Combined Use of Biochar and Microbial Agents Can Promote Lignocellulosic Degradation Microbial Community Optimization during Composting of Submerged Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Composting Design and Sampling
2.3. Measurements of Physical and Chemical Properties
2.4. Spectroscopic Metrics Characterization
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Statistical Analysis
3. Results and Discussion
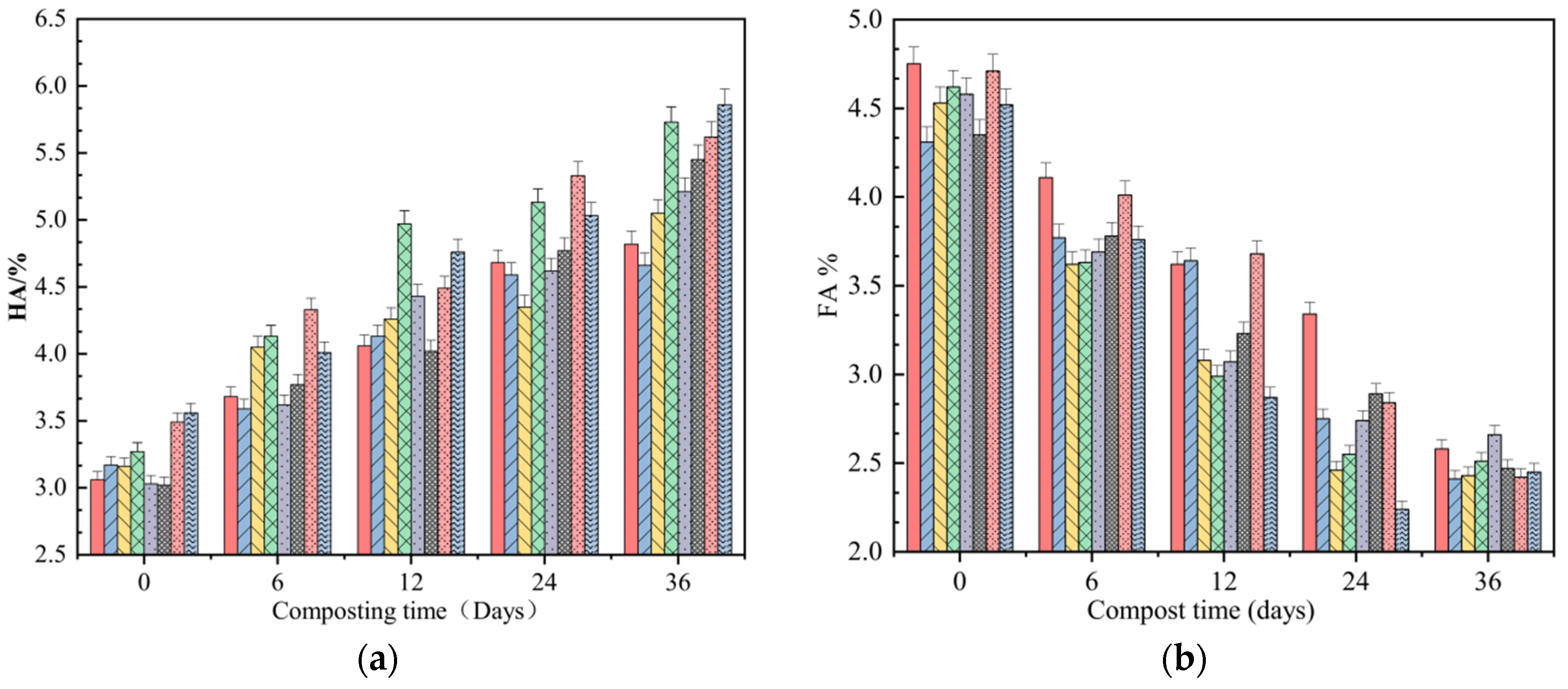
3.1. Composting Properties
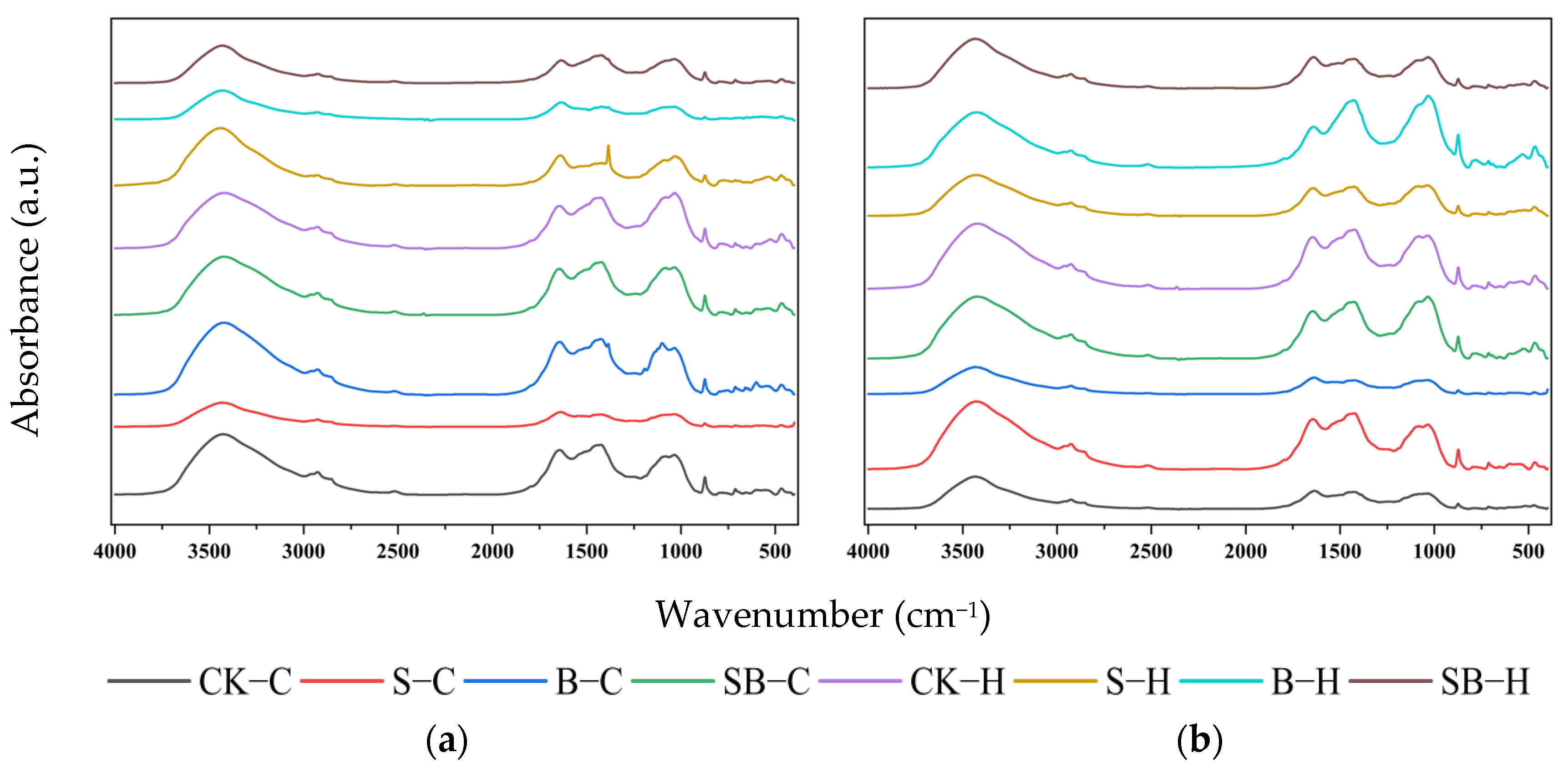
3.2. Infrared and SEM Characterization
3.3. Changes in Composition and Abundance of Microbial Community during Composting
3.3.1. Microbial Community Richness and Diversity
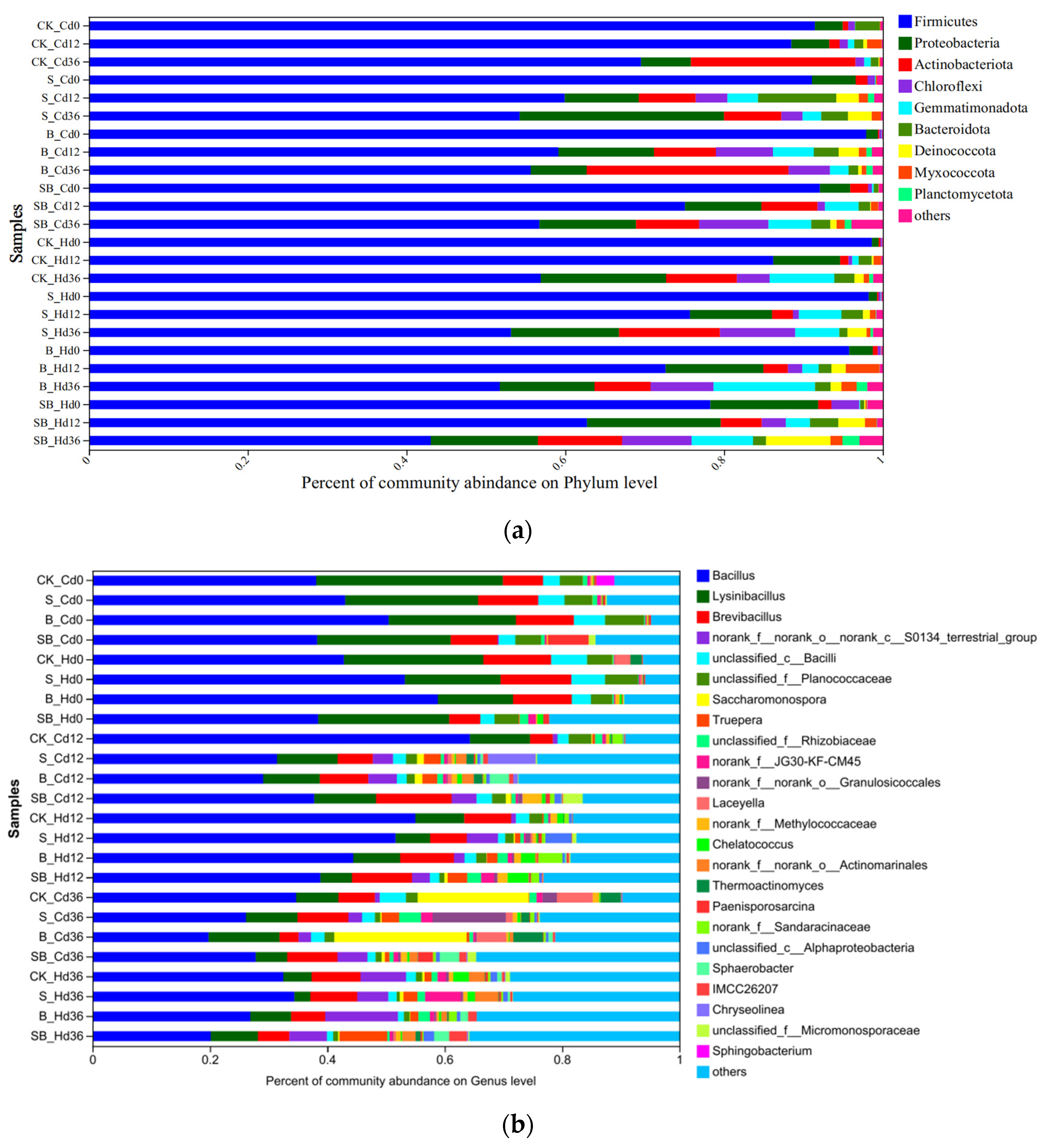
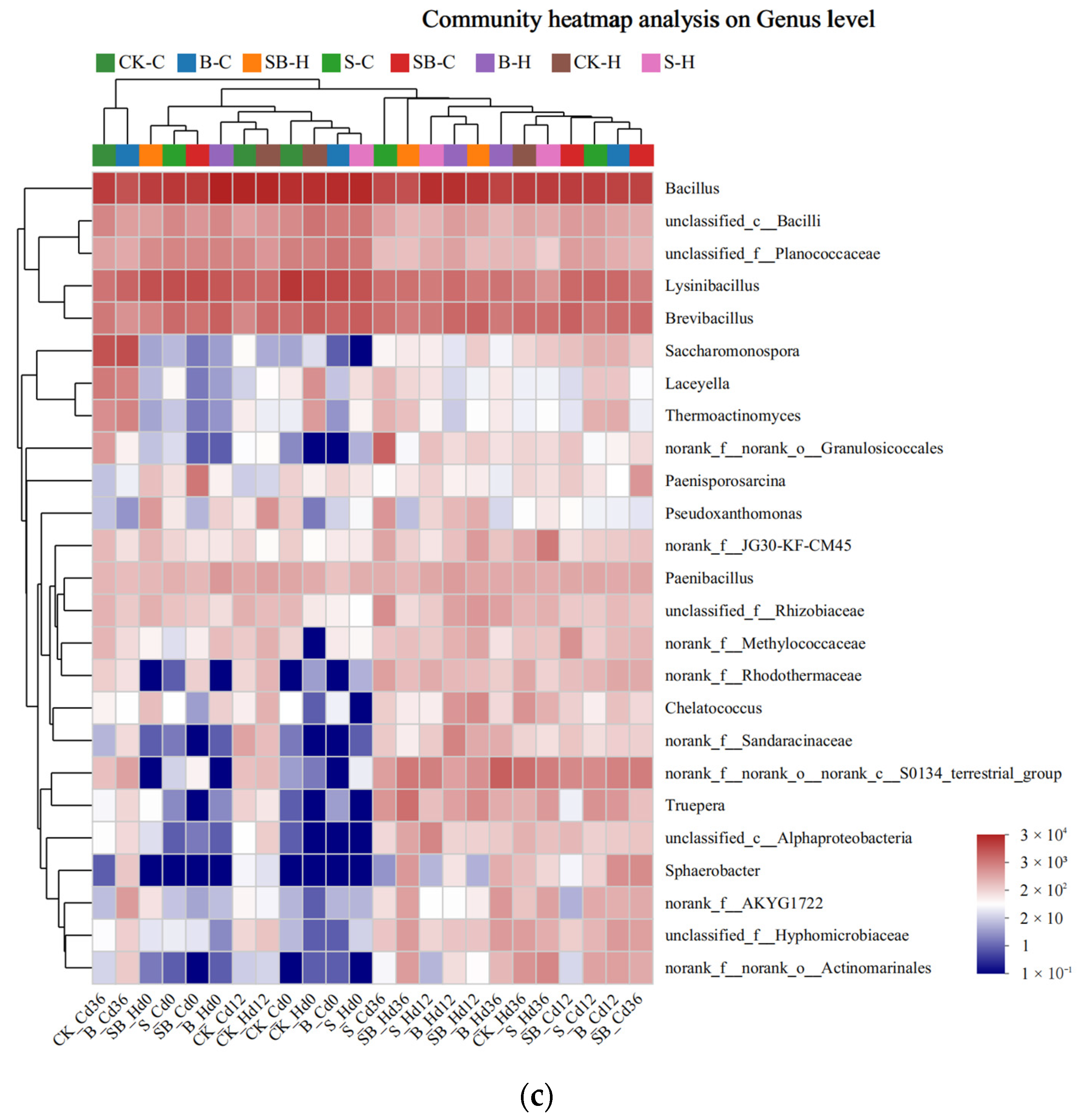
3.3.2. Analysis of the Microbial Community Composition
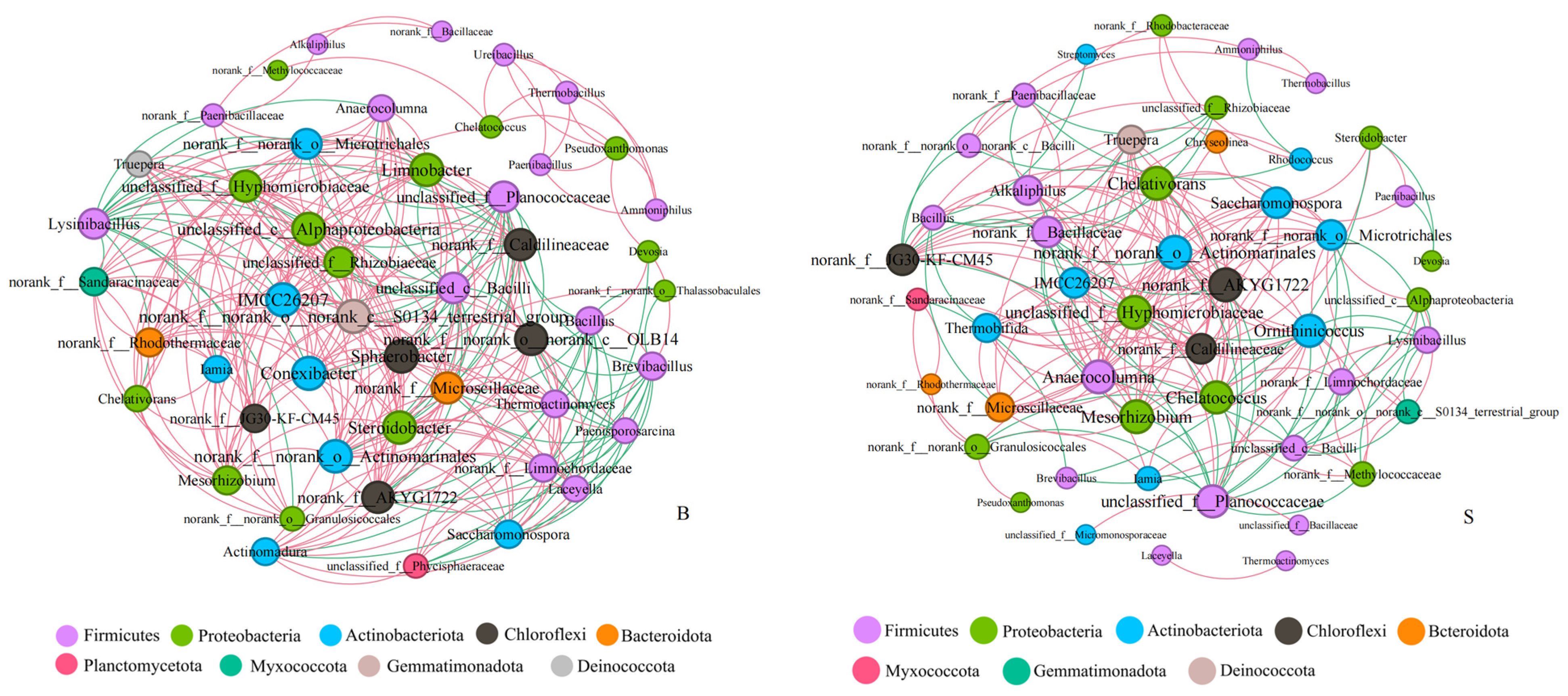
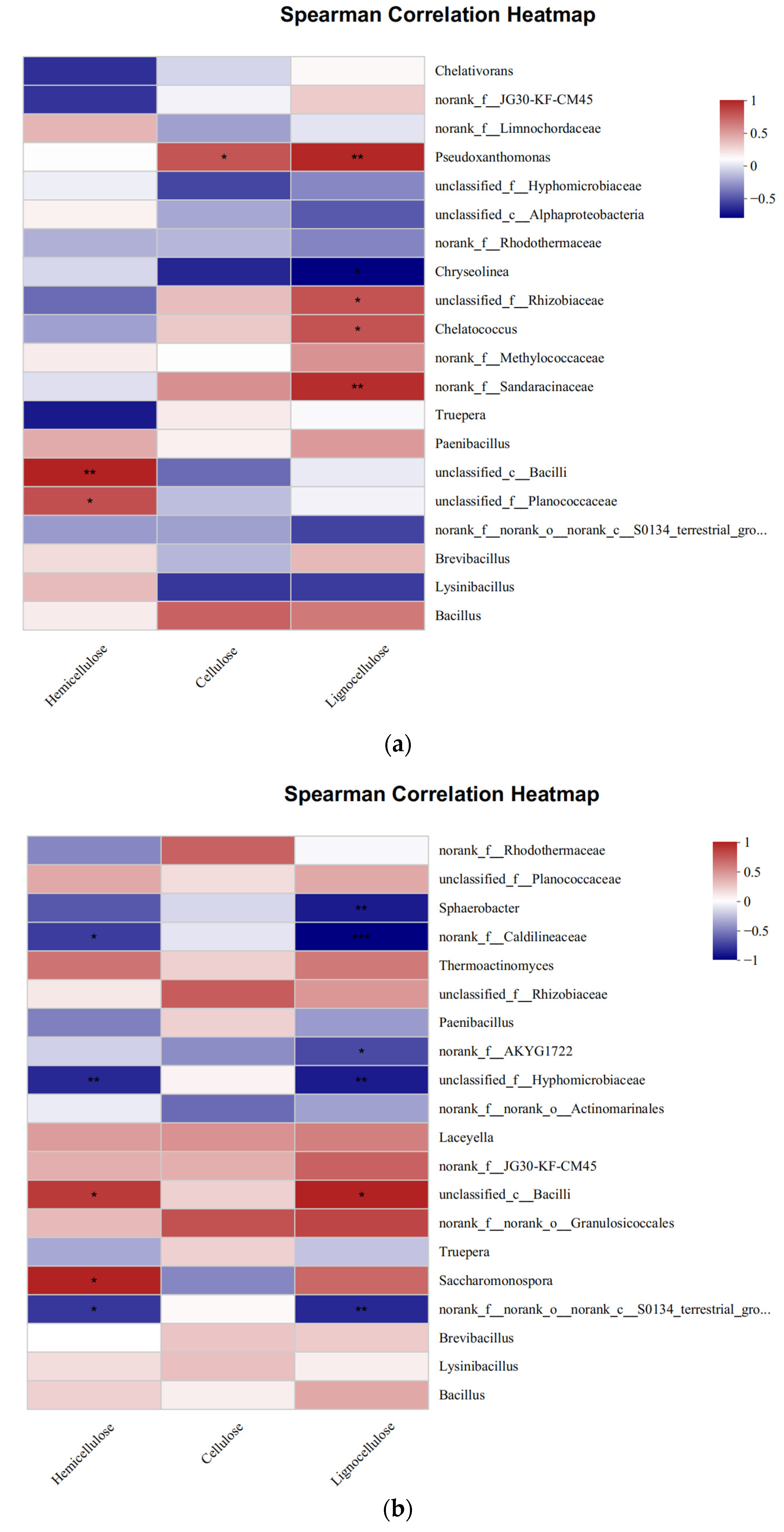
3.3.3. Relationship between the Bacteria Community and Environmental Factors
3.3.4. Analysis of the Factors That Affected Microbial Communities and Lignocellulose Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leloup, J.; Loy, A.; Knab, N.J.; Borowski, C.; Wagner, M.; Jørgensen, B.B. Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ. Microbiol. 2007, 9, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, G.; Fang, H.; Xu, S.; Bai, S. Effects of dissolved oxygen on the decomposers and decomposition of plant litter in lake ecosystem. J. Clean Prod. 2022, 372, 133837. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Gao, Q.; Li, D.; Chen, Z.; Wei, Z.; Zhang, Y.; Wang, F. Effect of Microbial Inoculation on Carbon Preservation during Goat Manure Aerobic Composting. Molecules 2021, 26, 4441. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Q.; Li, J.L.; Zhou, Q.; Liu, G.F.; Wang, T. Harmless disposal and resource utilization of wastes from the lake in China: Dewatering, composting and safety evaluation of fertilizer. Algal Res. 2019, 43, 101623. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Li, M.; Zheng, Y.; Ge, C.; Gu, J.; Li, H.; Duan, M.; Wang, X.; Chen, R. Research progress and prospects for using biochar to mitigate greenhouse gas emissions during composting: A review. Sci. Total Environ. 2021, 798, 149294. [Google Scholar] [CrossRef] [PubMed]
- Karnchanawong, S.; Mongkontep, T.; Praphunsri, K. Effect of green waste pretreatment by sodium hydroxide and biomass fly ash on composting process. J. Clean Prod. 2017, 146, 14–19. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Sun, Z.; Wang, S.; Shen, C.; Tang, Y.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Z.; Ma, T.; Zhang, X.; Wang, R.; Liu, Y.; Sun, B.; Xu, T.; Ding, G.; Wei, Y.; et al. Phosphorus excess changes rock phosphate solubilization level and bacterial community mediating phosphorus fractions mobilization during composting. Bioresour. Technol. 2021, 337, 125433. [Google Scholar] [CrossRef]
- Aycan DÜMENCİ, N.; CAGCAG YOLCU, O.; AYDIN TEMEL, F.; TURAN, N.G. Identifying the maturity of co-compost of olive mill waste and natural mineral materials: Modelling via ANN and multi-objective optimization. Bioresour. Technol. 2021, 338, 125516. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, C.; Zhang, L.; Ma, Y.; Li, G.; Nghiem, L.D.; Luo, W. Regulating bacterial dynamics by lime addition to enhance kitchen waste composting. Bioresour. Technol. 2021, 341, 125749. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, Y.; Wang, X.; Wei, Z.; Zhang, X.; Wu, J.; Xie, X.; Kang, K.; Yang, H.; Shi, M.; et al. Manganese dioxide driven the carbon and nitrogen transformation by activating the complementary effects of core bacteria in composting. Bioresour. Technol. 2021, 330, 124960. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Liu, H.; Song, C.; Wei, Z.; Chen, X.; Kang, K.; Yang, H. The action difference of metabolic regulators on carbon conversion during different agricultural organic wastes composting. Bioresour. Technol. 2021, 329, 124902. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Zhang, X.; Misselbrook, T.H.; Bai, Z.; Wang, H.; Ma, L. The effects of electric field assisted composting on ammonia and nitrous oxide emissions varied with different electrolytes. Bioresour. Technol. 2022, 344, 126194. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ji, K.; Su, L.; Wu, M.; Zhou, X.; Duan, E. Effects of FeSO4 dosage on nitrogen loss and humification during the composting of cow dung and corn straw. Bioresour. Technol. 2021, 341, 125867. [Google Scholar] [CrossRef]
- Valette, N.; Legout, A.; Goodell, B.; Alfredsen, G.; Auer, L.; Gelhaye, E.; Derrien, D. Impact of Norway spruce pre-degradation stages induced by Gloeophyllum trabeum on fungal and bacterial communities. Fungal Ecol. 2023, 61, 101188. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zhang, Z.; Wei, Y.; Wang, H.; Lu, Q.; Li, Y.; Wei, Z. Effect of thermo-tolerant actinomycetes inoculation on cellulose degradation and the formation of humic substances during composting. Waste Manag. 2017, 68, 64–73. [Google Scholar] [CrossRef]
- Loakasikarn, T.; Kubota, Y.; Koyama, M.; Nakasaki, K. Effect of seeding materials on organic matter degradation and microbial community succession during model organic waste composting. Biocatal. Agric. Biotechnol. 2021, 37, 102182. [Google Scholar] [CrossRef]
- Ballardo, C.; Barrena, R.; Artola, A.; Sánchez, A. A novel strategy for producing compost with enhanced biopesticide properties through solid-state fermentation of biowaste and inoculation with Bacillus thuringiensis. Waste Manag. 2017, 70, 53–58. [Google Scholar] [CrossRef]
- Greff, B.; Szigeti, J.; Nagy, Á.; Lakatos, E.; Varga, L. Influence of microbial inoculants on co-composting of lignocellulosic crop residues with farm animal manure: A review. J. Environ. Manag. 2022, 302, 114088. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuge, C.; Weng, Q.; Hu, B. Additional strains acting as key microbes promoted composting process. Chemosphere 2022, 287, 132304. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.; Zhang, H.; Mohamed, T.A.; Wei, Z. The key bacteria as the “Activator” promotes the rapid degradation of organic compounds during the start-up of low-temperature compost. Bioresour. Technol. 2021, 330, 124950. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Awasthi, M.K.; Bao, H.; Bie, J.; Lei, S.; Lv, J. Exploring the microbial mechanisms of organic matter transformation during pig manure composting amended with bean dregs and biochar. Bioresour. Technol. 2020, 313, 123647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.; Yao, W.; Han, C.; Sun, S.; Zhao, C. C, N, P, K stoichiometric characteristics of the “leaf-root-litter-soil” system in dryland plantations. Ecol. Indic. 2022, 143, 109371. [Google Scholar] [CrossRef]
- Ueda, M.U.; Kawabe, M.; Nakashizuka, T.; Kurokawa, H. Initial leaf litter traits affect soil microbial CO2 production: A laboratory experiment using the leaf litter of 41 temperate deciduous tree species. Appl. Soil Ecol. 2022, 180, 104605. [Google Scholar] [CrossRef]
- Carrasco-Barea, L.; Llorens, L.; Romaní, A.M.; Gispert, M.; Verdaguer, D. Litter decomposition of three halophytes in a Mediterranean salt marsh: Relevance of litter quality, microbial activity and microhabitat. Sci. Total Environ. 2022, 838, 155743. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1971–2010; Water-soluble-fertilizers-Determination of humic-acids content. Ministry of Agriculture and Rural Affairs of People’s Republic of China: Beijing, China, 2010.
- Chang, H.; Chen, C. Composting of Biosolids Enhanced by a Combined Pretreatment with Hydrogen Peroxide and Triton X-100. Waste Biomass Valorization 2015, 6, 45–51. [Google Scholar] [CrossRef]
- Shao, Y.; Bao, M.; Huo, W.; Ye, R.; Liu, Y.; Lu, W. Production of artificial humic acid from biomass residues by a non-catalytic hydrothermal process. J. Clean Prod. 2022, 335, 130302. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef]
- Xiao, X.; Xi, B.; He, X.; Zhang, H.; Li, D.; Zhao, X.; Zhang, X. Hydrophobicity-dependent electron transfer capacities of dissolved organic matter derived from chicken manure compost. Chemosphere 2019, 222, 757–765. [Google Scholar] [CrossRef]
- Moharana, P.C.; Biswas, D.R. Assessment of maturity indices of rock phosphate enriched composts using variable crop residues. Bioresour. Technol. 2016, 222, 1–13. [Google Scholar] [CrossRef]
- Ma, C.; Lo, P.K.; Xu, J.; Li, M.; Jiang, Z.; Li, G.; Zhu, Q.; Li, X.; Leong, S.Y.; Li, Q. Molecular mechanisms underlying lignocellulose degradation and antibiotic resistance genes removal revealed via metagenomics analysis during different agricultural wastes composting. Bioresour. Technol. 2020, 314, 123731. [Google Scholar] [CrossRef]
- Straathof, A.L.; Comans, R.N.J. Input materials and processing conditions control compost dissolved organic carbon quality. Bioresour. Technol. 2015, 179, 619–623. [Google Scholar] [CrossRef]
- Mujtaba, G.; Hayat, R.; Hussain, Q.; Ahmed, M. Physio-Chemical Characterization of Biochar, Compost and Co-Composted Biochar Derived from Green Waste. Sustainability 2021, 13, 4628. [Google Scholar] [CrossRef]
- Huang, F.; Yu, Y.; Huang, H. Temperature influence and distribution of bio-oil from pyrolysis of granular sewage sludge. J. Anal. Appl. Pyrolysis 2018, 130, 36–42. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.; Rene, E.R.; Wang, Z.; Zhou, L.; Zhang, Z.; Wang, Q. The degradation performance of different microplastics and their effect on microbial community during composting process. Bioresour. Technol. 2021, 332, 125133. [Google Scholar] [CrossRef]
- Huhe; Jiang, C.; Wu, Y.; Cheng, Y. Bacterial and fungal communities and contribution of physicochemical factors during cattle farm waste composting. Microbiologyopen 2017, 6, e00518. [Google Scholar] [CrossRef]
- Hou, T.; Zhou, Y.; Cao, X.; Li, W.; Zhang, S.; Zhao, Y.; Chen, L.; An, Q.; Meng, L. Effects of microbial inoculum on microbial community and enzyme activity involved in nitrogen-sulfur metabolism during sewage sludge composting. Sci. Total Environ. 2023, 858, 159954. [Google Scholar] [CrossRef]
- Song, B.; Almatrafi, E.; Sang, F.; Wang, W.; Zhang, C.; Shen, M.; Zhou, C.; Tang, X.; Zeng, G.; Gong, J. Managing Fenton-treated sediment with biochar and sheep manure compost: Effects on the evolutionary characteristics of bacterial community. J. Environ. Manag. 2022, 316, 115218. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, K.; Li, F.; Peng, H.; Lu, Y.; Zhang, L.; Pan, J.; Jiang, S.; Chen, A.; Yan, B.; et al. Characteristics of carbon, nitrogen, phosphorus and sulfur cycling genes, microbial community metabolism and key influencing factors during composting process supplemented with biochar and biogas residue. Bioresour. Technol. 2022, 366, 128224. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, P.; Li, Y.; Chen, L.; Jiang, H.; Liu, Y.; Luo, X. Effect of attapulgite on heavy metals passivation and microbial community during co-composting of river sediment with agricultural wastes. Chemosphere 2022, 299, 134347. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Liu, T.; Zhang, G.; Wu, S.; Xu, K.; Zhang, Y.; Wang, Q.; Kang, J.; Zhang, Z.; et al. Effect of inoculation with newly isolated thermotolerant ammonia-oxidizing bacteria on nitrogen conversion and microbial community during cattle manure composting. J. Environ. Manag. 2022, 317, 115474. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Chen, L.; Meng, L.; Zhang, S. Impacts of adding thermotolerant nitrifying bacteria on nitrogenous gas emissions and bacterial community structure during sewage sludge composting. Bioresour. Technol. 2023, 368, 128359. [Google Scholar] [CrossRef]
- Wang, C.; Wu, M.; Peng, C.; Yan, F.; Jia, Y.; Li, X.; Li, M.; Wu, B.; Xu, H.; Qiu, Z. Bacterial dynamics and functions driven by a novel microbial agent to promote kitchen waste composting and reduce environmental burden. J. Clean Prod. 2022, 337, 130491. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Wu, S.; He, Q.; Ling, Z.; Liu, T.; Wang, Q.; Zhang, Z.; Quan, F. Cattle manure compost humification process by inoculation ammonia-oxidizing bacteria. Bioresour. Technol. 2022, 344, 126314. [Google Scholar] [CrossRef]
- Tashiro, Y.; Kanda, K.; Asakura, Y.; Kii, T.; Cheng, H.; Poudel, P.; Okugawa, Y.; Tashiro, K.; Sakai, K. A Unique Autothermal Thermophilic Aerobic Digestion Process Showing a Dynamic Transition of Physicochemical and Bacterial Characteristics from the Mesophilic to the Thermophilic Phase. Appl. Environ. Microbiol. 2018, 84, e02537-17. [Google Scholar] [CrossRef]
- Liang, J.; Jin, Y.; Wen, X.; Mi, J.; Wu, Y. Adding a complex microbial agent twice to the composting of laying-hen manure promoted doxycycline degradation with a low risk on spreading tetracycline resistance genes. Environ. Pollut. 2020, 265, 114202. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Guo, X.; Wu, S. Probing changes in humus chemical characteristics in response to biochar addition and varying bulking agents during composting: A holistic multi-evidence-based approach. J. Environ. Manag. 2021, 300, 113736. [Google Scholar] [CrossRef]
- Yao, W.; Cai, D.; Huang, F.; Mohamed, T.A.; Li, P.; Qiao, X.; Wu, J. Promoting lignin exploitability in compost: A cooperative microbial depolymerization mechanism. Process Saf. Environ. Protect. 2023, 174, 856–868. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, F.; Sun, B.; Tian, Y.; Zhang, J.; Duan, J.; Bi, W.; Qin, J.; Xu, S. Effect of biochars with different particle sizes on fates of antibiotics and antibiotic resistance genes during composting of swine manure. Bioresour. Technol. 2023, 370, 128542. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Q.; Zhu, Q.; Niu, Q.; Yan, H.; Li, K.; Li, G.; Li, X.; Liu, H.; Liu, Y.; et al. Efficient decomposition of lignocellulose and improved composting performances driven by thermally activated persulfate based on metagenomics analysis. Sci. Total Environ. 2021, 794, 148530. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Sun, Z.; Shuai, W.; Xia, Z.; Xie, C.; Gou, M.; Tang, Y. Evaluation of physicochemical properties, bacterial community, and product fertility during rice straw composting supplemented with different nitrogen-rich wastes. Bioresour. Technol. 2023, 369, 128462. [Google Scholar] [CrossRef]
- Xu, S.; Lu, W.; Liu, Y.; Ming, Z.; Liu, Y.; Meng, R.; Wang, H. Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag. 2017, 63, 41–48. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Zhang, Z.; Wang, Q.; Shen, F.; Li, R.; Li, D.S.; Ren, X.; Wang, M.; Chen, H.; Zhao, J. New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresour. Technol. 2017, 238, 589–601. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Li, Y.; Zhong, C.; Tian, C. Effects of exogenous cellulose-degrading bacteria on humus formation and bacterial community stability during composting. Bioresour. Technol. 2022, 359, 127458. [Google Scholar] [CrossRef]
- Bello, A.; Han, Y.; Zhu, H.; Deng, L.; Yang, W.; Meng, Q.; Sun, Y.; Egbeagu, U.U.; Sheng, S.; Wu, X.; et al. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 2020, 721, 137759. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; Wang, B.; He, Q.; Wan, D. Using the modified pine wood as a novel recyclable bulking agent for sewage sludge composting: Effect on nitrogen conversion and microbial community structures. Bioresour. Technol. 2020, 309, 123357. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Huang, Y.; Lin, Q.; Huang, L.; Cheng, S.; Sun, S.; Zhu, Z. Addition of bacterial consortium produced high-quality sugarcane bagasse compost as an environmental-friendly fertilizer: Optimizing arecanut (Areca catechu L.) production, soil fertility and microbial community structure. Appl. Soil Ecol. 2023, 188, 104920. [Google Scholar] [CrossRef]
- Alfonzo, A.; Laudicina, V.A.; Muscarella, S.M.; Badalucco, L.; Moschetti, G.; Spanò, G.M.; Francesca, N. Cellulolytic bacteria joined with deproteinized whey decrease carbon to nitrogen ratio and improve stability of compost from wine production chain by-products. J. Environ. Manag. 2022, 304, 114194. [Google Scholar] [CrossRef]
- Huang, X.; He, Y.; Zhang, Y.; Lu, X.; Xie, L. Independent and combined effects of biochar and microbial agents on physicochemical parameters and microbial community succession during food waste composting. Bioresour. Technol. 2022, 366, 128023. [Google Scholar] [CrossRef]
- Meng, L.; Xu, C.; Wu, F. Microbial co-occurrence networks driven by low-abundance microbial taxa during composting dominate lignocellulose degradation. Sci. Total Environ. 2022, 845, 157197. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Xi, B.; Zhao, X.; He, X.; Huang, C.; Tan, W.; Gao, R.; Zhang, H.; Li, D. Successions and diversity of humic-reducing microorganisms and their association with physical-chemical parameters during composting. Bioresour. Technol. 2016, 219, 204–211. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L. Combined addition of biochar, lactic acid, and pond sediment improves green waste composting. Sci. Total Environ. 2022, 852, 158326. [Google Scholar] [CrossRef]
- Jia, P.; Wang, X.; Liu, S.; Hua, Y.; Zhou, S.; Jiang, Z. Combined use of biochar and microbial agent can promote lignocellulose degradation and humic acid formation during sewage sludge-reed straw composting. Bioresour. Technol. 2023, 370, 128525. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Wang, J.; Yu, J.; Hu, T.; Dai, X.; Xie, J.; Zhao, W. Microbial succession and molecular ecological networks response to the addition of superphosphate and phosphogypsum during swine manure composting. J. Environ. Manag. 2021, 279, 111560. [Google Scholar] [CrossRef]
- Jin, X.; Ai, W.; Zhang, Y.; Dong, W. Application of functional microbial agent in aerobic composting of wheat straw for waste recycling. Life Sci. Space Res. 2022, 33, 13–20. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Y.; Shan, G.; He, X.; Huang, J.; Li, Q. Inoculation with Compost-Born Thermophilic Complex Microbial Consortium Induced Organic Matters Degradation While Reduced Nitrogen Loss During Co-Composting of Dairy Manure and Sugarcane Leaves. Waste Biomass Valorization 2019, 10, 2467–2477. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Zhao, Y.; Zhang, S.; Meng, L. Evaluation of bacterial agent/nitrate coupling on enhancing sulfur conversion and bacterial community succession during aerobic composting. Bioresour. Technol. 2022, 362, 127848. [Google Scholar] [CrossRef]
- Li, F.; Ghanizadeh, H.; Cui, G.; Liu, J.; Miao, S.; Liu, C.; Song, W.; Chen, X.; Cheng, M.; Wang, P.; et al. Microbiome—Based agents can optimize composting of agricultural wastes by modifying microbial communities. Bioresour. Technol. 2023, 374, 128765. [Google Scholar] [CrossRef]
- Yu, J.; Gu, J.; Wang, X.; Lei, L.; Guo, H.; Song, Z.; Sun, W. Exploring the mechanism associated with methane emissions during composting: Inoculation with lignocellulose-degrading microorganisms. J. Environ. Manag. 2023, 325, 116421. [Google Scholar] [CrossRef]
- Jiang, X.; Deng, L.; Meng, Q.; Sun, Y.; Han, Y.; Wu, X.; Sheng, S.; Zhu, H.; Ayodeji, B.; Egbeagu, U.U.; et al. Fungal community succession under influence of biochar in cow manure composting. Environ. Sci. Pollut. Res. 2020, 27, 9658–9668. [Google Scholar] [CrossRef]





| Material | TOC (%) | TN (%) | C/N | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| Submerged plant | 84.62 ± 5.26 | 1.33 ± 0.09 | 63.62 | 15.8 ± 0.43 | 29.2 ± 0.76 | 8.90 ± 0.46 |
| Dry chicken dung | 63.11 ± 3.23 | 3.62 ± 0.22 | 17.43 | - | - | - |
| Wetland sediment | 39.64 ± 2.17 | 2.63 ± 0.19 | 15.13 | - | - | - |
| Compost material | 76.36 ± 4.35 | 2.69 ± 0.18 | 28.39 | 7.62 ± 0.26 | 15.9 ± 0.87 | 4.88 ± 0.39 |
| Group | Wetland Sediment (%) | Biochar (%) | Bacterial Type |
|---|---|---|---|
| CK-C | 0 | 0 | C |
| S-C | 10 | 0 | C |
| B-C | 0 | 2 | C |
| SB-C | 10 | 2 | C |
| CK-H | 0 | 0 | H |
| S-H | 10 | 0 | H |
| B-H | 0 | 2 | H |
| SB-H | 10 | 2 | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Su, Z.; Ren, S.; Zhang, P.; Li, H.; Guo, X.; Liu, L. Combined Use of Biochar and Microbial Agents Can Promote Lignocellulosic Degradation Microbial Community Optimization during Composting of Submerged Plants. Fermentation 2024, 10, 70. https://doi.org/10.3390/fermentation10010070
Wang H, Su Z, Ren S, Zhang P, Li H, Guo X, Liu L. Combined Use of Biochar and Microbial Agents Can Promote Lignocellulosic Degradation Microbial Community Optimization during Composting of Submerged Plants. Fermentation. 2024; 10(1):70. https://doi.org/10.3390/fermentation10010070
Chicago/Turabian StyleWang, Hongjie, Zhiwei Su, Shengnan Ren, Panyue Zhang, Hui Li, Xiaoping Guo, and Ling Liu. 2024. "Combined Use of Biochar and Microbial Agents Can Promote Lignocellulosic Degradation Microbial Community Optimization during Composting of Submerged Plants" Fermentation 10, no. 1: 70. https://doi.org/10.3390/fermentation10010070





