Influence of Animal/Plant Activated Biochar Properties on Methane Production from Corn Stalk by Anaerobic Fermentation
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Methods
2.2. ABC-Mediated Anaerobic Fermentation Studies
2.3. Analytical Methods
3. Effect of Animal/Plant ABC on the Characteristics of Anaerobic Fermentation Gas Production
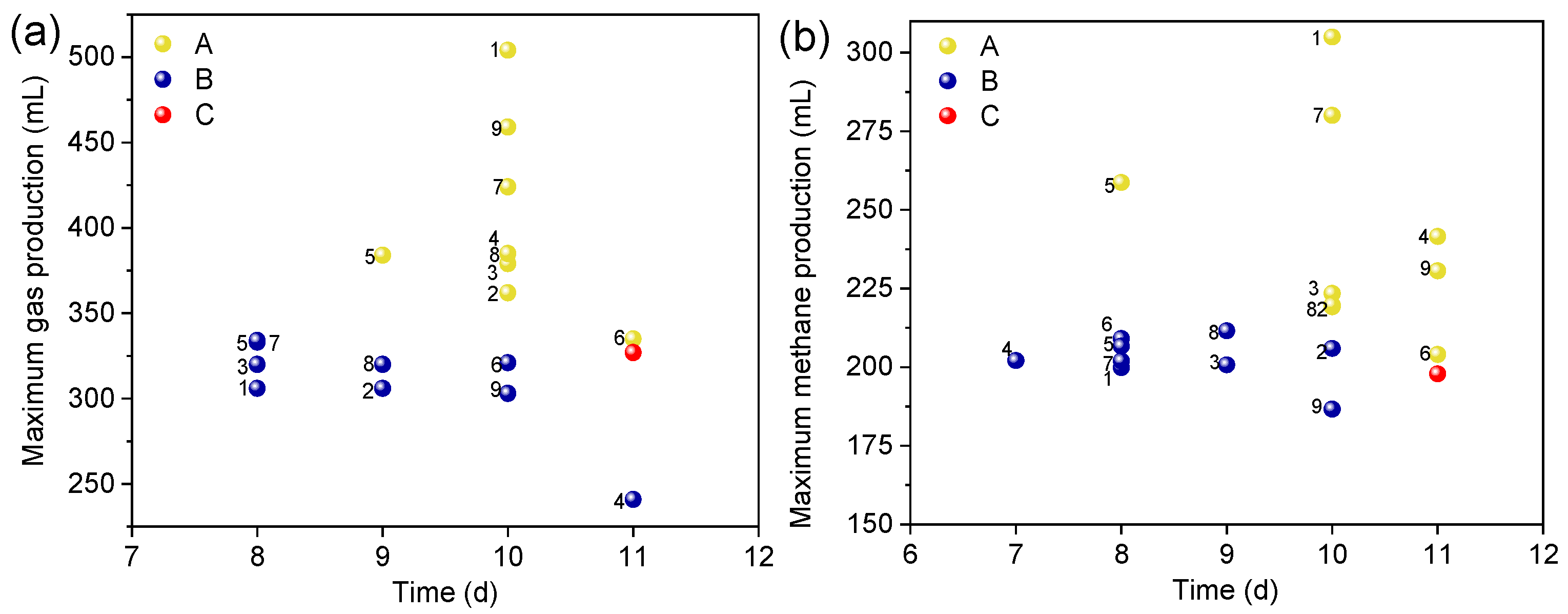
3.1. Effect of ABC on Gas and Methane Production Peak
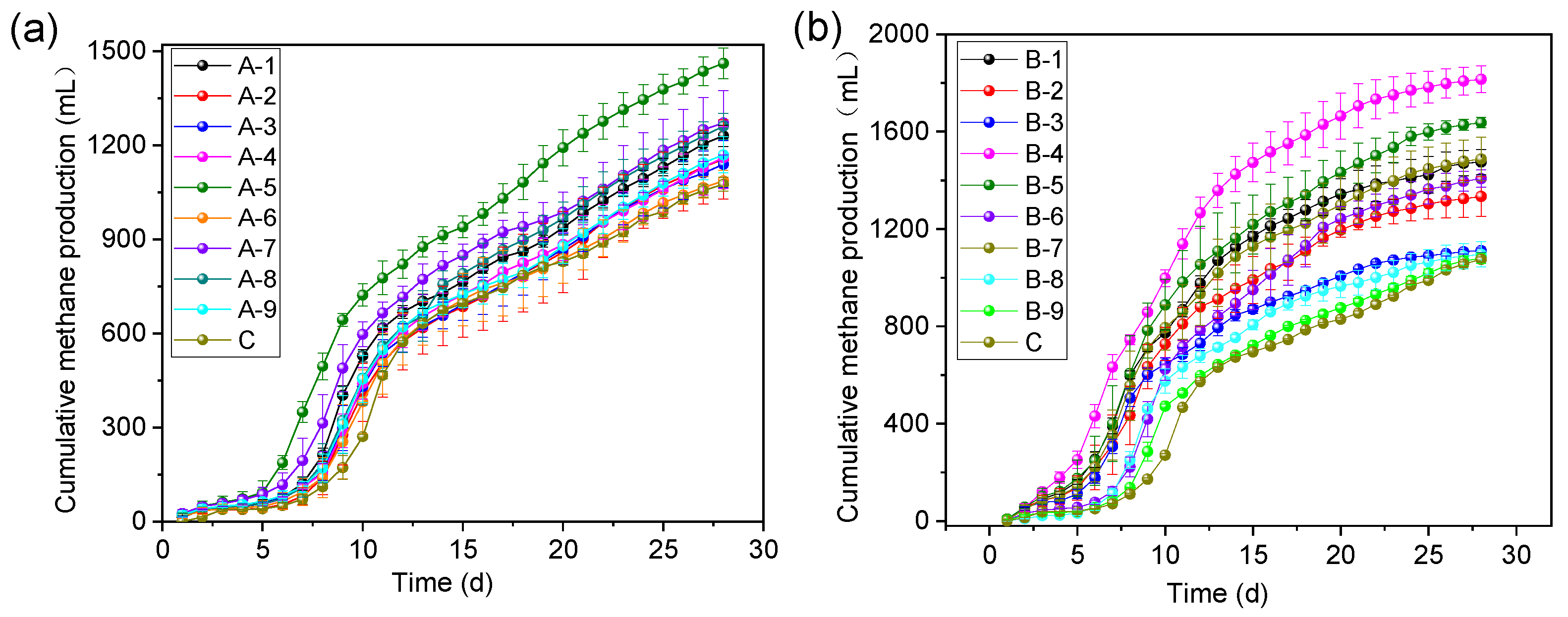
3.2. Effect of ABC on Gas and Methane Production
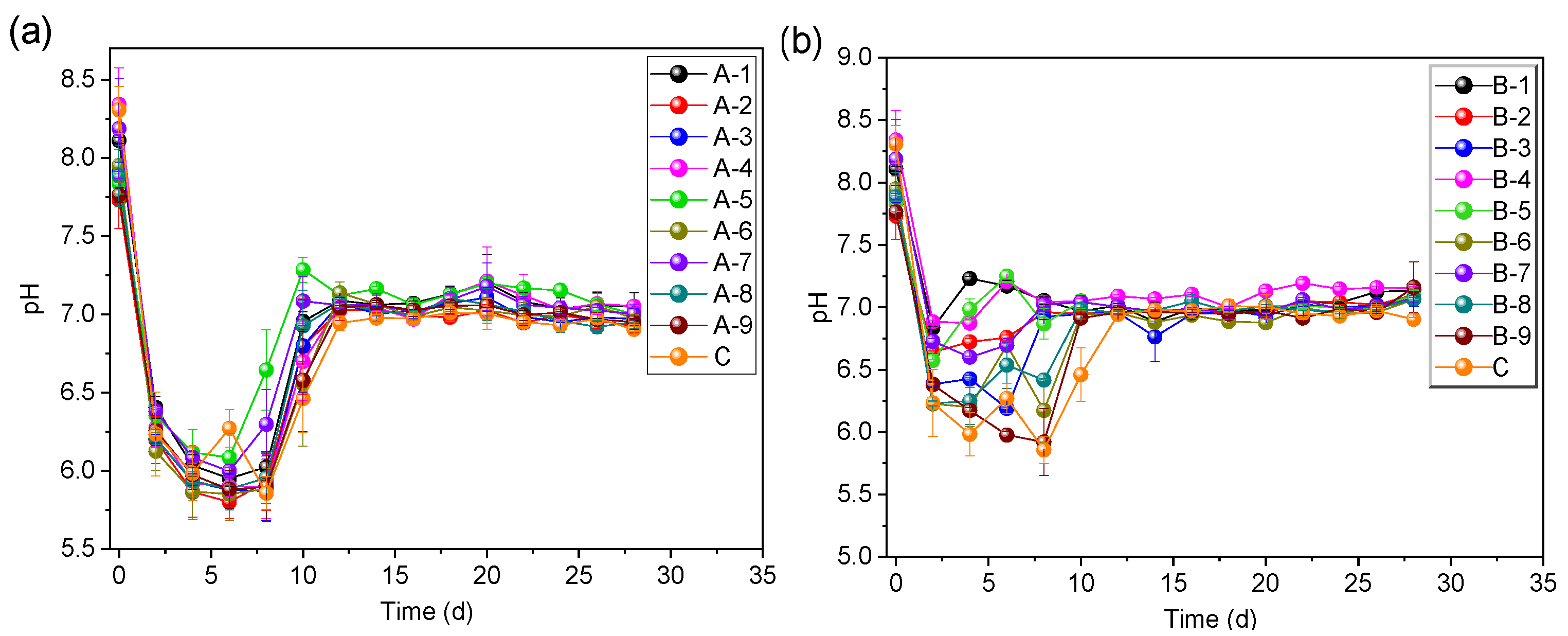
3.3. Effect of ABC on pH Value
4. Comparative Analysis of the Effects of the Properties of Activated Carbon from Plants/Animals ABC on Anaerobic Fermentation
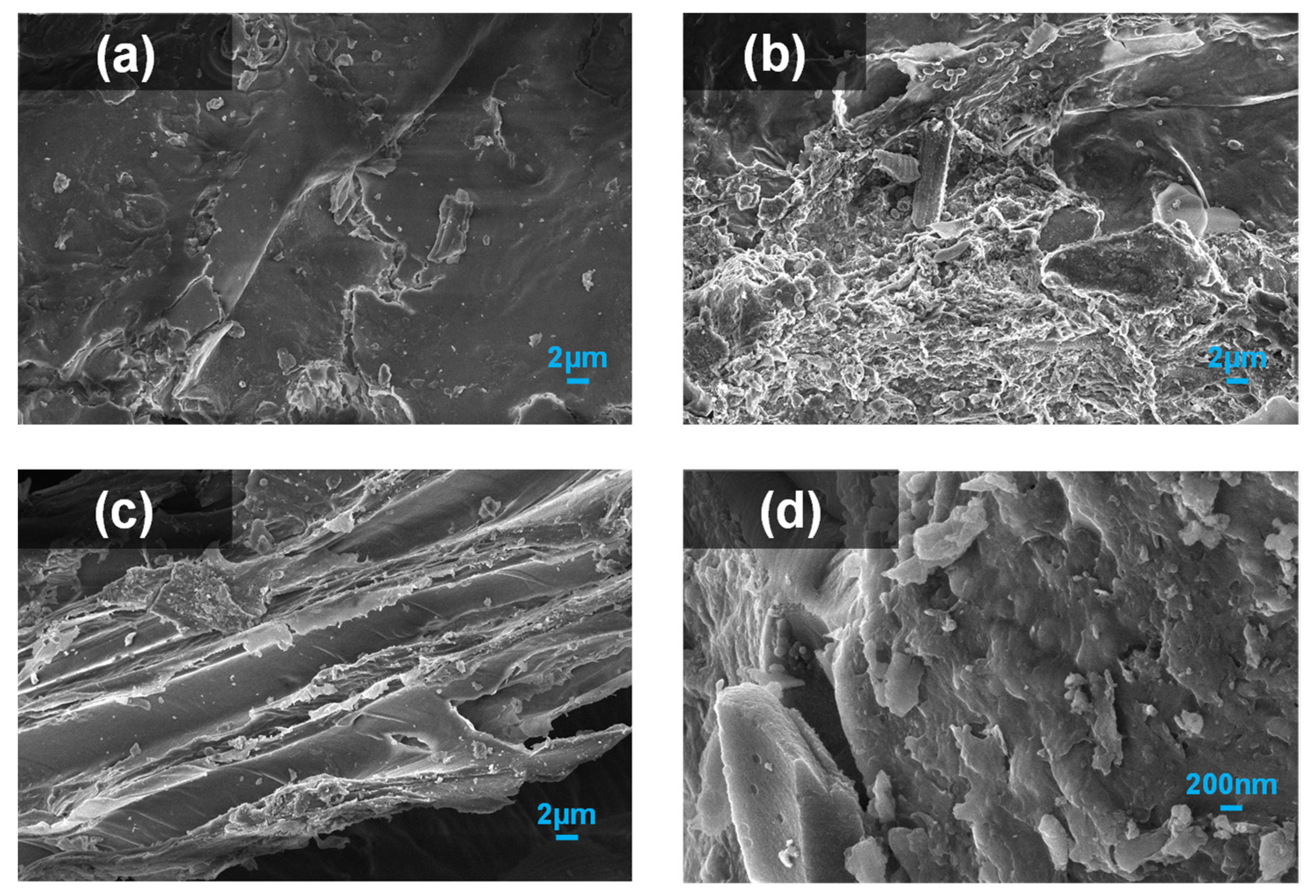
4.1. Structural Changes of Corn Stalk before and after Fermentation
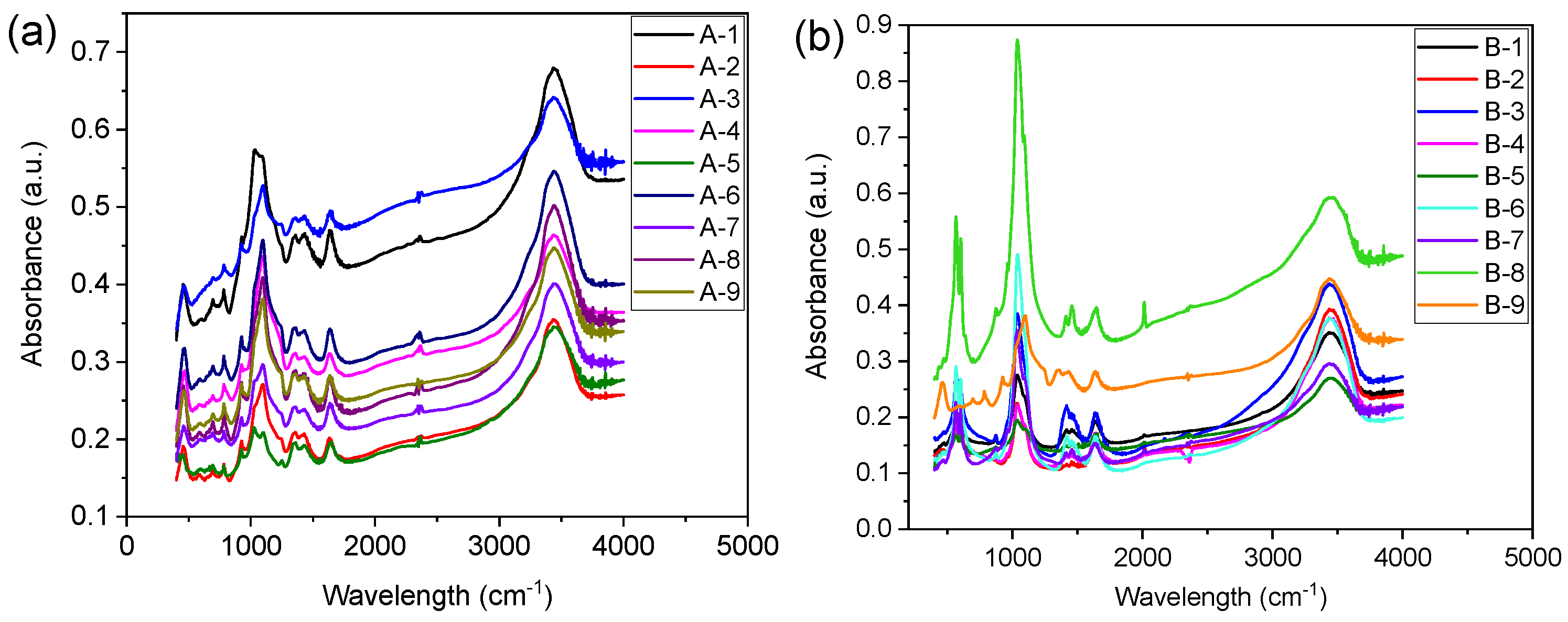
4.2. Effect of Surface Functional Groups on Anaerobic Fermentation Process
4.3. Effects of Specific Surface Area and Conductivity on Anaerobic Fermentation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiu, S.; Shahbazi, A.; Li, R. Characterization, Modification and Application of Biochar for Energy Storage and Catalysis: A Review. Trends Renew. Energy 2017, 3, 86–101. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. Bioenergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review. Bioenergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Binderless briquetting technology for lignite briquettes: A review. Energy Ecol. Environ. 2021, 6, 69–79. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef]
- Xu, J.; Mustafa, A.M.; Lin, H.; Choe, U.Y.; Sheng, K. Effect of hydrochar on anaerobic digestion of dead pig carcass after hydrothermal pretreatment. Waste Manag. 2018, 78, 849–856. [Google Scholar] [CrossRef]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Biochar-enhanced hydrogen production from organic fraction of municipal solid waste using co-culture of Enterobacter aerogenes and E. coli. Int. J. Hydrogen Energy 2017, 42, 18865–18874. [Google Scholar] [CrossRef]
- Xu, S.; He, C.; Luo, L.; Lü, F.; He, P.; Cui, L. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresour. Technol. 2015, 196, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Joseph, S.; Lehmann, J. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Cooney, M.J.; Lewis, K.; Harris, K.; Zhang, Q.; Yan, T. Start up performance of biochar packed bed anaerobic digesters. J. Water Process. Eng. 2016, 9, e7–e13. [Google Scholar] [CrossRef]
- Cabeza, I.; Waterhouse, T.; Sohi, S.; Rooke, J.A. Effect of biochar produced from different biomass sources and at different process temperatures on methane production and ammonia concentrations in vitro. Anim. Feed Sci. Technol. 2018, 237, 1–7. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chandiramouli, R. Investigation of NH3 adsorption behavior on graphdiyne nanosheet and nanotubes: A first-principles study. J. Mol. Liq. 2018, 249, 24–32. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, J.; Wen, C.; Sun, Z. Optimization and kinetic modeling of an enhanced bio-hydrogen fermentation with the addition of synergistic biochar and nickel nanoparticle. Int. J. Energy Res. 2019, 43, 983–999. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015, 17, 648–655. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, Y.; Yan, Y.; Feng, L.; Chen, Y.; Zhou, Q. New method for algae comprehensive utilization: Algae-derived biochar enhances algae anaerobic fermentation for short-chain fatty acids production. Bioresour. Technol. 2019, 289, 121637. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 2019, 7, 103067. [Google Scholar] [CrossRef]
- Kaur, G.; Johnravindar, D.; Wong, J.W.C. Enhanced volatile fatty acid degradation and methane production efficiency by biochar addition in food waste-sludge co-digestion: A step towards increased organic loading efficiency in co-digestion. Bioresour. Technol. 2020, 308, 123250. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Li, L.-L.; Tong, Z.-H.; Fang, C.-Y.; Chu, J.; Yu, H.-Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Zhou, H.; Brown, R.C.; Wen, Z. Biochar as an Additive in Anaerobic Digestion of Municipal Sludge: Biochar Properties and Their Effects on the Digestion Performance. ACS Sustain. Chem. Eng. 2020, 8, 6391–6401. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. 2016, 34, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.D.; Johnson, R.L.; Lehmann, J.; Olk, D.C.; Neves, E.G.; Thompson, M.L.; Schmidt-Rohr, K. Abundant and Stable Char Residues in Soils: Implications for Soil Fertility and Carbon Sequestration. Environ. Sci. Technol. 2012, 46, 9571–9576. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, Z.; Yang, S.; Fang, C.; Zou, X.; Luo, Y. Experimental co-digestion of corn stalk and vermicompost to improve biogas production. Waste Manag. 2010, 30, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Davraz, M.; Gunduz, L. Engineering properties of amorphous silica as a new natural pozzolan for use in concrete. Cem. Concr. Res. 2005, 35, 1251–1261. [Google Scholar] [CrossRef]



| Serial Number | Activation Temperature (°C) | Impregnation Ratio (g/g) | Activation Time (min) | Special Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|---|---|---|
| A-1 | 600 | 2 | 30 | 140.85 | 3.69 |
| A-2 | 700 | 4 | 30 | 90.65 | 4.84 |
| A-3 | 800 | 6 | 30 | 102.72 | 4.24 |
| A-4 | 700 | 2 | 60 | 134.57 | 6.09 |
| A-5 | 800 | 4 | 60 | 74.24 | 5.95 |
| A-6 | 600 | 6 | 60 | 166.60 | 4.19 |
| A-7 | 800 | 2 | 90 | 155.13 | 4.67 |
| A-8 | 600 | 4 | 90 | 123.10 | 2.39 |
| A-9 | 700 | 6 | 90 | 113.23 | 4.47 |
| B-1 | 600 | 2 | 30 | 146.35 | 6.32 |
| B-2 | 700 | 4 | 30 | 129.34 | 5.27 |
| B-3 | 800 | 6 | 30 | 98.93 | 7.93 |
| B-4 | 700 | 2 | 60 | 111.01 | 6.52 |
| B-5 | 800 | 4 | 60 | 127.28 | 5.33 |
| B-6 | 600 | 6 | 60 | 86.75 | 8.43 |
| B-7 | 800 | 2 | 90 | 120.26 | 8.24 |
| B-8 | 600 | 4 | 90 | 95.37 | 6.80 |
| B-9 | 700 | 6 | 90 | 84.95 | 9.36 |
| Samples | Moisture (%) | Total Solid (%) | Volatile Solid (%) | Ash (%) | C/N |
|---|---|---|---|---|---|
| Corn stalk | 3.1 | 96.9 | 91.9 | 5.1 | 38.04 |
| Inoculated sludge | 93.9 | 6.1 | 59.7 | 40.3 | 6.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Tian, S.; Liu, J.; Guo, P.-Y.; Shen, J. Influence of Animal/Plant Activated Biochar Properties on Methane Production from Corn Stalk by Anaerobic Fermentation. Fermentation 2022, 8, 397. https://doi.org/10.3390/fermentation8080397
Zhang Z, Tian S, Liu J, Guo P-Y, Shen J. Influence of Animal/Plant Activated Biochar Properties on Methane Production from Corn Stalk by Anaerobic Fermentation. Fermentation. 2022; 8(8):397. https://doi.org/10.3390/fermentation8080397
Chicago/Turabian StyleZhang, Zhen, Shujian Tian, Jun Liu, Peng-Yan Guo, and Jie Shen. 2022. "Influence of Animal/Plant Activated Biochar Properties on Methane Production from Corn Stalk by Anaerobic Fermentation" Fermentation 8, no. 8: 397. https://doi.org/10.3390/fermentation8080397






