (36.9) | 0.05 (0.1) | |
| isopentyl acetate | 1.55 (33.95) | 0.23 (45.1) | 1.54 (0.6) | 0.63 (0.1) | 0.21 (13.9) | 1.79 (0.2) | |
| terpenes | alpha-terpineol | bdl | bdl | bdl | bdl | bdl | bdl |
| beta-citronellol | 0.09 (1.6) | 0.1 (3.6) | 0.09 (0.4) | 0.09 (0.1) | 0.09 (4.2) | 0.09 (0.1) | |
| geraniol | bdl | bdl | bdl | bdl | bdl | bdl | |
| linalool | 0.004 (5.9) | 0.006 (40.6) | 0.004 (3.5) | 0.005 (4.6) | 0.007 (8.9) | 0.01 (0.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerőcs, A.; Nagy, T.; Nemes-Barnás, K.; Májer, J.; Szőke, B.Á.; Kővágó, R.; Magalhães, F.; Gibson, B.; Szekeres, A.; Juhász, Á.; et al. Characterization of Saccharomyces Strains Isolated from “Kéknyelű” Grape Must and Their Potential for Wine Production. Fermentation 2022, 8, 416. https://doi.org/10.3390/fermentation8080416
Gerőcs A, Nagy T, Nemes-Barnás K, Májer J, Szőke BÁ, Kővágó R, Magalhães F, Gibson B, Szekeres A, Juhász Á, et al. Characterization of Saccharomyces Strains Isolated from “Kéknyelű” Grape Must and Their Potential for Wine Production. Fermentation. 2022; 8(8):416. https://doi.org/10.3390/fermentation8080416
Chicago/Turabian StyleGerőcs, Annamária, Tibor Nagy, Katalin Nemes-Barnás, János Májer, Barna Árpád Szőke, Róbert Kővágó, Frederico Magalhães, Brian Gibson, András Szekeres, Ákos Juhász, and et al. 2022. "Characterization of Saccharomyces Strains Isolated from “Kéknyelű” Grape Must and Their Potential for Wine Production" Fermentation 8, no. 8: 416. https://doi.org/10.3390/fermentation8080416






