Pull-Out of Pristine and Functionalized Carbon Nanotubes from Cement: A Molecular Modelling Study
Abstract
:1. Introduction
2. Materials and Methods
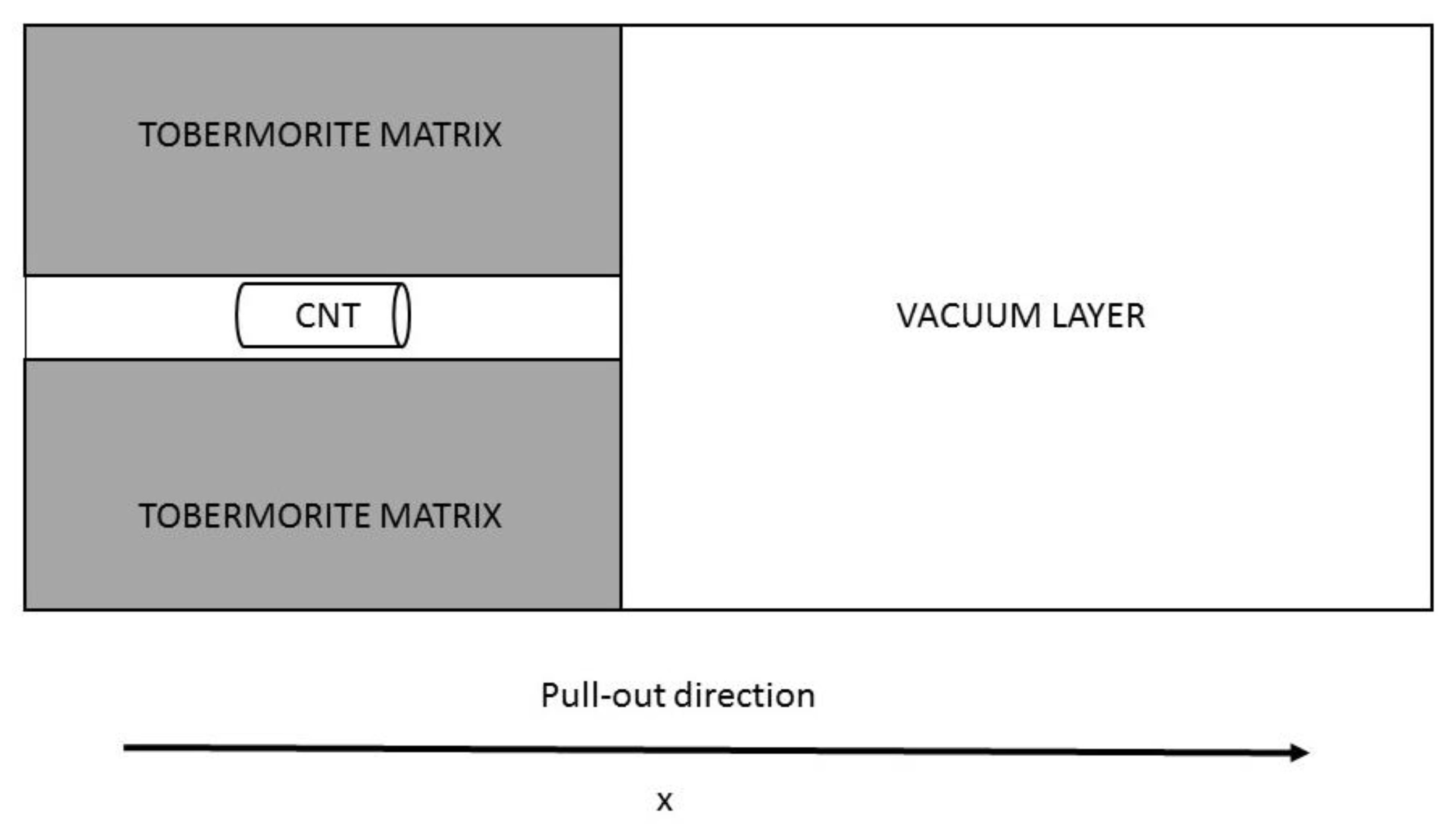
2.1. Model Systems
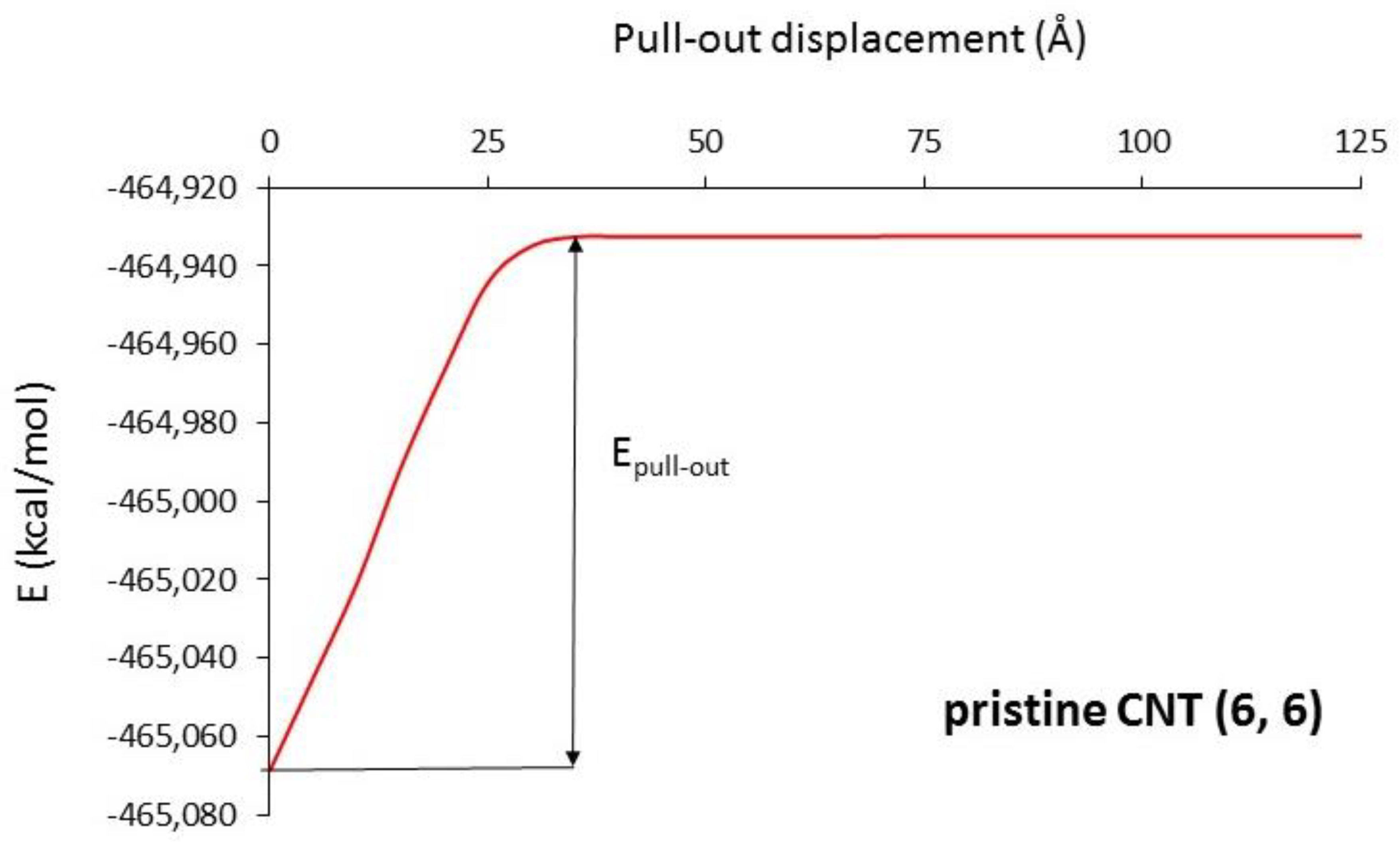
2.2. Calculation Method
3. Results and Discussion
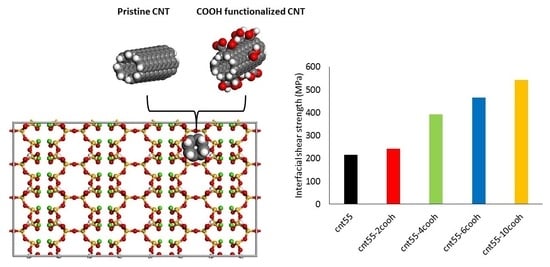
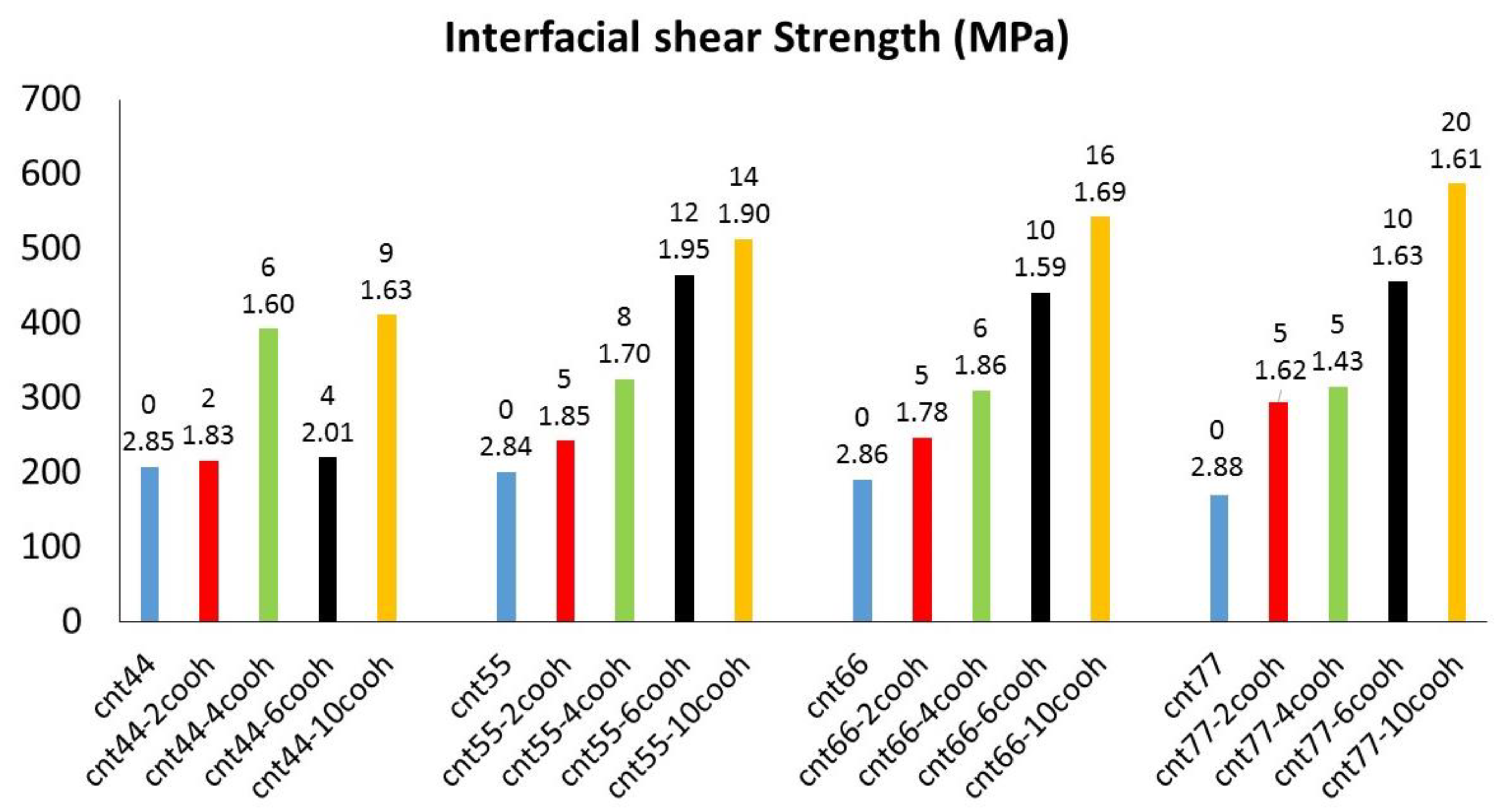
3.1. Interfacial Shear Strength
3.2. Non-Bonded Energy
3.3. Ca2+-O Interactions
4. Conclusions
- ISS decreased with increasing CNT radius for pristine CNTs and depended upon the number of H-bonds for functionalized CNTs.
- In the absence of chemical bonds between the CNT and the matrix, ISS values were positively correlated to ΔEnon-bonded energy.
- High, positive ΔEnon-bonded energy values were mainly derived from the destruction of H-bonds and electrostatic Ca2+-O interactions, as the CNT was pulled out from the matrix. Specifically, the complete pull-out configuration was less stable than the fully embedded configuration.
- The higher the number of carboxyl groups, the higher the ISS value, as the functionalization of CNTs with these polar groups resulted in both more H-bonds and more Ca2+-O interactions.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reales, O.A.M.; Toledo Filho, R.D. A review on the chemical, mechanical and microstructural characterization of carbon nanotubes-cement based composites. Constr. Build. Mater. 2017, 154, 697–710. [Google Scholar] [CrossRef]
- Shi, T.; Li, Z.; Guo, J.; Gong, H.; Gu, C. Research progress on CNTs/CNFs-modified cement-based composites—A review. Constr. Build. Mater. 2019, 202, 290–307. [Google Scholar] [CrossRef]
- Rocha, V.V.; Ludvig, P.; Trindade, A.C.C.; de Andrade Silva, F. The influence of carbon nanotubes on the fracture energy, flexural and tensile behavior of cement based composites. Constr. Build. Mater. 2019, 209, 1–8. [Google Scholar] [CrossRef]
- Makul, N. Advanced smart concrete—A review of current progress, benefits and challenges. J. Clean Prod. 2020, 274, 122899. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Tolkou, A.K.; Efstathiou, S.; Rahdar, A.; Favvas, E.P.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials in Cementitious Composites: An Update. Molecules 2021, 26, 1430. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Wang, P.M.; Zhao, X. Mechanical behavior and microstructure of cement composites incorporating surfacetreated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, P.M.; Zhao, X. Pressure-sensitive properties and microstructure of carbon nanotube reinforced cement composites. Cem. Concr. Compos. 2007, 29, 377–382. [Google Scholar] [CrossRef]
- Gao, F.; Tian, W.; Wang, Z.; Wang, F. Effect of diameter of multi-walled carbon nanotubes on mechanical properties and microstructure of the cement-based materials. Constr. Build. Mater. 2020, 260, 120452. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, H.; Zhou, Z.; Shi, X.; Li, L.; Fu, G. Roles of carbon nanotubes in reinforcing the interfacial transition zone and impermeability of concrete under different water-to-cement ratios. Constr. Build. Mater. 2021, 272, 121664. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Rudžionis, Z.; Rajapriya, R. The Effect of carbon nanotubes on the flowability, mechanical, microstructural and durability properties of cementitious composite: An overview. Sustainability 2020, 12, 8362. [Google Scholar] [CrossRef]
- Du, Y.; Gao, P.; Yang, J.; Shi, F.; Shabaz, M. Experimental Analysis of Mechanical Properties and Durability of Cement-Based Composite with Carbon Nanotube. Adv. Mater. Sci. Eng. 2021, 2021, 8777613. [Google Scholar] [CrossRef]
- Silvestro, L.; Ruviaro, A.; Lima, G.; de Matos, P.; de Azevedo, A.R.G.; Monteiro, S.N.; Gleize, P. Influence of Ultrasonication of Functionalized Carbon Nanotubes on the Rheology, Hydration, and Compressive Strength of Portland Cement Pastes. Materials 2021, 14, 5248. [Google Scholar] [CrossRef] [PubMed]
- Jongvivatsakul, P.; Thongchom, C.; Mathuros, A.; Prasertsri, T.; Adamu, M.; Orasutthikul, S.; Lenwari, A.; Charainpanitkul, T. Enhancing bonding behavior between carbon fiber-reinforced polymer plates and concrete using carbon nanotube reinforced epoxy composites. Case Stud. Constr. Mater. 2022, 17, e01407. [Google Scholar] [CrossRef]
- Yu, Z.; Lau, D. Evaluation on mechanical enhancement and fire resistance of carbon nanotube (CNT) reinforced concrete. Coupled Syst. Mech. 2017, 6, 335–349. [Google Scholar]
- Qin, R.; Zhou, A.; Yu, Z.; Wang, Q.; Lau, D. Role of carbon nanotube in reinforcing cementitious materials: An experimental and coarse-grained molecular dynamics study. Cem. Concr. Res. 2021, 147, 106517. [Google Scholar] [CrossRef]
- Sindu, B.S.; Sasmal, S. Molecular dynamics simulations for evaluation of surfactant compatibility and mechanical characteristics of carbon nanotubes incorporated cementitious composite. Constr. Build. Mater. 2020, 253, 119190. [Google Scholar]
- Balasubramaniam, B.; Mondal, K.; Ramasamy, K.; Palani, G.S.; Iyer, N.R. Hydration Phenomena of Functionalized Carbon Nanotubes (CNT)/Cement Composites. Fibers 2017, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Sarvandani, M.M.; Mahdikhani, M.; Aghabarati, H.; Fatmehsari, M.H. Effect of functionalized multi-walled carbon nanotubes on mechanical properties and durability of cement mortars. J. Build. Eng. 2021, 41, 102407. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltaniana, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916. [Google Scholar] [CrossRef]
- Liu, P. Modifications of carbon nanotubes with polymer. Eur. Polym. J. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Peng, H.; Alemany, L.B.; Margrave, J.L.; Khabashesku, V.N. Sidewall Carboxylic Acid Functionalization of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.T.; Seo, J.Y.; Park, S.H. The Characteristics of CNT/Cement Composites with Acid-Treated MWCNTs. Adv. Mater. Sci. Eng. 2015, 2015, 308725. [Google Scholar] [CrossRef]
- Ruan, Y.; Han, B.; Yu, X.; Zhang, W.; Wang, D. Carbon nanotubes reinforced reactive powder concrete. Compos. Part A Appl. Sci. Manuf. 2018, 112, 371–382. [Google Scholar] [CrossRef]
- Yilmaz, Y.L. Analyzing single fiber fragmentation test data by using stress transfer model. J. Compos. Mater. 2002, 36, 537–551. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Xing, Y.M.; Lei, Z.K. Interfacial stress transfer behavior in a specially-shaped fiber/matrix pullout test. Acta Mech. Sin. 2010, 26, 113–119. [Google Scholar] [CrossRef]
- Zu, M.; Li, Q.; Zhu, Y. The effective interfacial shear strength of carbon nanotube fibers in an epoxy matrix characterized by a microdroplet test. Carbon 2012, 50, 1271–1279. [Google Scholar] [CrossRef]
- Battisti, A.; Esqué-de los Ojos, D.; Ghisleni, R. Single fiber push-out characterization of interfacial properties of hierarchical CNT-carbon fiber composites prepared by electrophoretic deposition. Compos. Sci. Technol. 2014, 95, 121–127. [Google Scholar] [CrossRef]
- Rodríguez, M.; Molina-Aldareguía, J.M.; González, C. A methodology to measure the interface shear strength by means of the fiber push-in test. Compos. Sci. Technol. 2012, 72, 1924–1932. [Google Scholar] [CrossRef] [Green Version]
- Ji-Hong, J.; Chan, Y.; Chan-Gi, P. Bonding Characteristics of Macrosynthetic Fiber in Latex-Modified Fiber-Reinforced Cement Composites as a Function of Carbon Nanotube Content. Int. J. Polym. Sci. 2016, 2016, 7324975. [Google Scholar]
- Li, Y.; Wang, Q.; Wang, S. A review on enhancement of mechanical and tribological properties of polymer composites reinforced by carbon nanotubes and graphene sheet: Molecular dynamics simulations. Compos. B Eng. 2019, 160, 348–361. [Google Scholar] [CrossRef]
- Liao, K.; Li, S. Interfacial characteristics of a carbon nanotube–polystyrene composite system. Appl. Phys. Lett. 2001, 79, 4225. [Google Scholar] [CrossRef]
- Chowdhury, S.C.; Okabe, T. Computer simulation of carbon nanotube pull-out from polymer by the molecular dynamics method. Compos. Part A Appl. Sci. Manuf. 2007, 38, 747–754. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Peng, X.; Yan, C.; Liu, S.; Hu, N. Pull-out simulations on interfacial properties of carbon nanotube-reinforced polymer nanocomposites. Comput. Mater. Sci. 2011, 50, 1854–1860. [Google Scholar] [CrossRef]
- Chandra, Y.; Scarpa, F.; Adhikari, S.; Zhang, J.; Flores, E.S.; Peng, H.X. Pullout strength of graphene and carbon nanotube/epoxy composites. Compos. B Eng. 2016, 102, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Sharma, S. Molecular dynamics simulation of carbon nanotube pull-out from polyethylene matrix. Compos. Sci. Technol. 2017, 144, 169–177. [Google Scholar] [CrossRef]
- Fan, D.; Lue, L.; Yang, S. Molecular dynamics study of interfacial stress transfer in graphene-oxide cementitious composites. Comput. Mater. Sci. 2017, 139, 56–64. [Google Scholar] [CrossRef]
- Alkhateb, H.; Al-Ostaz, A.; Cheng, A.H.D.; Li, X. Materials genome for graphene-cement nanocomposites. J. Nanomechanics Micromechanics 2013, 3, 67–77. [Google Scholar] [CrossRef]
- Eftekhari, M.; Mohammadi, S. Molecular dynamics simulation of the nonlinear behavior of theCNT-reinforced calcium silicate hydrate (C–S–H) composite. Int. J. Impact. Eng. 2016, 82, 78–87. [Google Scholar]
- Kai, M.; Zhang, L.; Liew, K. Graphene and graphene oxide in calcium silicate hydrates: Chemical reactions, mechanical behaviors and interfacial sliding. Carbon 2019, 146, 181–193. [Google Scholar] [CrossRef]
- Lv, C.; Xue, Q.; Xia, D.; Ma, M.; Xie, J.; Chen, H. Effect of chemisorption on the interfacial bonding characteristics of graphene− polymer composites. J. Phys. Chem. C 2010, 114, 6588–6594. [Google Scholar] [CrossRef]
- Shiu, S.C.; Tsai, J.L. Characterizing thermal and mechanical properties of graphene/ epoxy nanocomposites. Compos. B Eng. 2014, 56, 691–697. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Wang, Q. Enhancement of tribological properties of polymer composites reinforced by functionalized graphene. Compos. B Eng. 2017, 120, 83–91. [Google Scholar] [CrossRef]
- Nikkhah, S.J.; Moghbeli, M.R.; Hashemianzadeh, S.M. Investigation of the interface polyethylene and functionalized graphene: A computer simulation study. Curr. Appl. Phys. 2015, 15, 1188–1199. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D. Molecular dynamics simulation of mechanical performance of graphene/graphene oxide paper based polymer composites. Carbon 2014, 67, 784–791. [Google Scholar] [CrossRef]
- Bauchy, M.; Qomi, M.A.; Ulm, F.J.; Pellenq, R.M. Order and Disorder in Calcium-silicate-hydrate. J. Chem. Phys. 2014, 140, 214503. [Google Scholar] [CrossRef] [Green Version]
- Richardson, I.G. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C–S–H: Applicability to hardened pastes of tricalcium silicate, -dicalcium silicate, Portland cement, and of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive molecular dynamics and experimental study of graphene-cement composites: Structure, dynamics and reinforcement mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Allen, A.J.; Thomas, J.J.; Jennings, H.M. Composition and density of nanoscale calcium–silicate–hydrate in cement. Nat. Mater. 2007, 6, 311–316. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Materials Studio Materials Modeling & Simulation Application|Dassault Systèmes BIOVIA. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio/ (accessed on 21 November 2022).
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Bhuvaneshwari, B.; Palani, G.S.; Karunya, R.; Iyer, N.R. Nano mechanical properties on the mineralogical array of calcium silicate hydrates and calcium hydroxide through molecular dynamics-CSIR-SERC. Curr. Sci. 2015, 108, 1058–1065. [Google Scholar]
- Du, J.; Bu, Y.; Shen, Z. Interfacial properties and nanostructural characteristics of epoxy resin in cement matrix. Constr. Build. Mater. 2018, 164, 103–112. [Google Scholar] [CrossRef]
- Al-Ostaz, A.; Wu, W.; Cheng, A.-D.; Song, C. A molecular dynamics and microporomechanics study on the mechanical properties of major constituents of hydrated cement. Compos. Part B Eng. 2010, 41, 543–549. [Google Scholar] [CrossRef]
- Hajilar, S.; Shafei, B. Nano-scale investigation of elastic properties of hydrated cement paste constituents using molecular dynamics simulations. Comput. Mater. Sci. 2015, 101, 216–226. [Google Scholar] [CrossRef]
- Tosi, M. Cohesion of ionic solids in the Born model. In Solid State Physics; Elsevier: Amsterdam, The Netherlands, 1964; Volume 16, pp. 1–120. [Google Scholar]
- Gou, J.; Minaie, B.; Wang, B.; Liang, Z.; Zhang, C. Computational and experimental study of interfacial bonding of single-walled nanotube reinforced composites. Comput. Mater. Sci. 2004, 31, 225–236. [Google Scholar] [CrossRef]
- Al-Muhit, B.; Sanchez, F. Nano-engineering of the mechanical properties of tobermorite 14 Å with graphene via molecular dynamics simulations. Constr. Build. Mater. 2020, 233, 117237. [Google Scholar] [CrossRef]
- Sanchez, F.; Zhang, L. Molecular dynamics modeling of the interface between surface functionalized graphitic structures and calcium–silicate–hydrate: Interaction energies, structure, and dynamics. J. Colloid. Interface Sci. 2008, 323, 349–358. [Google Scholar] [CrossRef]
- Merodio-Perea, R.G.; Páez-Pavón, A.; Lado-Touriño, I. Reinforcing cement with pristine and functionalized carbon nanotubes: Experimental and simulation studies. Int. J. Smart Nano Mater. 2020, 11, 370–386. [Google Scholar] [CrossRef]
- Wang, M.C.; Lai, Z.B.; Galpaya, D.; Yan, C.; Hu, N.; Zhou, L.M. Atomistic simulation of surface functionalization on the interfacial properties of graphene-polymer nanocomposites. J. Appl. Phys. 2014, 115, 123520. [Google Scholar] [CrossRef] [Green Version]
- Mani, A.; Sharma, S. Interfacial shear strength of carbon nanotube reinforced polymer composites: A review. Mater. Today Proc. 2022, 50, 1774–1780. [Google Scholar] [CrossRef]
- Lushnikova, A.; Zaoui, A. Improving mechanical properties of C–S–H from inserted carbon nanotubes. J. Phys. Chem. Solids 2017, 105, 72–80. [Google Scholar] [CrossRef]
- Katz, A.K.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium Ion Coordination: A CoMParison with That of Beryllium, Magnesium, and Zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Sowrey, F.E.; Skipper, L.J.; Pickup, D.M.; Drake, K.O.; Lin, Z.; Smith, M.E.; Newport, R.J. Systematic empirical analysis of calcium–oxygen coordination environment by calcium K-edge XANES. Phys. Chem. Chem. Phys. 2004, 6, 188–192. [Google Scholar] [CrossRef]
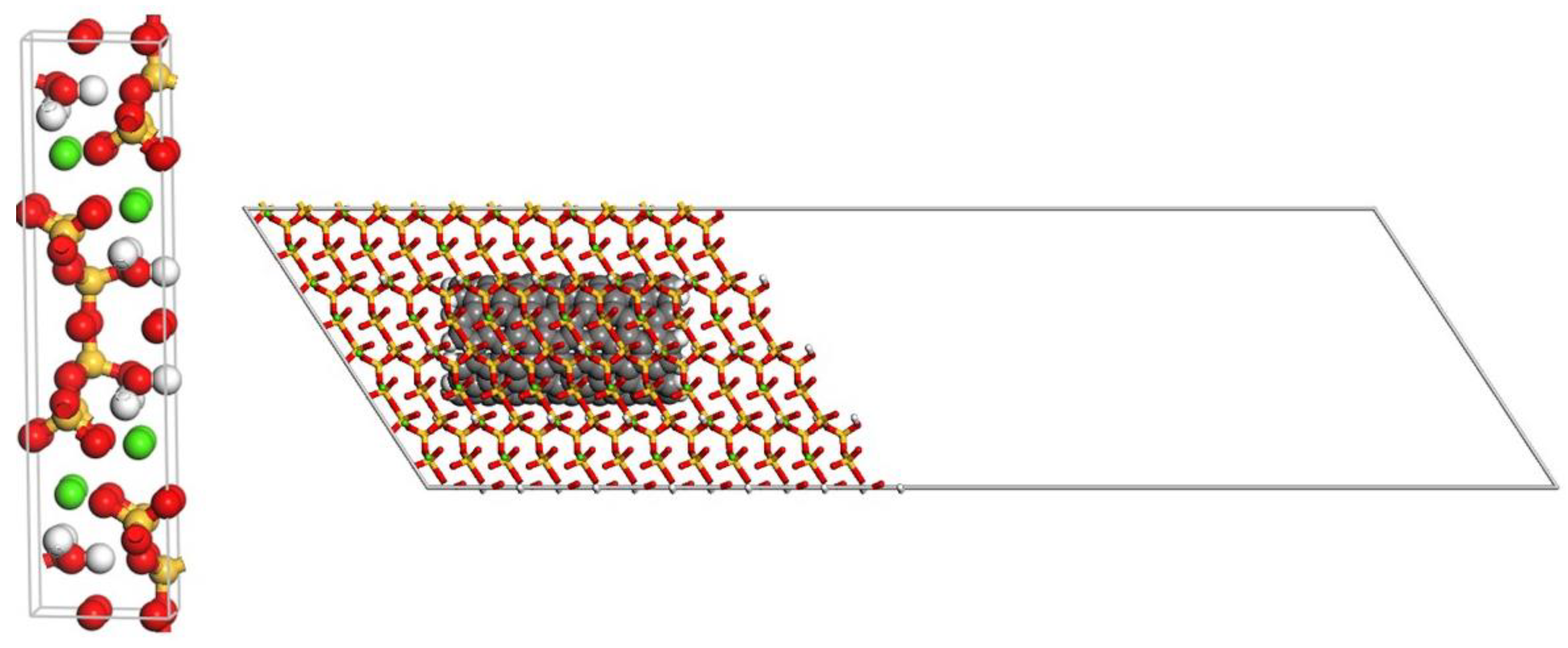
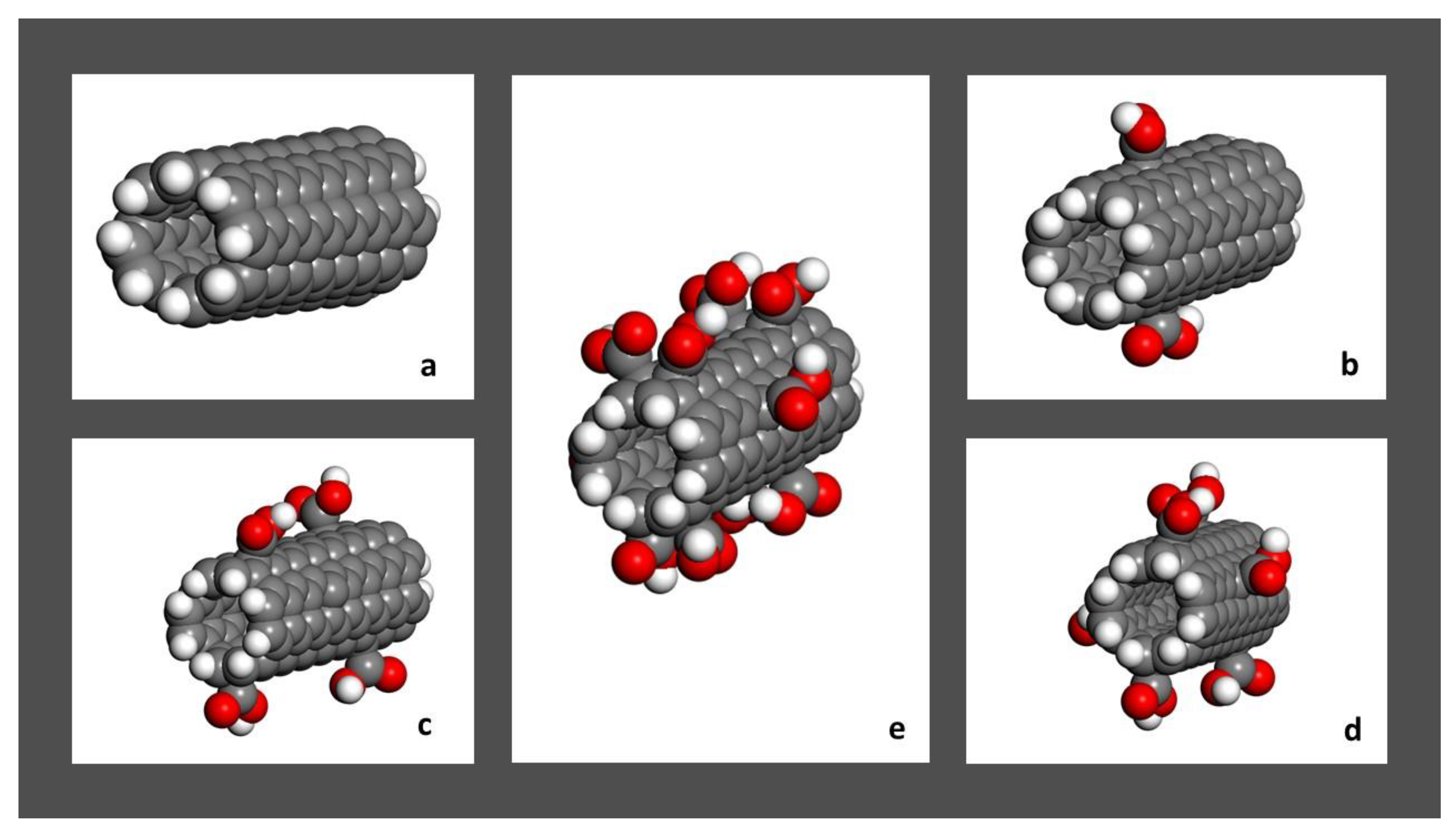




| System | Radius (Å) | Length (Å) | Number of COOH Groups |
|---|---|---|---|
| CNT (4, 4) | 2.71 | 0 | |
| CNT (5, 5) | 3.39 | 2 | |
| CNT (6, 6) | 4.07 | 9.84 | 4 |
| CNT (7, 7) | 4.74 | 10 |
| System | Mean Coordination Number | d (Å) | Number of Ca2+-Carboxylic O Close Contacts |
|---|---|---|---|
| cnt44-2cooh | 5.0 | 2.45 | 2 |
| cnt44-4cooh | 5.7 | 2.53 | 3 |
| cnt44-6cooh | 5.3 | 2.46 | 3 |
| cnt44-10cooh | 5.3 | 2.46 | 4 |
| cnt55-2cooh | 5.5 | 2.34 | 2 |
| cnt55-4cooh | 5.5 | 2.37 | 4 |
| cnt55-6cooh | 6.0 | 2.5 | 5 |
| cnt55-10cooh | 6.0 | 2.47 | 10 |
| cnt66-2cooh | 6.0 | 2.56 | 2 |
| cnt66-4cooh | 6.0 | 2.52 | 4 |
| cnt66-6cooh | 6.0 | 2.48 | 6 |
| cnt66-10cooh | 6.3 | 2.57 | 7 |
| cnt77-2cooh | 5.5 | 2.50 | 2 |
| cnt77-4cooh | 5.6 | 2.45 | 5 |
| cnt77-6cooh | 5.7 | 2.48 | 7 |
| cnt77-10cooh | 5.9 | 2.45 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lado-Touriño, I. Pull-Out of Pristine and Functionalized Carbon Nanotubes from Cement: A Molecular Modelling Study. C 2022, 8, 80. https://doi.org/10.3390/c8040080
Lado-Touriño I. Pull-Out of Pristine and Functionalized Carbon Nanotubes from Cement: A Molecular Modelling Study. C. 2022; 8(4):80. https://doi.org/10.3390/c8040080
Chicago/Turabian StyleLado-Touriño, Isabel. 2022. "Pull-Out of Pristine and Functionalized Carbon Nanotubes from Cement: A Molecular Modelling Study" C 8, no. 4: 80. https://doi.org/10.3390/c8040080