Optimizing Anthocyanin-Rich Black Cane (Saccharum sinensis Robx.) Silage for Ruminants Using Molasses and Iron Sulphate: A Sustainable Alternative
Abstract
:1. Introduction
2. Materials and Methods
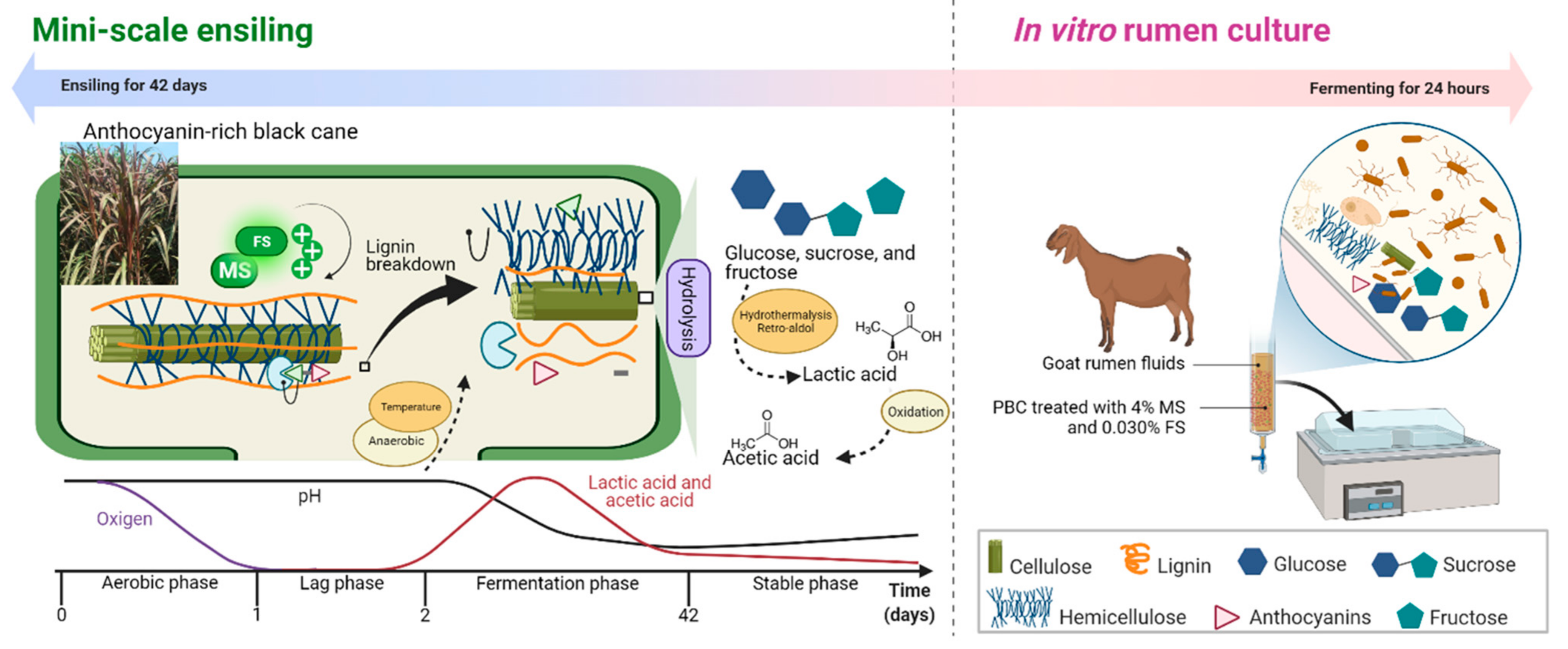
2.1. Roughage Harvesting and Mini-Scale Ensiling (Experiment 1)
2.2. Animal Management and In Vitro Rumen Culture (Experiment 2)
2.3. Sampling and Laboratory Analysis
2.4. DNA Extraction and Real-Time PCR Quantification
2.5. Statistical Analyses
3. Results
3.1. Composition of Anthocyanin-Rich Black Cane
3.2. Chemical Composition, Anthocyanin Content, and Ensiling Characteristics of Anthocyanin-Rich Black Cane
3.3. Effect of 4% MS + 0.030% FS Incorporated into Silages on Rumen Fermentation Profiles and Microbial Communities in Rumen Fluids
3.4. Suitability of 4% MS + 0.030% FS Incorporated into Silages for the Sustainable Mitigation of Ruminal Biogases
4. Discussion
4.1. Anthocyanin-Rich Black Cane Has a High Fiber Content and Abundance of Anthocyanin, as Well as High Lignification or Silicification of Lignocellulosic Biomass
4.2. Combination of Molasses and Ferrous Sulphate Improved Chemical Composition, Anthocyanin Content, and Ensiling Characteristics of Anthocyanin-Rich Black Cane
4.3. Combination of Molasses and Ferrous Sulphate Increases Total VFA Concentrations, Modulates Cellulolytic Bacteria, and Suppresses Methanogenic Bacteria in Rumen Fluid
4.4. The Incorporation of 4% MS + 0.030% FS into Silages Modulates the Sustainable Mitigation of the Ruminal Biogases Methane and Carbon Dioxide without Impairing Total Gas Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grivet, L.; Glaszmann, J.C.; D’Hont, A. Molecular Evidence of Sugarcane Evolution and Domestication. In Darwin’s Harvest; Columbia University Press: New York, NY, USA, 2006; pp. 49–66. [Google Scholar]
- Tian, X.Z.; Lu, Q.; Zhao, S.; Li, J.; Luo, Q.; Wang, X.; Zhang, Y.; Zheng, N. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of longissimus thoracis et lumborum muscle in goats. Animals 2021, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Suong, N.T.M.; Paengkoum, P.; Schonewille, J.T.; Purba, R.A.P.; Paengkoum, P. Growth performance, blood biochemical indices, rumen bacterial community, and carcass characteristics in goats fed anthocyanin-rich black cane silage. Front. Vet. Sci. 2022, 9, 880838. [Google Scholar] [CrossRef] [PubMed]
- Purba, R.A.P.; Paengkoum, S.; Yuangklang, C.; Paengkoum, P.; Salem, A.Z.M.; Liang, J.B. Mammary gene expressions and oxidative indicators in ruminal fluid, blood, milk, and mammary tissue of dairy goats fed a total mixed ration containing piper meal (Piper betle L.). Ital. J. Anim. Sci. 2022, 21, 129–141. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, S.; Han, Y.; Zhao, D.; Li, H.; Wang, Y.; Zhang, G.; Chen, C. Natural variation of lignocellulosic components in miscanthus biomass in china. Front. Chem. 2020, 8, 1028. [Google Scholar] [CrossRef] [PubMed]
- Vorlaphim, T.; Paengkoum, P.; Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Schonewille, J.T. Treatment of rice stubble with Pleurotus ostreatus and urea improves the growth performance in slow-growing goats. Animals 2021, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, H.; Zheng, R.; Lin, Z.; Huang, H. The enhancement of pretreatment and enzymatic hydrolysis of corn stover by FeSO4 pretreatment. Biochem. Eng. J. 2011, 56, 158–164. [Google Scholar] [CrossRef]
- Soudham, V.P.; Brandberg, T.; Mikkola, J.P.; Larsson, C. Detoxification of acid pretreated spruce hydrolysates with ferrous sulfate and hydrogen peroxide improves enzymatic hydrolysis and fermentation. Bioresour. Technol. 2014, 166, 559–565. [Google Scholar] [CrossRef]
- Bao, H.; Sagues, W.J.; Wang, Y.; Peng, W.; Zhang, L.; Yang, S.; Xiao, D.; Tong, Z. Depolymerization of lignin into monophenolics by ferrous/persulfate reagent under mild conditions. ChemSusChem 2020, 13, 6582–6593. [Google Scholar] [CrossRef]
- Suong, N.T.M.; Paengkoum, S.; Salem, A.Z.M.; Paengkoum, P.; Purba, R.A.P. Silage fermentation quality, anthocyanin stability, and in vitro rumen fermentation characteristic of ferrous sulfate heptahydrate-treated black cane (Saccharum sinensis R.). Front. Vet. Sci. 2022, 9, 896270. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of napier grasses at late maturity stage. Agriculture 2020, 10, 262. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A review regarding the use of molasses in animal nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.F. Safety and efficacy of iron compounds (E1) as feed additives for all animal species: Ferrous carbonate; ferric chloride, hexahydrate; ferrous fumarate; ferrous sulphate, heptahydrate; ferrous sulphate, monohydrate; ferrous chelate of amino acids, hydrate; ferrous chelate of glycine, hydrate, based on a dossier submitted by FEFANA asbl. EFSA J. 2016, 14, 4396. [Google Scholar]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Paengkoum, P. Milk fatty acid composition, rumen microbial population and animal performance in response to diets rich in linoleic acid supplemented with Piper betle leaves in Saanen goats. Anim. Prod. Sci. 2020. [Google Scholar] [CrossRef]
- Suong, N.T.M. Utilization of Anthocyanin-Rich Napier Grass Silage in Growing Goat Diets. Ph.D. Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2017. [Google Scholar]
- Xia, C.; Liang, Y.; Bai, S.; He, Y.; Muhammad, A.U.R.; Su, H.; Cao, B. Effects of harvest time and added molasses on nutritional content, ensiling characteristics and in vitro degradation of whole crop wheat. Asian-Australas J. Anim. Sci. 2018, 31, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, G.M.; Khatiwada, J.; Gueye, A. Effect of dietary supplementation of ferrous sulfate on performance and carcass characteristics of finishing lambs. J. Anim. Sci. Technol. 2012, 1, 7–12. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Paengkoum, P. Piper oil decreases in vitro methane production with shifting ruminal fermentation in a variety of diets. Int. J. Agric. Biol. 2021, 25, 231–240. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, P. Enhanced conjugated linoleic acid and biogas production after ruminal fermentation with Piper betle L. supplementation. Ciênc. Rural 2020, 50, e20191001. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, S.; Yuangklang, C.; Paengkoum, P. Flavonoids and their aromatic derivatives in Piper betle powder promote in vitro methane mitigation in a variety of diets. Cienc. Agrotec. 2020, 44, e012420. [Google Scholar] [CrossRef]
- Paengkoum, S.; Petlum, A.; Purba, R.A.P.; Paengkoum, P. Protein-binding affinity of various condensed tannin molecular weights from tropical leaf peel. J. Appl. Pharm. Sci. 2021, 11, 114–120. [Google Scholar]
- Orskov, E.R.; Mcdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. Camb. 1970, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis; AOAC International Suite 500: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergen fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cai, Y.; Du, Z.; Yamasaki, S.; Nguluve, D.; Tinga, B.; Macome, F.; Oya, T. Community of natural lactic acid bacteria and silage fermentation of corn stover and sugarcane tops in Africa. Asian-Australas J. Anim. Sci. 2020, 33, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Purba, R.A.P.; Paengkoum, S.; Paengkoum, P. Development of a simple high-performance liquid chromatography-based method to quantify synergistic compounds and their composition in dried leaf extracts of Piper sarmentosum Robx. Separations 2021, 8, 152. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, P. Bioanalytical HPLC method of Piper betle L. for quantifying phenolic compound, water-soluble vitamin, and essential oil in five different solvent extracts. J. Appl. Pharm. Sci. 2019, 9, 33–39. [Google Scholar]
- Kozaki, M.; Uchimura, T.; Okada, S. Experimental Manual of Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992; pp. 34–37. [Google Scholar]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Thongpea, S.; Ban, C. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J. Integr. Agric. 2018, 17, 2082–2095. [Google Scholar] [CrossRef] [Green Version]
- Song, T.H.; Han, O.K.; Park, T.; Kim, D.W.; Yoon, C.; Kim, K.J.; Park, K.H. Anthocyanin stability and silage fermentation quality of colored barley. J. Korean Grassl. Forage Sci. 2012, 32, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, J.K.; Scott, J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156. [Google Scholar] [CrossRef] [Green Version]
- Paengkoum, S.; Tatsapong, P.; Taethaisong, N.; Sorasak, T.; Purba, R.A.P.; Paengkoum, P. Empirical evaluation and prediction of protein requirements for maintenance and growth of 18–24 months old thai swamp buffaloes. Animals 2021, 11, 1405. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Atikah, I.N.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Jahromi, M.F.; Ivan, M.; Samsudin, A.A. Profiling of rumen fermentation, microbial population and digestibility in goats fed with dietary oils containing different fatty acids. BMC Vet. Res. 2018, 14, 344. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedure of Statistics. A Biometrical Approach, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical characteristics of selected grass species from polish meadows and their potential utilization for energy generation purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Li, X.; Ma, Z.; Yao, S. Bioactivity-guided systematic extraction and purification supported by multitechniques for sugarcane flavonoids and anthocyanins. Food Bioprod. Process. 2015, 94, 547–554. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Tu, B.; Li, X.; Jia, C.; Song, H. Determination and comparison of flavonoids and anthocyanins in Chinese sugarcane tips, stems, roots and leaves. J. Sep. Sci. 2010, 33, 1216–1223. [Google Scholar] [CrossRef]
- Oba, M. Review: Effects of feeding sugars on productivity of lactating dairy cows. Can. J. Anim. Sci. 2011, 91, 37–46. [Google Scholar] [CrossRef]
- Fernández, A.M.; Cabezuelo, A.B.S.; de la Roza Delgado, M.B.; Arrojo, M.A.G.; Gutiérrez, A.A. Modelling a quantitative ensilability index adapted to forages from wet temperate areas. Span. J. Agric. Res. 2013, 11, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. In Silage Science and Technology; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Hosoda, K.; Eruden, B.; Matsuyama, H.; Shioya, S. Silage fermentative quality and characteristics of anthocyanin stability in anthocyanin-rich corn (Zea mays L.). Asian-Australas J. Anim. Sci. 2009, 22, 528–533. [Google Scholar] [CrossRef]
- Pace, E.; Jiang, Y.; Clemens, A.; Crossman, T.; Rupasinghe, H.P.V. Impact of thermal degradation of cyanidin-3-o-glucoside of haskap berry on cytotoxicity of hepatocellular carcinoma hepg2 and breast cancer mda-mb-231 cells. Antioxidants 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gu, M.; Gong, P.; Wang, J.; Hu, Y.; Hu, Y.; Tan, X.; Wei, J.; Yang, H. Glycosides changed the stability and antioxidant activity of pelargonidin. LWT 2021, 147, 111581. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Zhou, D.; Long, Q.M.; Wang, X.; Lu, Q.; Wen, G.L. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and rumen microbiota in goats. Front. Vet. Sci. 2021, 8, 715710. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, A.L.; Adeyemi, K.D.; Samsudin, A.A.; Goh, Y.M.; Alimon, A.R.; Sazili, A.Q. Effects of dietary supplementation of leaves and whole plant of Andrographis paniculata on rumen fermentation, fatty acid composition and microbiota in goats. BMC Vet. Res. 2017, 13, 349. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Meng, Q.; Li, S.; Ren, L.; Zhou, B.; Schonewille, T.; Zhou, Z. Effects of diets supplemented with ensiled mulberry leaves and sun-dried mulberry fruit pomace on the ruminal bacterial and archaeal community composition of finishing steers. PLoS ONE 2016, 11, e0156836. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Fields, C.J.; Lepercq, P.; Ruiz, P.; Forano, E.; White, B.A.; Mosoni, P. In vivo competitions between Fibrobacter succinogenes, Ruminococcus flavefaciens, and Ruminoccus albus in a gnotobiotic sheep model revealed by multi-omic analyses. mBio 2021, 12, e03533-20. [Google Scholar] [PubMed]
- Patra, A.K.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Zhao, Z.; Zhang, Y. Insight into ferrihydrite effects on methanogenesis in UASB reactors treating high sulfate wastewater: Reactor performance and microbial community. Environ. Sci. Water Res. Technol. 2020, 6, 1794–1803. [Google Scholar] [CrossRef]
- Elliott, C.L.; Edwards, J.E.; Wilkinson, T.J.; Allison, G.G.; McCaffrey, K.; Scott, M.B.; Rees-Stevens, P.; Kingston-Smith, A.H.; Huws, S.A. Using ‘omic approaches to compare temporal bacterial colonization of Lolium perenne, Lotus corniculatus, and Trifolium pratense in the rumen. Front. Microbiol. 2018, 9, 2184. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.G.D.; Henrique, D.S.; Abreu, M.L.C.; Fluck, A.C.; Mayer, L.R.R.; Costa, O.A.D.; Atoji-Henrique, K. Evaluation of mathematical models to describe gas production kinetics of some tropical and temperate forages. Rev. Bras. Zootec. 2020, 49, 379. [Google Scholar] [CrossRef]
- Ban, C.; Paengkoum, S.; Yang, S.; Tian, X.Z.; Thongpea, S.; Purba, R.A.P.; Paengkoum, P. Feeding meat goats mangosteen (Garcinia mangostana L.) peel rich in condensed tannins, flavonoids, and cinnamic acid improves growth performance and plasma antioxidant activity under tropical conditions. J. Appl. Anim. Res. 2022, 50, 307–315. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joblin, K.N. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 1999, 50, 1307–1314. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.J.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef] [PubMed]
 ) and ensiled forms (NA,
) and ensiled forms (NA,  ; 4% MS + 0.030% FS,
; 4% MS + 0.030% FS,  ), at three time points (6, 12, 24 h) during in vitro rumen incubation: (a) total gas production; (b) carbon dioxide production; (c) methane production. The values shown are the means, with standard errors represented by vertical bars. Significantly different values are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, p > 0.05.
), at three time points (6, 12, 24 h) during in vitro rumen incubation: (a) total gas production; (b) carbon dioxide production; (c) methane production. The values shown are the means, with standard errors represented by vertical bars. Significantly different values are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, p > 0.05.
 ) and ensiled forms (NA,
) and ensiled forms (NA,  ; 4% MS + 0.030% FS,
; 4% MS + 0.030% FS,  ), at three time points (6, 12, 24 h) during in vitro rumen incubation: (a) total gas production; (b) carbon dioxide production; (c) methane production. The values shown are the means, with standard errors represented by vertical bars. Significantly different values are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, p > 0.05.
), at three time points (6, 12, 24 h) during in vitro rumen incubation: (a) total gas production; (b) carbon dioxide production; (c) methane production. The values shown are the means, with standard errors represented by vertical bars. Significantly different values are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, p > 0.05.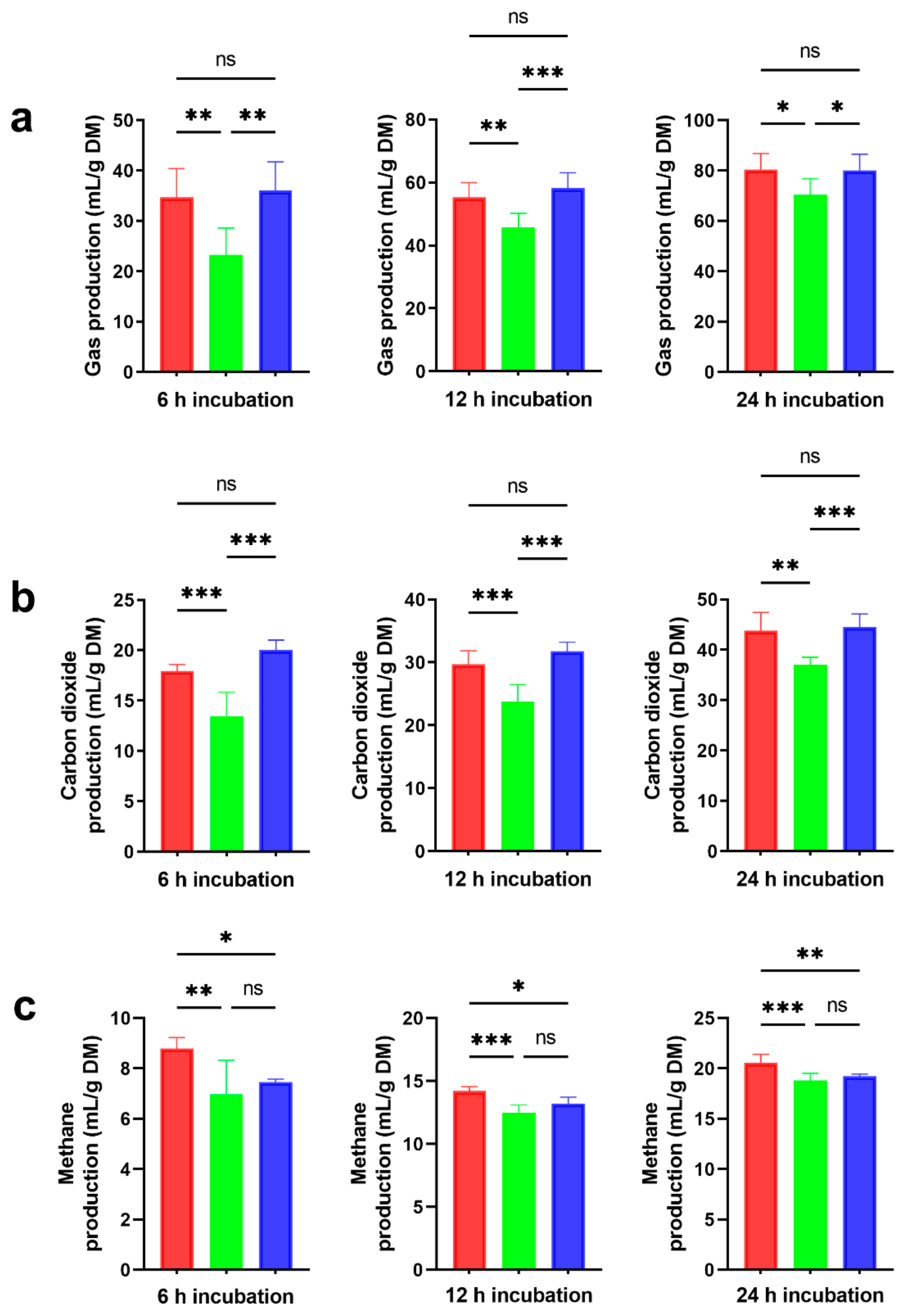
| Item 1 | DM (g/kg FM) | OM (g/kg DM) | CP (g/kg DM) | NDF (g/kg DM) | ADF (g/kg DM) | ADL (g/kg DM) | HC (g/kg DM) | CEL (g/kg DM) | WSC (g/kg DM) |
|---|---|---|---|---|---|---|---|---|---|
| Pre-ensiled materials (PBC) | 154.34 | 868.12 | 76.24 | 794.91 | 490.11 | 58.43 | 304.80 | 431.68 | 26.13 |
| Silage additives | |||||||||
| MS 0% FS 0% (NA) | 154.52 | 859.72 | 73.83 | 790.50 | 490.90 | 61.26 | 299.60 | 429.64 | 10.42 |
| MS 4% | 151.06 | 855.04 | 73.01 | 780.61 | 488.82 | 57.37 | 291.79 | 431.45 | 11.25 |
| MS 8% | 152.62 | 855.96 | 72.95 | 780.45 | 487.93 | 57.36 | 292.52 | 430.57 | 12.06 |
| FS 0.015% | 156.13 | 841.25 | 75.01 | 788.75 | 480.76 | 50.09 | 307.99 | 430.67 | 11.52 |
| FS 0.030% | 161.42 | 834.53 | 76.17 | 789.44 | 490.81 | 49.97 | 298.63 | 440.84 | 12.42 |
| MS 4% FS 0.015% | 162.21 | 868.72 | 76.02 | 790.64 | 489.34 | 56.53 | 301.30 | 432.81 | 21.27 |
| MS 4% FS 0.030% | 169.94 | 870.52 | 76.55 | 790.52 | 490.00 | 55.73 | 300.52 | 434.27 | 23.94 |
| MS 8% FS 0.015% | 163.64 | 869.64 | 75.16 | 789.56 | 489.74 | 58.03 | 299.82 | 431.71 | 22.04 |
| MS 8% FS 0.030% | 165.25 | 870.06 | 74.98 | 780.30 | 489.84 | 57.35 | 290.46 | 432.49 | 24.03 |
| SEM | 0.109 | 0.103 | 0.095 | 0.393 | 0.083 | 0.072 | 0.043 | 0.066 | 0.090 |
| Contrast p-values | |||||||||
| PBC vs. Silage additives | <0.001 | <0.001 | 0.086 | 0.148 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| NA vs. MS FS | <0.001 | <0.001 | 0.089 | 0.280 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| NA vs. MS | 0.219 | 0.102 | 0.780 | 0.089 | 0.481 | 0.384 | <0.001 | 0.840 | <0.001 |
| NA vs. FS | <0.001 | <0.001 | 0.419 | 0.926 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| NA vs. MS FS | <0.001 | <0.001 | 0.150 | 0.567 | 0.560 | <0.001 | <0.001 | <0.001 | <0.001 |
| PBC vs. MS FS | <0.001 | 0.692 | 0.635 | 0.310 | 0.899 | <0.001 | <0.001 | <0.001 | <0.001 |
| MS 4% vs. MS 8% | 0.648 | 0.772 | 0.999 | 0.999 | 0.999 | 0.999 | 0.810 | 0.999 | 0.002 |
| FS 0.015% vs. FS 0.030% | 0.005 | <0.001 | 0.917 | 0.999 | 0.002 | 0.999 | <0.001 | 0.004 | <0.001 |
| Item 1 | pH | Anthocyanin Content (mg/g DM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C3G | P3G | Del | Peo3G | M3G | Cya | Pel | Mal | Total | ||
| Pre-ensiled materials (PBC) | 5.42 | 0.047 | 0.094 | 0.080 | 0.138 | 0.115 | 0.158 | 0.080 | 0.393 | 1.105 |
| Silage additives | ||||||||||
| MS 0% FS 0% (NA) | 4.76 | 0.014 | 0.021 | 0.049 | 0.047 | 0.041 | 0.074 | 0.005 | 0.040 | 0.291 |
| MS 4% | 4.17 | 0.016 | 0.034 | 0.055 | 0.063 | 0.058 | 0.045 | 0.006 | 0.064 | 0.341 |
| MS 8% | 3.74 | 0.018 | 0.037 | 0.057 | 0.064 | 0.071 | 0.057 | 0.009 | 0.077 | 0.390 |
| FS 0.015% | 4.68 | 0.015 | 0.026 | 0.061 | 0.059 | 0.080 | 0.077 | 0.005 | 0.082 | 0.405 |
| FS 0.030% | 4.21 | 0.015 | 0.027 | 0.072 | 0.072 | 0.089 | 0.081 | 0.007 | 0.119 | 0.482 |
| MS 4% FS 0.015% | 3.76 | 0.020 | 0.039 | 0.085 | 0.126 | 0.097 | 0.174 | 0.008 | 0.122 | 0.671 |
| MS 4% FS 0.030% | 3.71 | 0.022 | 0.041 | 0.098 | 0.133 | 0.129 | 0.187 | 0.008 | 0.129 | 0.747 |
| MS 8% FS 0.015% | 3.72 | 0.020 | 0.045 | 0.113 | 0.13 | 0.138 | 0.172 | 0.010 | 0.132 | 0.760 |
| MS 8% FS 0.030% | 3.69 | 0.022 | 0.049 | 0.136 | 0.142 | 0.144 | 0.185 | 0.009 | 0.138 | 0.825 |
| SEM | 0.036 | 0.002 | 0.007 | 0.014 | 0.016 | 0.022 | 0.019 | 0.004 | 0.017 | 0.038 |
| Contrast p-values | ||||||||||
| PBC vs. Silage additives | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.020 | <0.001 | <0.001 | <0.001 | <0.001 |
| NA vs. MS FS | <0.001 | 0.131 | 0.095 | 0.004 | <0.001 | 0.015 | <0.001 | 0.158 | <0.001 | <0.001 |
| NA vs. MS | <0.001 | 0.414 | 0.241 | 0.898 | 0.472 | 0.436 | 0.187 | 0.163 | 0.108 | 0.002 |
| NA vs. FS | 0.002 | 0.946 | 0.630 | 0.236 | 0.480 | 0.191 | 0.971 | 0.361 | <0.001 | <0.001 |
| NA vs. MS FS | <0.001 | 0.208 | 0.054 | 0.003 | 0.003 | 0.034 | 0.006 | 0.270 | <0.001 | <0.001 |
| PBC vs. MS FS | <0.001 | <0.001 | <0.001 | 0.160 | 0.979 | 0.708 | 0.896 | 0.003 | <0.001 | 0.007 |
| MS 4% vs. MS 8% | <0.001 | 0.740 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.249 | 0.763 | 0.044 |
| FS 0.015% vs. FS 0.030% | <0.001 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.596 | 0.038 | 0.002 |
| Item 1 | Microorganism (CFU/g Fresh Matter) | ||||
|---|---|---|---|---|---|
| Lactic Acid Bacteria | Coliform Bacteria | Aerobic Bacteria | Yeasts | Molds | |
| Pre-ensiled materials (PBC) | 2.4 × 104 | 2.2 × 107 | 4.5 × 106 | 2.6 × 108 | nd |
| Silage additives | |||||
| MS 0% FS 0% (NA) | 5.3 × 107 | 2.5 × 104 | 8.8 × 105 | 4.1 × 106 | nd |
| MS 4% | 1.2 × 108 | 2.3 × 104 | 7.5 × 104 | nd | nd |
| MS 8% | 3.5 × 108 | 2.1 × 104 | 3.3 × 104 | nd | nd |
| FS 0.015% | 8.4 × 107 | 2.2 × 104 | 2.0 × 105 | nd | nd |
| FS 0.030% | 1.1 × 108 | 2.1 × 104 | 6.3 × 104 | nd | nd |
| MS 4% FS 0.015% | 2.4 × 108 | 1.8 × 103 | 4.1 × 104 | nd | nd |
| MS 4% FS 0.030% | 5.4 × 108 | 1.3 × 103 | 3.1 × 104 | nd | nd |
| MS 8% FS 0.015% | 3.9 × 108 | 1.7 × 103 | 3.1 × 104 | nd | nd |
| MS 8% FS 0.030% | 5.5 × 108 | 1.3 × 103 | 3.0 × 104 | nd | nd |
| SEM | 2.032 | 0.980 | 0.878 | 0.430 | 0.000 |
| Contrast p-values | |||||
| PBC vs. Silage additives | <0.001 | <0.001 | <0.001 | <0.001 | 0.000 |
| NA vs. MS FS | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| NA vs. MS | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| NA vs. FS | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| NA vs. MS FS | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| PBC vs. MS FS | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| MS 4% vs. MS 8% | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| FS 0.015% vs. FS 0.030% | <0.001 | <0.001 | <0.001 | 0.000 | 0.000 |
| Item 1 | Organic Compound (g/kg DM) | ||||
|---|---|---|---|---|---|
| Ammonia Nitrogen | Lactic Acid | Acetic ACID | Propionic Acid | Butyric Acid | |
| Silage additives | |||||
| MS 0% FS 0% (NA) | 0.31 | 35.16 | 26.71 | nd | nd |
| MS 4% | 0.29 | 49.25 | 27.54 | nd | nd |
| MS 8% | 0.21 | 68.41 | 27.37 | nd | nd |
| FS 0.015% | 0.27 | 43.37 | 28.28 | nd | nd |
| FS 0.030% | 0.25 | 48.34 | 29.66 | nd | nd |
| MS 4% FS 0.015% | 0.26 | 53.63 | 28.82 | nd | nd |
| MS 4% FS 0.030% | 0.27 | 80.47 | 28.86 | nd | nd |
| MS 8% FS 0.015% | 0.31 | 72.21 | 28.14 | nd | nd |
| MS 8% FS 0.030% | 0.28 | 83.29 | 28.73 | nd | nd |
| SEM | 0.040 | 0.196 | 0.097 | 0.000 | 0.000 |
| Contrast p-values | |||||
| NA vs. MS FS | 0.846 | <0.001 | <0.001 | 0.000 | 0.000 |
| NA vs. MS | 0.282 | <0.001 | 0.136 | 0.000 | 0.000 |
| NA vs. FS | 0.654 | <0.001 | <0.001 | 0.000 | 0.000 |
| NA vs. MS FS | 0.917 | <0.001 | <0.001 | 0.000 | 0.000 |
| MS 4% vs. MS 8% | 0.250 | <0.001 | 0.897 | 0.000 | 0.000 |
| FS 0.015% vs. FS 0.030% | 0.999 | <0.001 | <0.001 | 0.000 | 0.000 |
| Item 1 | Time (h) | PBC | NA | MS 4% FS 0.030% | SEM | p-Value |
|---|---|---|---|---|---|---|
| pH | 6 | 6.84 | 6.86 | 6.55 | 0.106 | 0.391 |
| 12 | 6.74 | 6.74 | 6.48 | 0.196 | 0.635 | |
| 24 | 6.55 | 6.71 | 6.44 | 0.271 | 0.791 | |
| Ammonia nitrogen (mg/dL) | 6 | 14.22 | 14.93 | 15.86 | 0.970 | 0.666 |
| 12 | 15.62 | 15.04 | 15.97 | 0.561 | 0.549 | |
| 24 | 15.96 | 15.67 | 16.01 | 0.286 | 0.687 | |
| Total volatile fatty acids (mmol/L) | 6 | 93.16 a | 80.56 b | 91.99 a | 1.793 | 0.001 |
| 12 | 105.75 a | 92.97 b | 108.16 a | 1.017 | <0.001 | |
| 24 | 114.88 a | 100.05 b | 115.48 a | 0.613 | <0.001 | |
| Acetic acid (mol/100 mol) | 6 | 51.01 a | 49.94 b | 51.16 a | 0.287 | 0.018 |
| 12 | 51.64 a | 50.91 b | 51.48 a | 0.136 | 0.010 | |
| 24 | 51.58 a | 50.65 b | 51.50 a | 0.161 | 0.002 | |
| Propionic acid (mol/100 mol) | 6 | 37.31 | 37.81 | 37.67 | 0.166 | 0.166 |
| 12 | 35.18 | 35.85 | 35.56 | 0.256 | 0.218 | |
| 24 | 35.16 | 35.76 | 35.6 | 0.162 | 0.081 | |
| Butyric acid (mol/100 mol) | 6 | 11.68 | 12.25 | 11.17 | 0.292 | 0.106 |
| 12 | 13.18 | 13.24 | 12.96 | 0.099 | 0.373 | |
| 24 | 13.26 | 13.59 | 12.9 | 0.171 | 0.056 | |
| Ratio acetic acid to propionic acid | 6 | 1.37 a | 1.32 b | 1.36 a | 0.007 | 0.001 |
| 12 | 1.47 a | 1.42 b | 1.45 a | 0.006 | 0.003 | |
| 24 | 1.47 a | 1.42 b | 1.45 a | 0.005 | <0.001 |
| Item 1 | Time (h) | PBC | NA | MS 4% FS 0.030% | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total bacteria | 6 | 9.26 | 9.29 | 9.38 | 0.597 | 0.991 |
| 12 | 9.74 | 9.35 | 9.51 | 0.493 | 0.885 | |
| 24 | 9.77 | 9.45 | 9.31 | 0.356 | 0.706 | |
| Ruminococcus albus | 6 | 7.65 a | 7.33 b | 7.75 a | 0.028 | 0.001 |
| 12 | 7.72 a | 7.45 b | 7.87 a | 0.038 | 0.007 | |
| 24 | 7.88 a | 7.63 b | 7.94 a | 0.016 | <0.001 | |
| Ruminococcus flavefaciens | 6 | 5.71 a | 4.22 b | 6.60 a | 0.268 | 0.003 |
| 12 | 6.62 | 5.97 | 6.86 | 0.272 | 0.135 | |
| 24 | 6.94 | 6.78 | 7.09 | 0.160 | 0.572 | |
| Fibrobacter succinogenes | 6 | 7.91 | 7.87 | 7.70 | 0.157 | 0.674 |
| 12 | 8.40 | 8.28 | 8.41 | 0.049 | 0.402 | |
| 24 | 8.58 | 8.39 | 8.51 | 0.047 | 0.247 | |
| Butyrivibrio fibrisolvens | 6 | 6.72 | 6.96 | 6.94 | 0.094 | 0.311 |
| 12 | 7.03 | 7.16 | 7.00 | 0.158 | 0.831 | |
| 24 | 6.99 | 6.89 | 7.02 | 0.087 | 0.683 | |
| Megasphaera elsdenii | 6 | 3.69 | 3.71 | 3.72 | 0.131 | 0.995 |
| 12 | 3.97 | 3.73 | 3.71 | 0.111 | 0.535 | |
| 24 | 3.67 | 3.68 | 3.94 | 0.174 | 0.695 | |
| Streptococus bovis | 6 | 5.60 | 4.53 | 4.66 | 0.337 | 0.153 |
| 12 | 5.56 | 5.42 | 5.36 | 0.391 | 0.972 | |
| 24 | 5.44 | 5.58 | 5.24 | 0.204 | 0.665 | |
| Methanogen | 6 | 5.77 a | 5.38 b | 5.42 b | 0.056 | 0.006 |
| 12 | 6.03 a | 5.65 b | 5.72 b | 0.039 | 0.007 | |
| 24 | 6.95 a | 6.62 b | 6.57 b | 0.050 | 0.015 | |
| Protozoa | 6 | 6.04 | 6.35 | 6.13 | 0.314 | 0.782 |
| 12 | 6.05 | 6.19 | 6.21 | 0.252 | 0.933 | |
| 24 | 6.33 | 6.31 | 6.00 | 0.155 | 0.467 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suong, N.T.M.; Paengkoum, S.; Purba, R.A.P.; Paengkoum, P. Optimizing Anthocyanin-Rich Black Cane (Saccharum sinensis Robx.) Silage for Ruminants Using Molasses and Iron Sulphate: A Sustainable Alternative. Fermentation 2022, 8, 248. https://doi.org/10.3390/fermentation8060248
Suong NTM, Paengkoum S, Purba RAP, Paengkoum P. Optimizing Anthocyanin-Rich Black Cane (Saccharum sinensis Robx.) Silage for Ruminants Using Molasses and Iron Sulphate: A Sustainable Alternative. Fermentation. 2022; 8(6):248. https://doi.org/10.3390/fermentation8060248
Chicago/Turabian StyleSuong, Ngo Thi Minh, Siwaporn Paengkoum, Rayudika Aprilia Patindra Purba, and Pramote Paengkoum. 2022. "Optimizing Anthocyanin-Rich Black Cane (Saccharum sinensis Robx.) Silage for Ruminants Using Molasses and Iron Sulphate: A Sustainable Alternative" Fermentation 8, no. 6: 248. https://doi.org/10.3390/fermentation8060248









