Optimization of the Enzymatic Synthesis of Pentyl Oleate with Lipase Immobilized onto Novel Structured Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Immobilization
2.3. Lipase Quantification
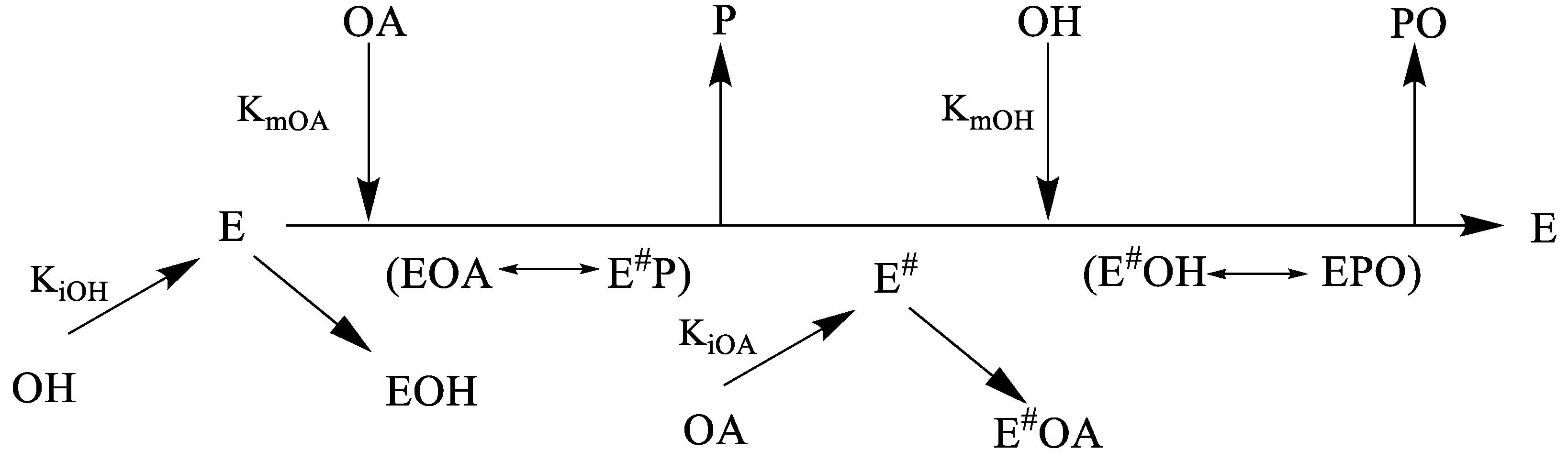
2.4. Biocatalyzed PO Synthesis
2.5. Experimental Factorial Design and Statistical Analysis
3. Results and Discussion
3.1. Reaction Parameters Effect on Pentyl Oleate Conversion
3.1.1. Temperature Effect
3.1.2. Initial Oleic Acid Concentration Effect
3.1.3. Substrate Molar Ratio Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michele, A.; Dibenedetto, A.; Dumeignill, F. Biorefinery: From Biomass to Chemicals and Fuels; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Cavani, F. Chemicals and Fuels from Bio-Based Building Blocks. Focus Catal. 2016, 2016, 7. [Google Scholar]
- Bouaid, A.; Acherki, H.; García, A.; Martinez, M.; Aracil, J. Enzymatic butanolysis of coconut oil. Biorefinery approach. Fuel 2017, 209, 141–149. [Google Scholar] [CrossRef]
- Zaidi, A.; Gainer, J.L.; Carta, G.; Mrani, A.; Kadiri, T.; Belarbi, Y.; Mir, A. Esterification of fatty acids using nylon-immobilized lipase in n-hexane: Kinetic parameters and chain-length effects. J. Biotechnol. 2002, 93, 209–216. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chabouni, M.; Gargouri, Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: A comparative study between n-hexane and solvent-free system. Enzym. Microb. Technol. 2004, 35, 355–363. [Google Scholar] [CrossRef]
- Lage, F.A.P.; Bassi, J.J.; Corradini, M.C.C.; Todero, L.M.; Luiz, J.H.H.; Mendes, A.A. Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzym. Microb. Technol. 2016, 84, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, N.; Abdul Rahman, M.B.; Basri, M.; Salleh, A.B.; Abd-Aziz, S. Lipase-catalyzed dimethyl adipate synthesis: Response surface modeling and kinetics. Biotechnol. J. 2010, 5, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Errazu, A.; Ferreira, M.L. Effect of several reaction parameters in the solvent-free ethyl oleate synthesis using Candida rugosa lipase immobilised on polypropylene. Biochem. Eng. J. 2005, 25, 69–77. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Screening of Lipases with Unusual High Activity in the sn-2 Esterification of 1,3-Dicaprin under Mild Operating Conditions. J. Agric. Food Chem. 2017, 65, 5010–5017. [Google Scholar] [CrossRef]
- Sun, S.; Hu, B. Enzymatic preparation of novel caffeoyl structured lipids using monoacylglycerols as caffeoyl acceptor and transesterification mechanism. Biochem. Eng. J. 2017, 124, 78–87. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of lipase on mesoporous silica nanoparticles with hierarchical fibrous pore. J. Mol. Catal. B Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Nyari, N.L.D.; Fernandes, I.A.; Bustamante-Vargas, C.E.; Steffens, C.; De Oliveira, D.; Zeni, J.; Rigo, E.; Dallago, R.M. In situ immobilization of Candida antarctica B lipase in polyurethane foam support. J. Mol. Catal. B Enzym. 2016, 124, 52–61. [Google Scholar] [CrossRef]
- Tufvesson, P.; Törnvall, U.; Carvalho, J.; Karlsson, A.J.; Hatti-Kaul, R. Towards a cost-effective immobilized lipase for the synthesis of specialty chemicals. J. Mol. Catal. B Enzym. 2011, 68, 200–205. [Google Scholar] [CrossRef]
- Santos, J.C.; De Castro, H.F. Optimization of lipase-catalysed synthesis of butyl butyrate using a factorial design. World J. Microbiol. Biotechnol. 2006, 22, 1007–1011. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar Garlapati, V.; Banerjee, R.; Nag, A. Enzymatic synthesis of fruit flavor esters by immobilized lipase from Rhizopus oligosporus optimized with response surface methodology. J. Mol. Catal. B Enzym. 2009, 60, 57–63. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag. 2016, 47, 98–104. [Google Scholar] [CrossRef]
- Cavallaro, V.; Ercoli, D.R.; Tonetto, G.M.; Ferreira, M.L. Simple and economical CALB/polyethylene/aluminum biocatalyst for fatty acid esterification. Polym. Adv. Technol. 2018, 19, 1002–1006. [Google Scholar] [CrossRef]
- Paiva, A.L.; Balcão, V.M.; Malcata, F.X. Kinetics and mechanisms of reactions catalyzed by immobilized lipases. Enzym. Microb. Technol. 2000, 27, 187–204. [Google Scholar] [CrossRef]
- Marangoni, A. Enzyme Kinetics: A Modern Approach; John Wiley & sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Serri, N.A.; Kamaruddin, A.H.; Long, W.S. Studies of reaction parameters on synthesis of Citronellyl laurate ester via immobilized Candida rugosa lipase in organic media. Bioprocess Biosyst. Eng. 2006, 29, 253–260. [Google Scholar] [CrossRef]
- Chowdary, G.V.; Prapulla, S.G. Kinetic study on lipase-catalyzed esterification in organic solvents. Indian J. Chem. 2005, 44, 2322–2327. [Google Scholar]
- Shieh, C.J.; Liao, H.F.; Lee, C.C. Optimization of lipase-catalyzed biodiesel by response surface methodology. Bioresour. Technol. 2003, 88, 103–106. [Google Scholar] [CrossRef]
- Shnyrov, V.L.; Martínez, L.D.; Roig, M.G.; Lyubarev, A.E.; Kurganov, B.I.; Villar, E. Irreversible thermal denaturation of lipase B from Candida rugosa. Thermochim. Acta 1999, 325, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Jumbri, K.; Al-Haniff Rozy, M.F.; Ashari, S.E.; Mohamad, R.; Basri, M.; Fard Masoumi, H.R. Optimisation and characterisation of lipase catalysed synthesis of a kojic monooleate ester in a solvent-free system by response surface methodology. PLoS ONE 2015, 10, e0144664. [Google Scholar] [CrossRef] [PubMed]
- Hari Krishna, S.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl butyrate: A kinetic study. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 2001, 1547, 262–267. [Google Scholar] [CrossRef]
- Segel, I.H. Enzyme Kinetics—Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; Wiley Interscience Publication: Hoboken, NJ, USA, 1993; ISBN 978-0-471-30309-1. [Google Scholar]
- Dumont, T.; Barth, D.; Corbier, C.; Branlant, G.; Perrut, M. Enzymatic reaction kinetic: Comparison in an organic solvent and in supercritical carbon dioxide. Biotechnol. Bioeng. 1992, 40, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Ching, C.B.; Lim, E.; Ang, C.H. Kinetic study of enantioselective esterification of ketoprofen with n-propanol catalysed by an lipase in an organic medium. Biotechnol. Lett. 1997, 19, 1051–1055. [Google Scholar] [CrossRef]
- Gogoi, S.; Hazarika, S.; Rao, P.G.; Dutta, N.N. Esterification of lauric acid with lauryl alcohol using cross-linked enzyme crystals: Solvent effect and kinetic study. Biocatal. Biotransform. 2006, 24, 343–351. [Google Scholar] [CrossRef]
- Shu, C.; Cai, J.; Huang, L.; Zhu, X.; Xu, Z. Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth. J. Mol. Catal. B Enzym. 2011, 72, 139–144. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Zhou, L.; Gao, J. Optimization and kinetic study of immobilized lipase-catalyzed synthesis of ethyl lactate. Biocatal. Biotransform. 2010, 28, 279–287. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhou, L.; Gao, J. Enzymatic esterification of ammonium lactate with ethanol in organic solvent: Kinetic study. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Lopresto, C.G.; Calabrò, V.; Woodley, J.M.; Tufvesson, P. Kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2014, 110, 64–71. [Google Scholar] [CrossRef]
- Meunier, S.M.; Rajabzadeh, A.R.; Legge, R.L. Kinetic modelling of the production of methyl oleate by Celite supported lipase sol-gels. Biochem. Eng. J. 2014, 85, 63–70. [Google Scholar] [CrossRef]
- Sengupta, A.; Dey, T.; Ghosh, M.; Ghosh, J.; Ghosh, S. Enzymatic Synthesis of Furfuryl Alcohol Ester with Oleic Acid by Candida antarctica Lipase B and Its Kinetic Study. J. Inst. Eng. Ser. E 2013, 93, 31–36. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Chatterji, A.; Rathod, V.K. Kinetics of Enzymatic Synthesis of Cinnamyl Butyrate by Immobilized Lipase. Appl. Biochem. Biotechnol. 2017, 193, 792–806. [Google Scholar] [CrossRef] [PubMed]





| Run Number | Experimental Factors | Response | ||
|---|---|---|---|---|
| T (°C) | iOA (mg) | MR | Converted OA (mg) | |
| 1 | 25 | 75 | 1 | 7.4 |
| 2 | 65 | 225 | 1 | 47.9 |
| 3 | 65 | 75 | 3 | 29.2 |
| 4 | 65 | 75 | 1 | 18.6 |
| 5 | 45 | 150 | 2 | 28.8 |
| 6 | 25 | 225 | 3 | 24.8 |
| 7 | 65 | 225 | 3 | 61.9 |
| 8 | 25 | 225 | 1 | 24.1 |
| 9 | 45 | 150 | 2 | 30.0 |
| 10 | 25 | 75 | 3 | 20.7 |
| 11 * | 25 | 150 | 3 | 30.0 |
| 12 * | 25 | 150 | 1 | 39.9 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: T | 812.045 | 1 | 812.045 | 74.43 | 0.0033 |
| B: iOA | 856.98 | 1 | 856.98 | 78.55 | 0.0030 |
| C: MR | 186.245 | 1 | 186.245 | 17.07 | 0.0257 |
| AB | 212.18 | 1 | 212.18 | 19.45 | 0.0216 |
| AC | 14.045 | 1 | 14.045 | 1.29 | 0.3390 |
| BC | 10.58 | 1 | 10.58 | 0.97 | 0.3973 |
| Total error | 32.729 | 3 | 10.9027 | ||
| Total (corr.) | 2124.8 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallaro, V.; Tonetto, G.; Ferreira, M.L. Optimization of the Enzymatic Synthesis of Pentyl Oleate with Lipase Immobilized onto Novel Structured Support. Fermentation 2019, 5, 48. https://doi.org/10.3390/fermentation5020048
Cavallaro V, Tonetto G, Ferreira ML. Optimization of the Enzymatic Synthesis of Pentyl Oleate with Lipase Immobilized onto Novel Structured Support. Fermentation. 2019; 5(2):48. https://doi.org/10.3390/fermentation5020048
Chicago/Turabian StyleCavallaro, Valeria, Gabriela Tonetto, and María Luján Ferreira. 2019. "Optimization of the Enzymatic Synthesis of Pentyl Oleate with Lipase Immobilized onto Novel Structured Support" Fermentation 5, no. 2: 48. https://doi.org/10.3390/fermentation5020048
APA StyleCavallaro, V., Tonetto, G., & Ferreira, M. L. (2019). Optimization of the Enzymatic Synthesis of Pentyl Oleate with Lipase Immobilized onto Novel Structured Support. Fermentation, 5(2), 48. https://doi.org/10.3390/fermentation5020048




