Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Beers
2.2. Malt
2.3. Hops
2.4. Yeasts
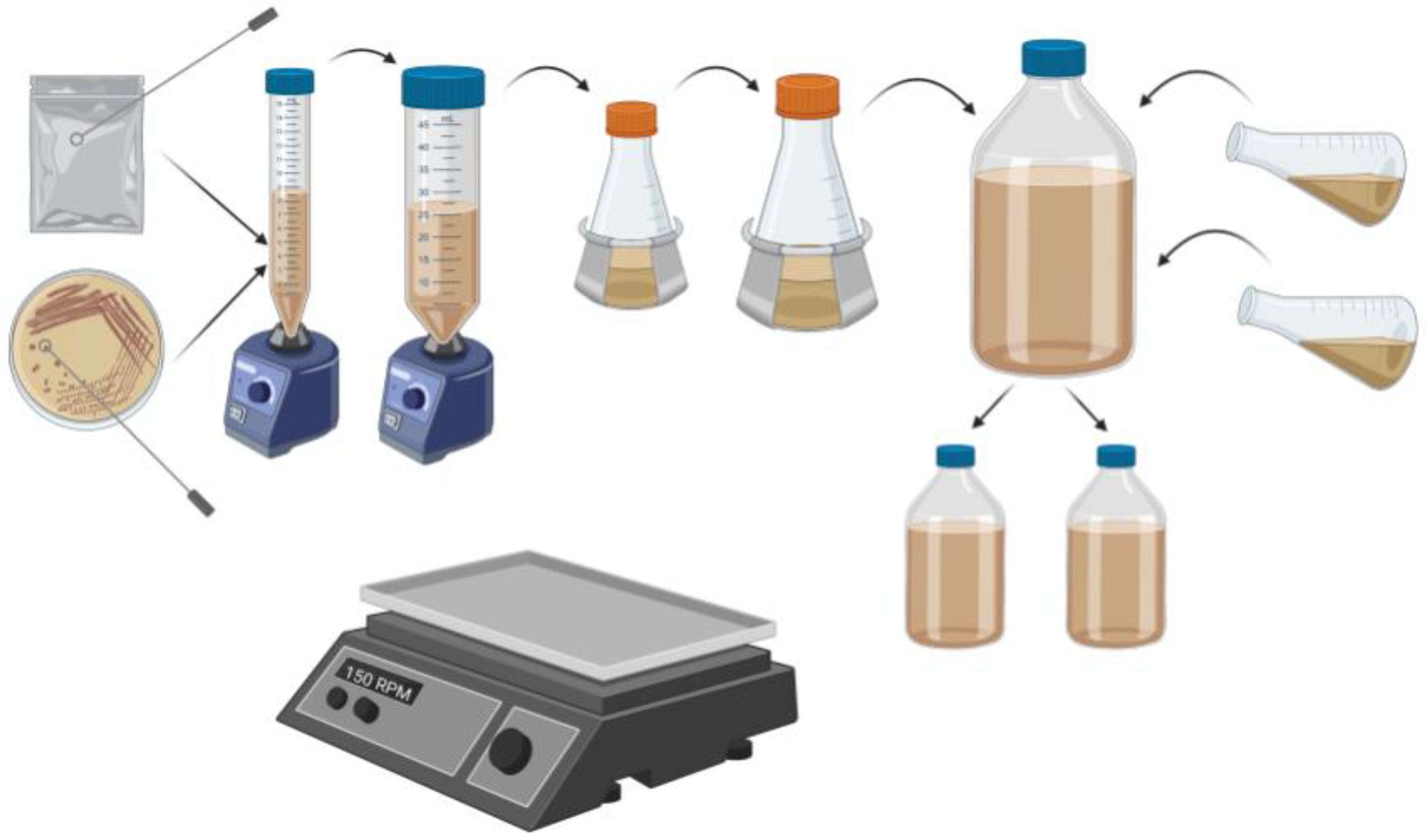
2.5. Yeast Propagation
2.6. Sample Collection and Preparation
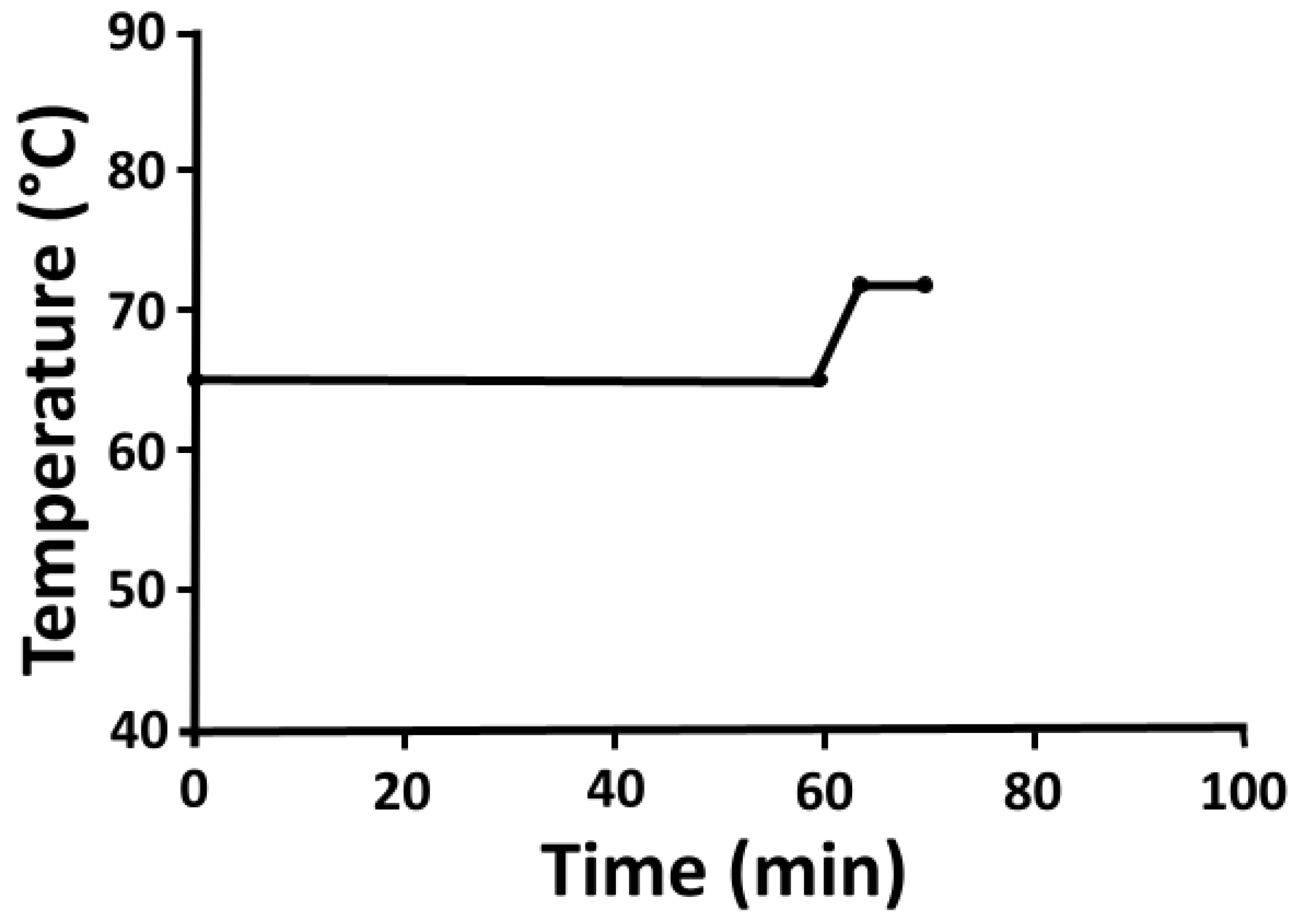
2.7. Pilot Fermentations
2.8. Bench-Top Fermentations
2.9. Analytical Measurements
2.10. Statistical Analysis
3. Results and Discussion
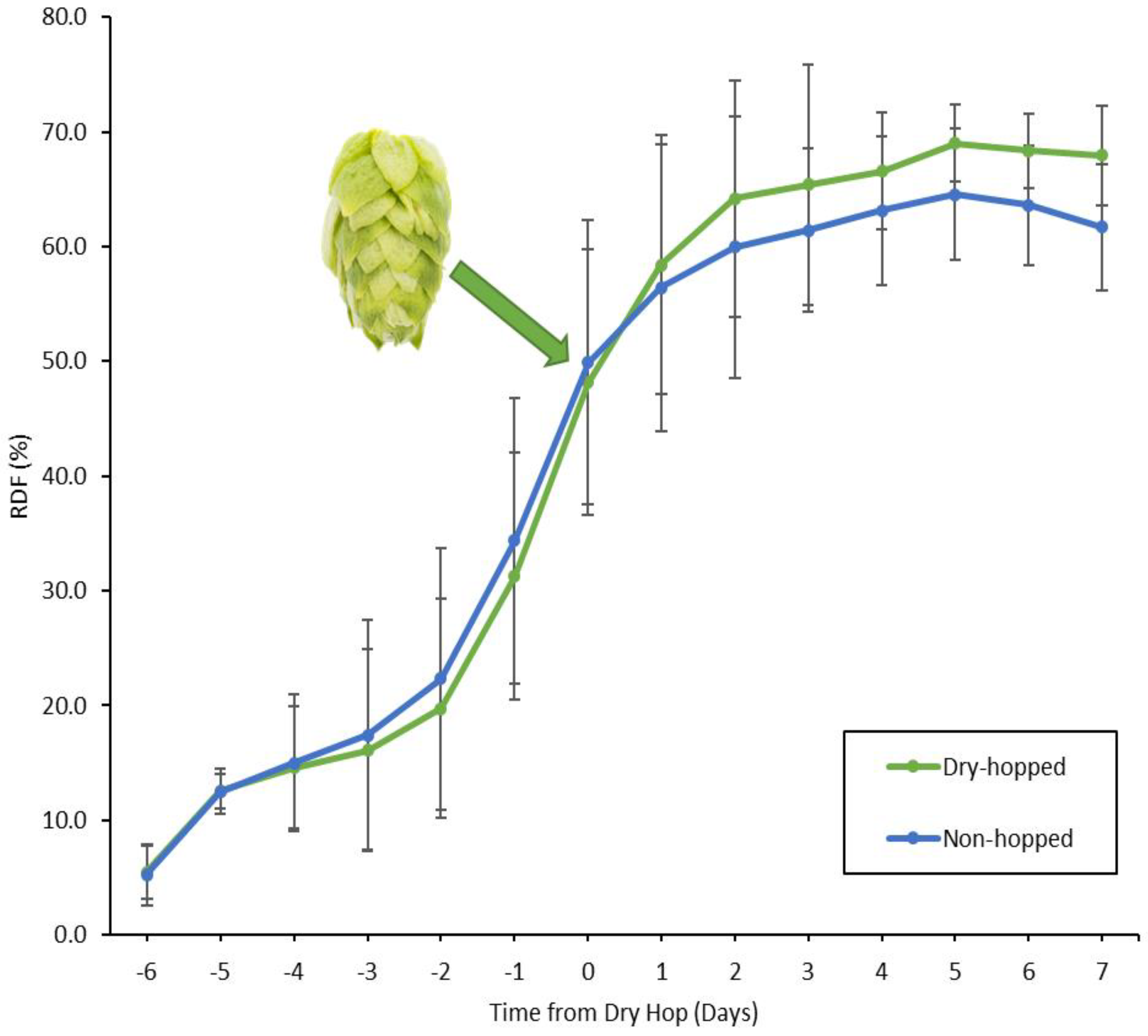
3.1. Pilot Fermentations
3.2. Bench-Top Fermentations
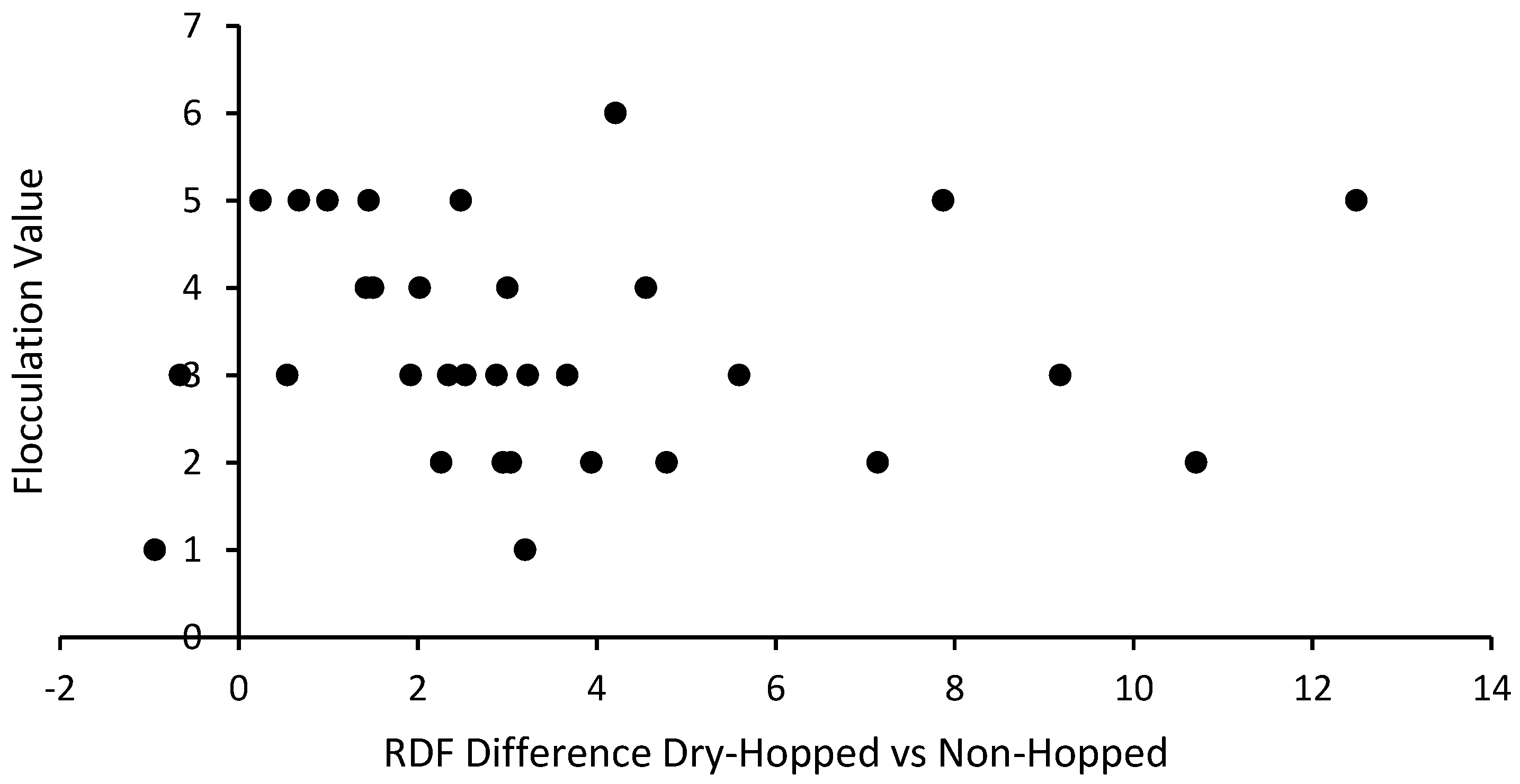
3.3. Flocculation and Attenuation
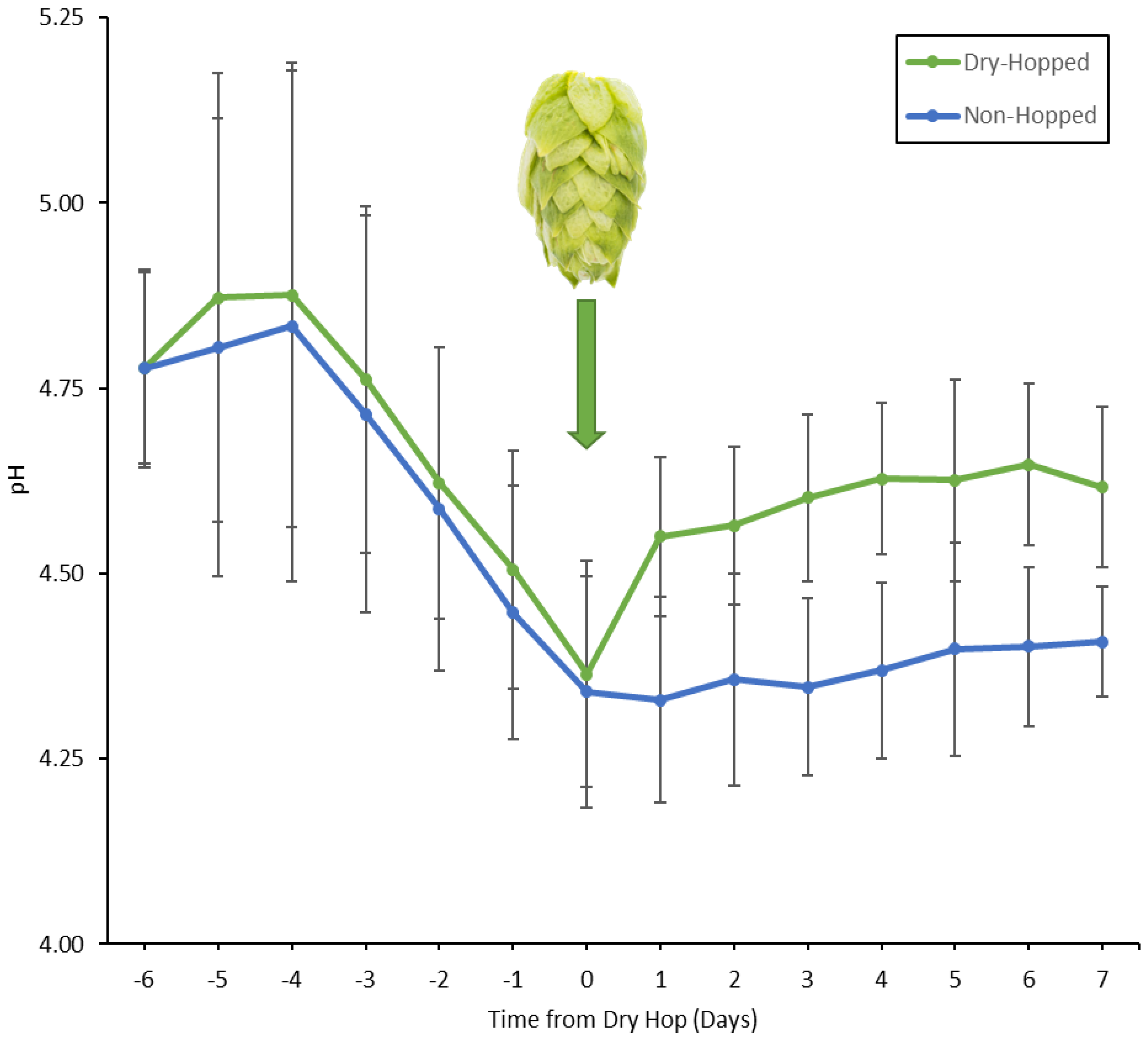
3.4. pH and Dry-Hopping
3.5. Biological Replicates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moritz, E.R.; Morris, G.H. A Text-Book of the Science of Brewing; Spon: London, UK, 1891. [Google Scholar]
- Schönberger, C.; Kostelecky, T. 125th Anniversary Review: The Role of Hops in Brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing & Malting; 6th Revised English; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei in Berlin (VLB): Berlin, Germany, 2019. [Google Scholar]
- Brown, H.; Morris, G. On Certain Functions of Hops Used in the Dry-Hopping of Beers. Trans. Inst. Brew 1893, 6, 94–106. [Google Scholar]
- How Hoppy Beer Production Has Redefined Hop Quality and a Discussion of Agricultural and Processing Strategies to Promote It. Tech. Q. 2019. [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Investigating the Factors Impacting Aroma, Flavor, and Stability in Dry-Hopped Beers. MBAA Tech. Q. 2019, 56, 13–23. [Google Scholar] [CrossRef]
- Hauser, D.G.; Van Simaeys, K.R.; Lafontaine, S.R.; Shellhammer, T.H. A Comparison of Single-Stage and Two-Stage Dry-Hopping Regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251–260. [Google Scholar] [CrossRef]
- Dykstra, J. The Beer Connoisseur; CafeMedia: New York, NY, USA, 2020; pp. 18–29. [Google Scholar]
- National Beer Sales & Production Data|Brewers Association. Available online: https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed on 11 January 2020).
- Bud Light Crisp. Available online: https://www.budlight.com/en/our-beers/crisp.html (accessed on 31 March 2021).
- Guinness® Nitro IPA|Guinness®. Available online: https://www.guinness.com/en/our-beers/guinness-nitro-ipa/ (accessed on 30 March 2021).
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Otter, G.E.; Taylor, L. Determination of the sugar composition of wort and beer by gas liquid chromatography. J. Inst. Brew. 1967, 73, 570–576. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001; p. 656. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principles of Malting and Brewing; American Society of Brewing Chemists, Ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2006; p. 246. [Google Scholar]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of Hop-Derived Monoterpene Alcohols by Lager Yeast and Their Contribution to the Flavor of Hopped Beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. [Google Scholar] [CrossRef] [PubMed]
- Olodokun, O.; Cowley, T.; James, S.; Smart, K.A. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Kirkendall, J.A.; Mitchell, C.A.; Chadwick, L.R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. [Google Scholar] [CrossRef]
- Janicki, J.; Kotasthane, W.V.; Parker, A.; Walker, T.K. The DIAStatic activity of hops, together with a note on maltase in hops. J. Inst. Brew. 1941, 47, 24–36. [Google Scholar] [CrossRef]
- U.S. Department of the Treasury. The Beverage Alcohol Manual (BAM): A Practical Guide; Basic Mandatory Labeling Information for Malt Bev-Erages; US Gov., Tax and Trade Bureau: Washington, DC, USA, 2007; Chapter 1; Volume 3, Section 5; p. 7.
- Otter, G.E.; Taylor, L. Estimation and occurrence of acetaldehyde in beer. J. Inst. Brew. 1971, 77, 467–472. [Google Scholar] [CrossRef]
- Wainwright, T. diacetyl-a review: Part i-analytical and biochemical considerations: Part ii-brewing experience. J. Inst. Brew. 1973, 79, 451–470. [Google Scholar] [CrossRef]
- Tian, J. Determination of several flavours in beer with headspace sampling–gas chromatography. Food Chem. 2010, 123, 1318–1321. [Google Scholar] [CrossRef]
- Technical Committee. Alcohol. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Cutaia, A.J.; Munroe, J.H. A Method for the Consistent Estimation of Real Degree of Fermentation. J. Am. Soc. Brew. Chem. 1979, 37, 188–189. [Google Scholar] [CrossRef]
- Huerta-Zurita, R.; Horsley, R.D.; Schwarz, P.B. Is the Apparent Degree of Fermentation a Reliable Estimator of Fermentability? J. Am. Soc. Brew. Chem. 2019, 77, 1–9. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Beauchamp, A.; Kravitz, M.; Vaughn, C.; Cilurzo, V. Hop Creep: What It Is and Approaches to Man-Aging It; Brewers’ Association: Boulder, CO, USA, 2020. [Google Scholar]
- Bruner, J.; Williams, J.; Fox, G. Further Exploration of Hop Creep Variability with Humulus lupulus Cultivars and Proposed Method for Determination of Secondary Fermentation. Tech. Q. 2020, 57, 57. [Google Scholar] [CrossRef]
- Stokholm, A.; Lindsey, N.R.; Shellhammer, T.H. Evaluating a benchtop fermentation method for estimating dextrin degra-dation by hop’ ‘ diastatic enzymes during dry-hopping. Brew. Sci. 2020, 73, 140–148. [Google Scholar]
- Gallagher, L.W.; Silberstein, R.; Prato, L.; Vogt, H. ‘Butta 12’, a two-rowed malting barley adapted to the California Central Valley with proven floor-malting success and craft brewer acceptance. J. Plant Regist. 2020, 14, 250–265. [Google Scholar] [CrossRef]
- Bruner, J.; Fox, G. Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations. Fermentation 2020, 6, 116. [Google Scholar] [CrossRef]
- Schisler, D.O. Comparison of Revised Yeast Counting Methods. J. Am. Soc. Brew. Chem. 1986, 44, 81–85. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeastSaccharomyces pastorianus. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Gibson, B.R.; Storgårds, E.; Krogerus, K.; Vidgren, V. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental speciesSaccharomyces eubayanus. Yeast 2013, 30, 255–266. [Google Scholar] [CrossRef]
- Yamashita, I.; Suzuki, K.; Fukui, S. Nucleotide sequence of the extracellular glucoamylase gene STA1 in the yeast Saccharomyces diastaticus. J. Bacteriol. 1985, 161, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Fukui, S.; Yabuuchi, S.; Aoyagi, S.; Tsumura, Y. Expression of theSaccharomyces Diastaticus STA1Gene in Brewing Yeasts. J. Am. Soc. Brew. Chem. 1989, 47, 87–91. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.L.; Chambers, P.J. Introducing a New Breed of Wine Yeast: Interspecific Hybridisation between a Commercial Saccharomyces cerevisiae Wine Yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef] [Green Version]
- Bellon, J.; Schmidt, S.; Solomon, M. Case study: Development of Saccharomyces cerevisiae × Saccharomyces mikatae wine yeast hybrids and their potential to deliver alternative wine styles. AWRI Tech. Rev. 2019, 241, 6–11. [Google Scholar]
- Technical Committee. Yeast Fermentable Extract. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Technical Committee. End fermentation (yeast fermentable extract). In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- D’Hautcourt, O.; Smart, K.A. Measurement of Brewing Yeast Flocculation. J. Am. Soc. Brew. Chem. 1999, 57, 123–128. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Yeast flocculation: What brewers should know. Appl. Microbiol. Biotechnol. 2003, 61, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. Yeast Flocculation—Sedimentation and Flotation. Ferment 2018, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Bendiak, D.S. Quantification of the Helm’s Flocculation Test. J. Am. Soc. Brew. Chem. 1994, 52, 120–122. [Google Scholar] [CrossRef]
- Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers. Tech. Q. 2016, 53, 23–27. [CrossRef]
- Maye, J.P.; Smith, R.; Leker, J. Dry Hopping and Its Effect on Beer Bitterness, the IBU Test, and pH. BrauW. Int. 2018, 2018, 25–29. [Google Scholar]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Prediction of ester production in industrial beer fermentation. Enzym. Microb. Technol. 1994, 16, 66–71. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Fusel Alcohols Production in Beer Fermentation Processes. Process. Biochem. 1994, 29, 303–309. [Google Scholar] [CrossRef]
| Yeast Name | Scientific Name | Comparable Strains | Origin | Flocculation | Attenuation |
|---|---|---|---|---|---|
| BY881 | S. cerevisiae | (R&D) ** | Oakland, CA, USA | Medium | 75–85% |
| SafAle™ BE-134 | S. cerevisiae var. diastaticus | WLP566 | Belgium | Low | 89–93% |
| SafAle™ BE-256 | S. cerevisiae | WLP530; WY3787 | Belgium | High | 82–86% |
| SafAle™ K-97 | S. cerevisiae | WLP029 | Koln, Germany | Medium High | 80–84% |
| SafAle™ S-33 | S. cerevisiae | WLP005 | England | Medium Low | 68–72% |
| SafAle™ T-58 | S. cerevisiae | WLP565 | Belgium | Medium Low | 72–78% |
| SafAle™ US-05 * | S. cerevisiae | WLP001; UCDFST 96-12 | USA | Medium | 78–82% |
| SafLager™ W 34/70 | S. pastorianus | WLP830 | Germany | High | 80–84% |
| SafŒno™ BC S103 | S. bayanus | ** | France | High | High |
| SafŒno™ CK S102 | S. cerevisiae | ** | France | High | High |
| SafŒno™ HD T18 | S. cerevisiae x S. bayanus | (R&D) ** | Marcq-en-Baroeul, France | Medium | High |
| SafSpirit™ USW-6 | S. cerevisiae | ** | USA | Medium Low | High |
| UCDFST 01-135 | S. bayanus | CBS 380; DVBPG 6171 | Turbid Beer—Italy | Medium | Moderate |
| UCDFST 01-157 | S. pastorianus | CBS 1538; DBVPG 6047 | Carlsberg—Denmark | Medium High | 72–78% |
| UCDFST 01-161 | S. paradoxus | CBS 432; DBVPG 6411 | Northeast Europe | Medium | Moderate |
| UCDFST 11-510 | S. mikatae | CBS 8839; NCYC 2888 | Soil—Japan | Medium | Moderate Low |
| UCDFST 11-512 | S. uvarum | CBS 395; DBVPG 6179 | Fruit—Scandinavia | High | Moderate |
| UCDFST 11-515 | S. kudriavzevii | CBS 8840, NCYC 2889 | Western Europe | Medium High | Moderate |
| UCDFST 21-101 | S. cerevisiae var. chevalieri | CBS 400; DBVPG 6174 | Ivory Coast | Medium Low | 15% |
| UCDFST 77-65 | Saccharomyces cerevisiae | WLP076 | Santa Rosa, CA, USA | Medium | 70–74% |
| UCDFST 96-12 | S. cerevisiae | WLP001; US-05 | Chico, CA, USA | Medium Low | 73–78% |
| WLP001 | S. cerevisiae | US-05; UCDFST 96-12 | Chico, CA, USA | Medium | 73–80% |
| WLP002 | S. cerevisiae | UCD 96-17; WY1968 | London, UK | Very High | 63–70% |
| WLP013 | S. cerevisiae | OYL003; UCDFST 96-11 | London, UK | Medium | 67–75% |
| WLP030 | S. cerevisiae | WY1275 | Trent, UK | High | 72–78% |
| WLP066 | S. cerevisiae | A38; OYL011 | London, UK | Medium Low | 75–82% |
| WLP090 | S. cerevisiae | OYL043 | San Diego, CA, USA | Medium High | 76–83% |
| WLP095 | S. cerevisiae | OYL052; GY054 | Burlington, VT, USA | Medium High | 75–80% |
| WLP351 | S. bayanus | UCDFST 02-124; OYL025 | Weiss—Germany | Low | 75–82% |
| WLP518 | S. cerevisiae | NCYC 4285 | Kveik—Norway | High | 70–80% |
| Yeast Name | Non-Hopped | Dry-Hopped | Difference | RDF (%) | |
|---|---|---|---|---|---|
| BY881 | 2.26 | ||||
| SafAle™ BE-134 | −0.94 | ||||
| SafAle™ BE-256 | 1.45 | ||||
| SafAle™ K-97 | 4.55 | ||||
| SafAle™ S-33 | 3.04 | ||||
| SafAle™ T-58 | 7.14 | 75% | |||
| SafAle™ US-05 * | 2.56 | ||||
| SafLager™ W-34/70 | 2.48 | ||||
| SafŒno™ BC S103 | 12.49 | ||||
| SafŒno™ CK S102 | 0.67 | ||||
| SafŒno™ HD T18 | 2.88 | ||||
| SafSpirit™ USW-6 | 2.95 | ||||
| UCDFST 01-135 | 3.67 | ||||
| UCDFST 01-157 | 2.02 | ||||
| UCDFST 01-161 | 0.54 | ||||
| UCDFST 11-510 | −0.66 | 65% | |||
| UCDFST 11-512 | 0.24 | ||||
| UCDFST 11-515 | 1.42 | ||||
| UCDFST 21-101 | 10.70 | ||||
| UCDFST 77-65 | 9.18 | ||||
| UCDFST 96-12 | 4.78 | ||||
| WLP001 | 2.34 | ||||
| WLP002 | 4.21 | ||||
| WLP013 | 5.59 | ||||
| WLP030 | 0.99 | ||||
| WLP066 | 3.94 | 55% | |||
| WLP090 | 1.50 | ||||
| WLP095 | 3.00 | ||||
| WLP351 | 3.20 | ||||
| WLP518 | 7.87 |
| Yeast Name | Non-Hopped | Dry-Hopped | Difference | RDF (%) | |
|---|---|---|---|---|---|
| BY881 | 20.68 | ||||
| SafAle™ BE-134 | −3.07 | ||||
| SafAle™ BE-256 | 3.25 | ||||
| SafAle™ K-97 | 20.71 | ||||
| SafAle™ S-33 | 5.40 | ||||
| SafAle™ T-58 | 5.16 | 75% | |||
| SafAle™ US-05 * | 3.50 | ||||
| SafLager™ W-34/70 | 2.90 | ||||
| SafŒno™ BC S103 | 10.41 | ||||
| SafŒno™ CK S102 | 4.94 | ||||
| SafŒno™ HD T18 | 1.83 | ||||
| SafSpirit™ USW-6 | 3.13 | ||||
| UCDFST 01-135 | 4.33 | ||||
| UCDFST 01-157 | −42.97 | ||||
| UCDFST 01-161 | −0.37 | ||||
| UCDFST 11-510 | 1.68 | 65% | |||
| UCDFST 11-512 | 31.85 | ||||
| UCDFST 11-515 | 38.13 | ||||
| UCDFST 21-101 | 1.49 | ||||
| UCDFST 77-65 | 11.23 | ||||
| UCDFST 96-12 | 43.33 | ||||
| WLP001 | 3.39 | ||||
| WLP002 | 2.69 | ||||
| WLP013 | 2.43 | ||||
| WLP030 | 1.78 | ||||
| WLP066 | 1.97 | 55% | |||
| WLP090 | 4.15 | ||||
| WLP095 | 0.37 | ||||
| WLP351 | 1.92 | ||||
| WLP518 | 9.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation 2021, 7, 66. https://doi.org/10.3390/fermentation7020066
Bruner J, Marcus A, Fox G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation. 2021; 7(2):66. https://doi.org/10.3390/fermentation7020066
Chicago/Turabian StyleBruner, James, Andrew Marcus, and Glen Fox. 2021. "Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains" Fermentation 7, no. 2: 66. https://doi.org/10.3390/fermentation7020066







