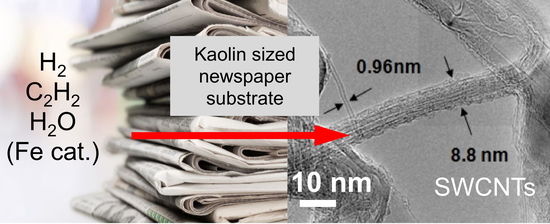
From Newspaper Substrate to Nanotubes—Analysis of Carbonized Soot Grown on Kaolin Sized Newsprint
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. CNT Growth
2.3. Characterization
3. Results and Discussion
3.1. SWCNT Growth on Paper Substrates
3.2. What Determines the Activity of a Paper Substrate for SWCNT Growth?
3.3. Characterization of Carbon Soot
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campidelli, S.; Klumpp, C.; Bianco, A.; Guldi, D.M.; Prato, M. Functionalization of CNT: Synthesis and applications in photovoltaics and biology. J. Phys. Org. Chem. 2006, 19, 531–539. [Google Scholar] [CrossRef]
- Avouris, P.; Freitag, M.; Perebeinos, V. Carbon-nanotube photonics and optoelectronics. Nat. Photon. 2008, 2, 341–350. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Azhang, J.; Willett, T.T.; Gelwick, S.A.; Haroz, E.H.; Kono, J.; Hauge, R.H.; Wong, M.S. Using nonionic surfactants for production of semiconductor-type carbon nanotubes by gel-based affinity chromatography. Nanomat. Nanotech. 2014, 4, 19. [Google Scholar] [CrossRef]
- Gomez, V.; Irusta, S.; Adams, W.W.; Hauge, R.H.; Dunnill, C.W.; Barron, A.R. Enhanced carbon nanotubes purification by physic-chemical treatment with microwave and Cl2. RSC Adv. 2016, 6, 11895–11902. [Google Scholar] [CrossRef]
- Zhang, K.S.; Pham, D.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Smalley, P.; Kennedy, K.; Brinson, B.; Billups, W.E.; Hauge, R.; et al. Overcoming catalyst residue inhibition of the functionalization of single-walled carbon nanotubes via the Billups-Birch reduction. ACS Appl. Mater. Interfaces 2017, 9, 37972–37980. [Google Scholar] [CrossRef]
- Chiang, I.W.; Brinson, B.E.; Smalley, R.E.; Margrave, J.L.; Hauge, R.H. Purification and characterization of single-wall carbon nanotubes. J. Phys. Chem. B 2001, 105, 1157–1161. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Pantano, A.; Parks, D.M.; Boyce, M.C. Mechanics of deformation of single- and multi-wall carbon nanotubes. J. Mech. Phys. Solids 2004, 52, 789–821. [Google Scholar] [CrossRef]
- Carlson, T.F.; Yang, H.H.H.; Wampler, W.A. Carbon Black with Attached Carbon Nanotubes and Method of Manufacture. U.S. Patent 20080233402A1, 25 September 2008. [Google Scholar]
- Asthana, A.; Maitra, T.; Buchel, R.; Tiwari, M.K.; Poulikakos, D. Multifunctional superhydrophobic polymer/carbon nanocomposites: Graphene, carbon nanotubes, or carbon black? ACS Appl. Mater. Interfaces 2014, 6, 8859–8867. [Google Scholar] [CrossRef]
- Bokobza, L.; Rahmani, M.; Belin, C.; Brunell, J.-L.; Bounia, N.-E.E. Blends of carbon blacks and multiwall carbon nanotubes as reinforcing fillers for hydrocarbon rubbers. J. Polym. Sci. B 2008, 46, 1939–1951. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.-P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef] [PubMed]
- Orbaek, A.W.; Barron, A.R. Towards a ‘catalyst activity map’ regarding the nucleation and growth of single walled carbon nanotubes. J. Exp. Nanosci. 2015, 10, 66–76. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Aggarwal, N.; Barron, A.R. The development of a ‘process map’ for the growth of carbon nanomaterials from ferrocene by injection CVD. J. Mater. Chem. A 2013, 1, 14122–14132. [Google Scholar] [CrossRef]
- Bronikowski, M.J.; Willis, P.A.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vac. Sci. Technol. A 2001, 19, 1800–1805. [Google Scholar] [CrossRef]
- Craddock, J.D.; Weisenberger, M.C. Harvesting of large, substrate-free sheets of vertically aligned multiwall carbon nanotube arrays. Carbon 2015, 81, 839–841. [Google Scholar] [CrossRef]
- Hedayati, A.; Barnett, C.J.; Swan, G.; White, A.O. Chemical recycling of consumer-grade black plastic into electrically conductive carbon nanotubes. C 2019, 5, 32–38. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Li, Y.; Kim, W.; Zhang, Y.; Rolandi, M.; Wang, D.; Dai, H. Growth of single-walled carbon nanotubes from discrete catalytic nanoparticles of various sizes. J. Phys. Chem. B 2001, 105, 11424–11431. [Google Scholar] [CrossRef]
- Fan, S.; Chapline, M.G.; Franklin, N.R.; Tombler, T.W.; Cassell, A.M.; Dai, H. Self-oriented regular arrays of carbon nanotubes and their field emission properties. Science 1999, 293, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Amama, P.B.; Pint, C.L.; Kim, S.M.; McJilton, L.; Eyink, K.G.; Stach, E.A.; Hauge, R.H.; Maruyama, B. Influence of alumina type on the evolution and activity of alumina-supported Fe catalysts in single-walled carbon nanotube carpet growth. ACS Nano 2010, 4, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, S.; Schnepp, Z.; Giordano, C. From paper to to structured carbon electrodes by inkjet printing. Angew. Chem. Int. Ed. 2013, 52, 2355–2358. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Li, F.; Pint, C.L.; Mayo, J.T.; Fisher, E.Z.; Tour, J.M.; Colvin, V.L.; Hauge, R.H. Uniform large diameter carbon nanotubes in vertical arrays from premade near-monodisperse nanoparticles. Chem. Mater. 2011, 23, 3466–3475. [Google Scholar] [CrossRef]
- Pint, C.L.; Pheasant, S.T.; Parra-Vasquez, A.N.G.; Horton, C.; Xu, Y.; Hauge, R.H. Investigation of optimal parameters for oxide-assisted growth of vertically aligned single-walled carbon nanotubes. J. Phys. Chem. C 2009, 113, 4125–4133. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Raja, P.M.V.; Esquenazi, G.L.; Barron, A.R. The safe handling of bulk low-density nanomaterials. SN Appl. Sci. 2019, 1, 644. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.-D.R.; Bachilo, S.M.; Ghosh, S.; Arepalli, S.; Weisman, R.B. Efficient spectrofluorimetric analysis of single-walled carbon nanotube samples. Anal. Chem. 2011, 83, 7431–7437. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Rao, A.M.; Richter, E.; Bandow, S.; Chase, B.; Eklund, P.C.; Williams, K.A.; Fang, S.; Subbaswamy, K.R.; Menon, M.; Thess, A.; et al. Diameter-selective raman scattering from vibrational modes in carbon nanotubes. Science 1997, 275, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, T.D.; Hankins, J.M.; Veitch, F.P. Deterioration of Book and Record Papers; Technical Bulletin No. 541; US Department of Agriculture: Washington, DC, USA, 1936.
- Barrow, W.J. Manuscripts and Documents; Their Deterioration and Restoration; University of Virginia Press: Charlottesville, NC, USA, 1955. [Google Scholar]
- MacInnes, A.N.; Barron, A.R. A spectroscopic evaluation of the efficacy of two mass deacidification processes for paper. J. Mater. Chem. 1992, 2, 1049–1056. [Google Scholar] [CrossRef]
- Schmude, K.G. Can library collections survive? The problem of paper deterioration. Aust. Libr. J. 1984, 33, 15–22. [Google Scholar] [CrossRef]
- Bundy, W.M.; Ishley, J.N. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Ma, D.; Carter, R.D.; Haefner, D.; Dogariu, A. The influence of fine kaolin and ground calcium carbonates on the efficiency and distribution of fluorescence whitening agents (FWA) in paper coating. Nord. Pulp Pap. Res. J. 2008, 23, 327–332. [Google Scholar] [CrossRef]
- Stempkowska, A.; Mastalska-Popławska, J.; Izak, P.; Ogłaza, L.; Turkowska, M. Stabilization of kaolin clay slurry with sodium silicate of different silicate moduli. Appl. Clay Sci. 2017, 146, 147–151. [Google Scholar] [CrossRef]
- Brown, R. Physical and chemical aspects of the use of fillers in paper. In Paper Chemistry; Roberts, J.C., Ed.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Gournisa, D.; Karakassidesa, M.A.; Bakasb, T.; Boukosc, N.; Petridis, D. Catalytic synthesis of carbon nanotubes on clay minerals. Carbon 2002, 40, 2641–2646. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszyñska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Gerspacher, D.M. Carbon black characterization. In Internal Report; Sid Richardson Carbon Co.: Fort Worth, TX, USA, 1999. [Google Scholar]
- Choi, D.H.; Wang, Q.; Azuma, Y.; Majima, Y.; Warner, J.H.; Miyata, Y.; Shinohara, H.; Kitaura, R. Fabrication and characterization of fully flattened carbon nanotubes: A new graphene nanoribbon analogue. Sci. Rep. 2013, 3, 1617. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Saito, R.; Jorio, A. Semiconducting carbon nanotubes. In Proceedings of the 27th International Conference on the Physics of Semiconductors (ICPS-27), Flagstaff, AZ, USA, 26–30 July 2004; pp. 25–31. [Google Scholar]
- Jorio, A.; Filho, A.G.S.; Dresselhaus, G.; Dresselhaus, M.S.; Swan, A.K.; Unlu, M.S.; Goldberg, B.B.; Pimenta, M.A.; Hafner, J.H.; Lieber, C.M.; et al. G-band resonant Raman study of 62 isolated single-wall carbon nanotubes. Phys. Rev. B 2002, 65, 155412. [Google Scholar] [CrossRef]
- Motta, M.; Moisala, A.; Kinloch, I.A.; Windle, A.H. Highperformance fibres from ‘dog bone’: Carbon nanotubes. Adv. Mater. 2007, 19, 3721–3726. [Google Scholar] [CrossRef]




| Peak | 514 nm | 633 nm | 780 nm |
|---|---|---|---|
| D | 1399 | 1306 | 1290 |
| G | 1594 | 1591 | 1591 |
| 2D | 2652 | 2597 | 2566 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinson, B.E.; Gangoli, V.S.; Kumar, A.; Hauge, R.H.; Adams, W.W.; Barron, A.R. From Newspaper Substrate to Nanotubes—Analysis of Carbonized Soot Grown on Kaolin Sized Newsprint. C 2019, 5, 66. https://doi.org/10.3390/c5040066
Brinson BE, Gangoli VS, Kumar A, Hauge RH, Adams WW, Barron AR. From Newspaper Substrate to Nanotubes—Analysis of Carbonized Soot Grown on Kaolin Sized Newsprint. C. 2019; 5(4):66. https://doi.org/10.3390/c5040066
Chicago/Turabian StyleBrinson, Bruce E., Varun Shenoy Gangoli, Anjli Kumar, Robert H. Hauge, W. Wade Adams, and Andrew R. Barron. 2019. "From Newspaper Substrate to Nanotubes—Analysis of Carbonized Soot Grown on Kaolin Sized Newsprint" C 5, no. 4: 66. https://doi.org/10.3390/c5040066