1. Introduction
Carbonization is the first step in the production of synthetic graphite. A liquid crystalline state or mesophase formation during carbonization is a key process that determines the property of graphitizable carbon [
1,
2,
3,
4]. The discovery of the carbonaceous mesophase is credited to Taylor, who in 1961 observed naturally occurring anisotropic spheres in the Wongawillie coal seam in New South Wales, Australia [
5]. Mesophase is utilized to produce various forms of carbon including cokes [
6,
7,
8], carbon fibers [
9,
10], and porous carbon via templating [
11,
12]. The nature of mesophase and thus the resulting carbon product is dependent upon the extent of planarity of intermediate compounds formed, rates of carbonization, fluidity and extent of fluidity, and possible effect of solids on mesophase formation and coalescence [
6,
7,
13,
14,
15]. Model polycyclic aromatic hydrocarbon (PAH) compounds have been utilized to study mesophase. Carbonization of biphenyl results in an isotropic non-graphitizable carbon [
6,
7,
15]. Walker and colleagues co-carbonized anthracene and phenanthrene with biphenyl to observe potential retardation of mesophase development and resulting graphitizability by its introduction [
7,
14,
15]. The anthracene-biphenyl system produced a coke of in-homogeneous optical texture with regions that were similar to pure anthracene and pure biphenyl carbonization. The phenanthrene-biphenyl system exhibited a greater homogeneous texture and the observed optical texture of the anisotropic regions dropped sharply with increased additions of biphenyl. The in-homogeneous texture from anthracene-biphenyl and the homogenous texture from phenanthrene-biphenyl is due to the differences in carbonization reactivity between the compounds. Biphenyl carbonization reactivity is three orders of magnitude less than anthracene and similar to phenanthrene [
7]. Anthracene finishes carbonization before biphenyl has a chance to influence mesophase and actively participates with phenanthrene carbonization.
Therefore, mesophase derived carbon can be pre-engineered with proper selection and concentrations of precursor PAHs. Carbonized products from PAHs with differing carbonization reactivity will produce a coke comprised of segmented components that can be traced back to the precursor. Post carbonization heat treatment can then be used to accentuate the structural difference found in the material. Recently, it has been demonstrated that carbon materials can be heated to graphitization temperature in a millisecond under the action of a CO
2 laser [
16]. In this study a porous graphite is produced by rapid laser heating a coke derived from selective model compounds. The coke contains regions of pitch from slow carbonizing PAHs that are vaporized. The well-developed coke from highly reactive anthracene is annealed under the action of CO
2 laser heating.
Porous carbons have received a lot of attention due to their use in many applications, including separations, absorbents, catalyst supports, and electrodes in energy storage media [
11]. A truly porous graphite would make a good lithiated anode for lithium ion hybrid battery-supercapacitors. A hybrid battery-capacitor combines the best properties of the two devices. Lithium ion batteries have high energy density, but suffer from low power by virtue of the reversible Coulombic reactions that occur at both electrodes [
17]. The high energy density of the lithium ion battery is owed to the intercalation of lithium within the graphite anode material. In contrast, electrochemical double-layer capacitors store energy by accumulation of ions on the surface of high surface area electrode materials. The supercapacitor has high power density, but low energy storage capacity. Most current capacitor research effort is focused on the cathode and activated carbons for their extremely high surface areas [
18,
19]. Efforts to combine the high energy density of the battery and high power density of the supercapacitor into a single device is an emerging area of active research [
17,
20,
21]. As the charge capacity in hybrid battery-supercapacitors is proportional to the amount of each electrode component, the power and energy performance is decoupled [
17,
20]. However, these electrode types are not separate, but necessarily for their dual function integrated into one hybrid electrode [
22]. Specifically, the ideal hybrid electrode requires a graphitic character for high energy density while possessing a high surface area along with porosity for power performance. Moreover, the galleries must be externally accessible to the lithium ions.
Synthesis of carbons with such ordered and uniform pores is a challenge. Activation is the most common means to prepare porous carbon, but results in pores with tortuous connectivity and lack of order. Macroporous carbons with large pore sizes are commonly produced via emulsion templating by polymerization of a continuous phase of a high internal phase emulsion [
23,
24]. As an alternative, templates provide a way to synthesize ordered carbons with controlled meso- and micropore sizes [
12,
25]. Templated porous carbon was pioneered by Knox et al. in 1986 [
26]. Since then templating has been used to synthesize a wide range of porous carbons. However, obtaining a truly porous graphitic material by templating remains challenging [
11,
25]. Typical carbon precursors used in templating, like polyfurfuryl alcohol, acrylonitrile, and phenolic resins result in non-graphitizing carbons. Graphitizing precursors that pass through a fluid mesophase can be utilized to prepare a graphitizable porous carbon via templating. However, the template guides mesophase development and results in the carbon basal planes aligning normally to the template walls. Thus, the interlamellar galleries are not accessible as desired for a hybrid battery-capacitor electrode.
As shown here, rapid thermal processing of pre-engineered carbons bypasses the need for templating and the added processing complexity therein. The laser synthesized porous graphite has been studied as anode material for lithium ion capacitors. In general, porous graphitic anodes are very interesting materials for use in both lithium ion batteries and lithium ion capacitors. The presence of mesopores in the graphite can minimize the lithium diffusion length while also providing greater gallery access area for lithium storage.
3. Results and Discussion
Anthracene goes through an extended mesophase during carbonization as evidenced by the high optical anisotropy shown in the polarized light micrograph,
Figure 1. As such, anthracene coke is highly graphitizable and forms a synthetic graphite upon graphitization heat treatment [
14,
15,
28,
29]. Optical texture is classified in
Appendix A.
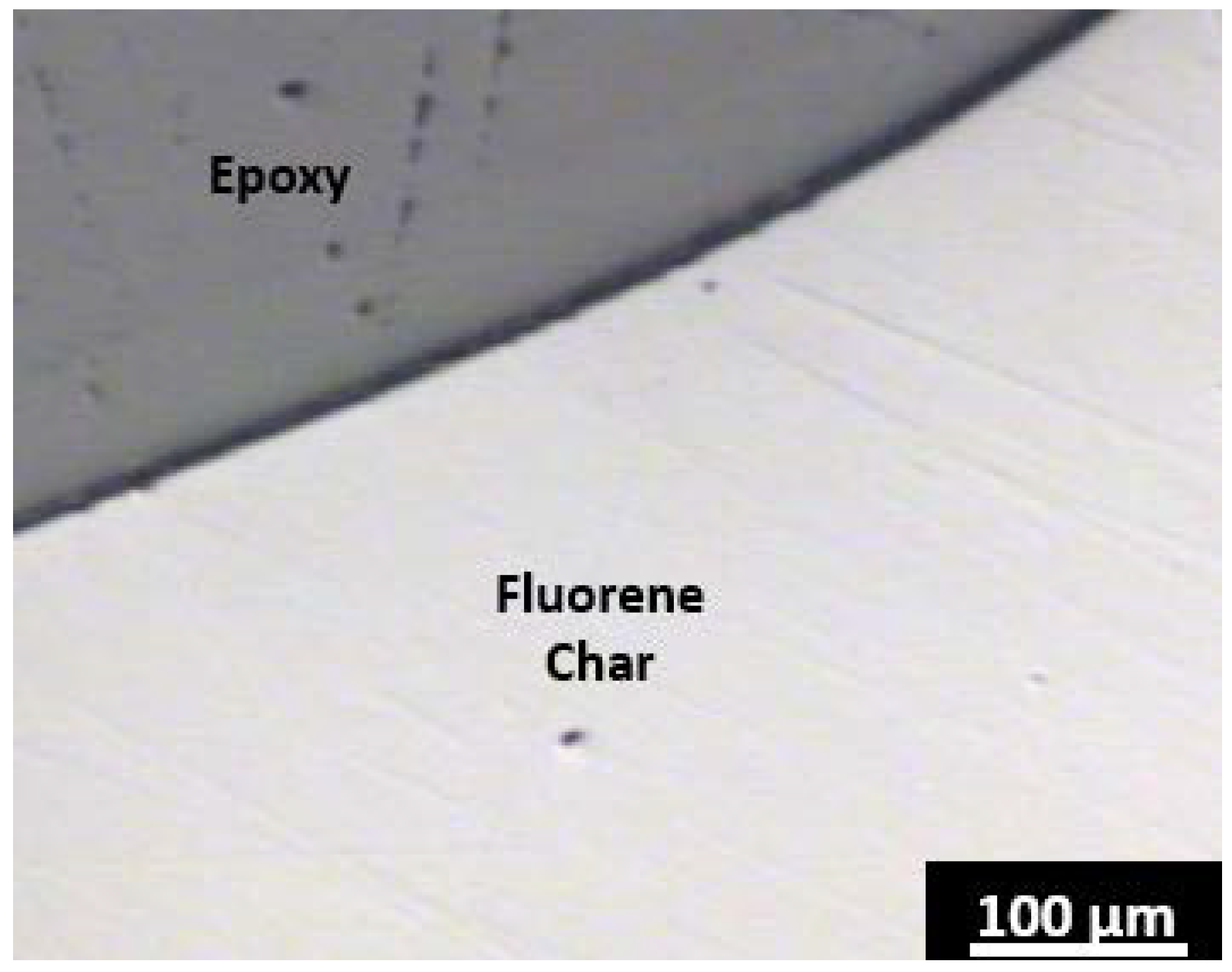
Fluorene, a five-membered ring containing PAH, yields only tar and no solid carbon after carbonization at 500 °C for 5 h. To maintain a prolonged fluid phase that promotes potential mesophase formation, fluorene was again carbonized at 500 °C and a solid carbon product was obtained after 12 h. The carbon yield was very high ~75% (anthracene coke ~50%). However, no optical anisotropy was observed under the polarized light microscope. The polarized light micrograph from fluorene char in
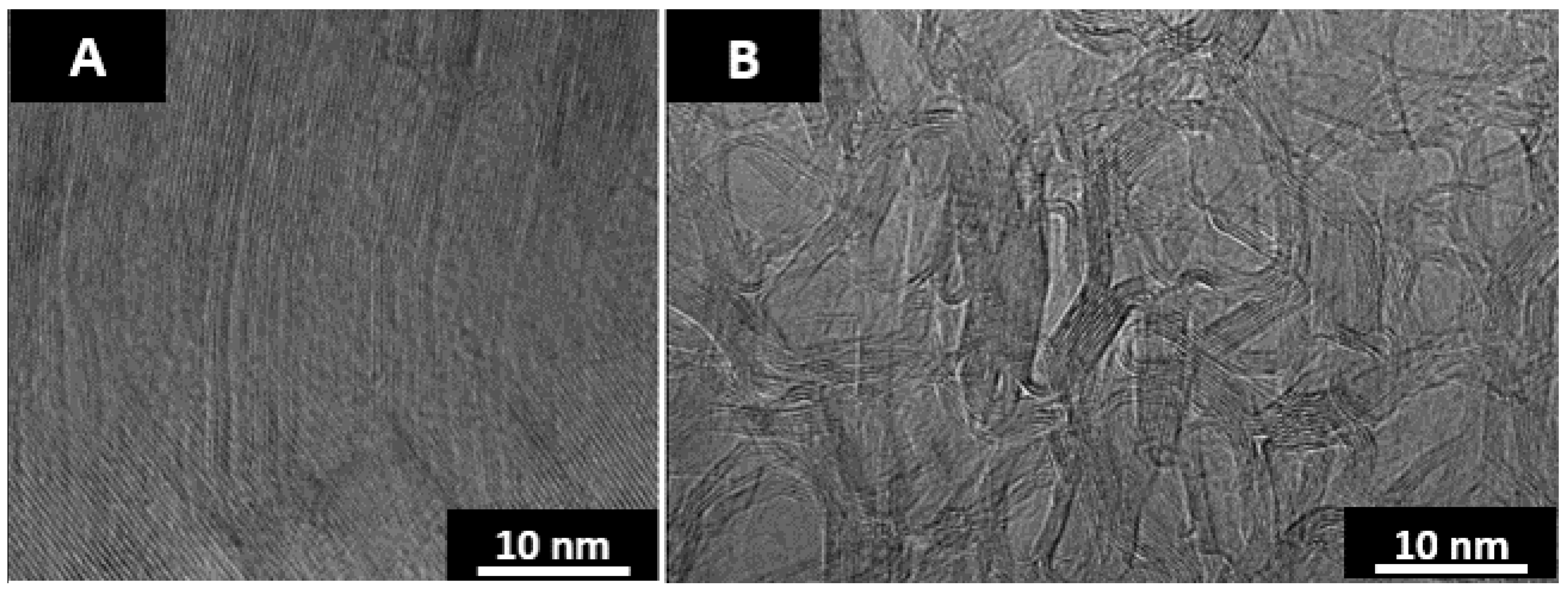
Figure 2 is absent of colored regions representative of anisotropy. The nanostructure of the 2 materials after furnace annealing at 2600 °C for 1 h is displayed by the TEM micrographs in
Figure 3.
As seen in the TEM micrographs, anthracene coke is a highly ordered graphitic structure and fluorene char is a highly disordered material with a chaotic nanostructure. The curvature found in heat-treated fluorene char is believed to be due to the inclusion of curvature inducing pentagonal ring structures [
30,
31,
32,
33,
34,
35,
36].
A 1:1 blend of fluorene and anthracene by weight was co-carbonized at 500 °C for 5 h. Due to the difference in reactivity of the compounds at 500 °C, it was expected that the surface would be heterogeneous and contain optical anisotropic regions formed from anthracene carbonization and pitch from fluorene carbonization. Indeed, pitch was found in abundant supply, shown on the edges of the micrograph in
Figure 4 (black regions), as were abundant flow domains from anthracene.
It has been demonstrated that optical anisotropy is typically locked in place upon the completion of mesophase development [
27]. However, heat treatment of the co-carbonized material partially destroyed the optical texture as shown in
Figure 5. The optical textures after heat treatment at 2600 °C for 1 h are mosaics (left) and small domains (right). No flow domains were preserved upon heating and thus fluorene pitch must have been embedded in the flow domains in
Figure 4. The smaller textures are the remnants of the original anthracene flow domains. At the nanoscale, clear heterogeneity exists, as shown in the TEM micrograph in
Figure 6.
The 2 regions are anthracene coke derived synthetic graphite as displayed in the right hand side of
Figure 6A and disordered regions as shown on the left in
Figure 6A and with a higher magnification in
Figure 6B. The disordered regions were likely present in the virgin coke as fluorene derived pitch. Since the disordered regions shown in the TEM micrograph are below the detection size limit of a light microscope, pitch was not observed dispersed throughout the flow domains in
Figure 4. The structure of the disordered region is very similar to that observed from heat-treated fluorene char. Upon furnace heat treatment, the pitch forms the disordered carbon regions causing stress between adjacent graphitic regions that results in misalignment. This misalignment translates to a decreased optical texture size as observed in the polarized light micrographs from before and after heat treatment.
CO
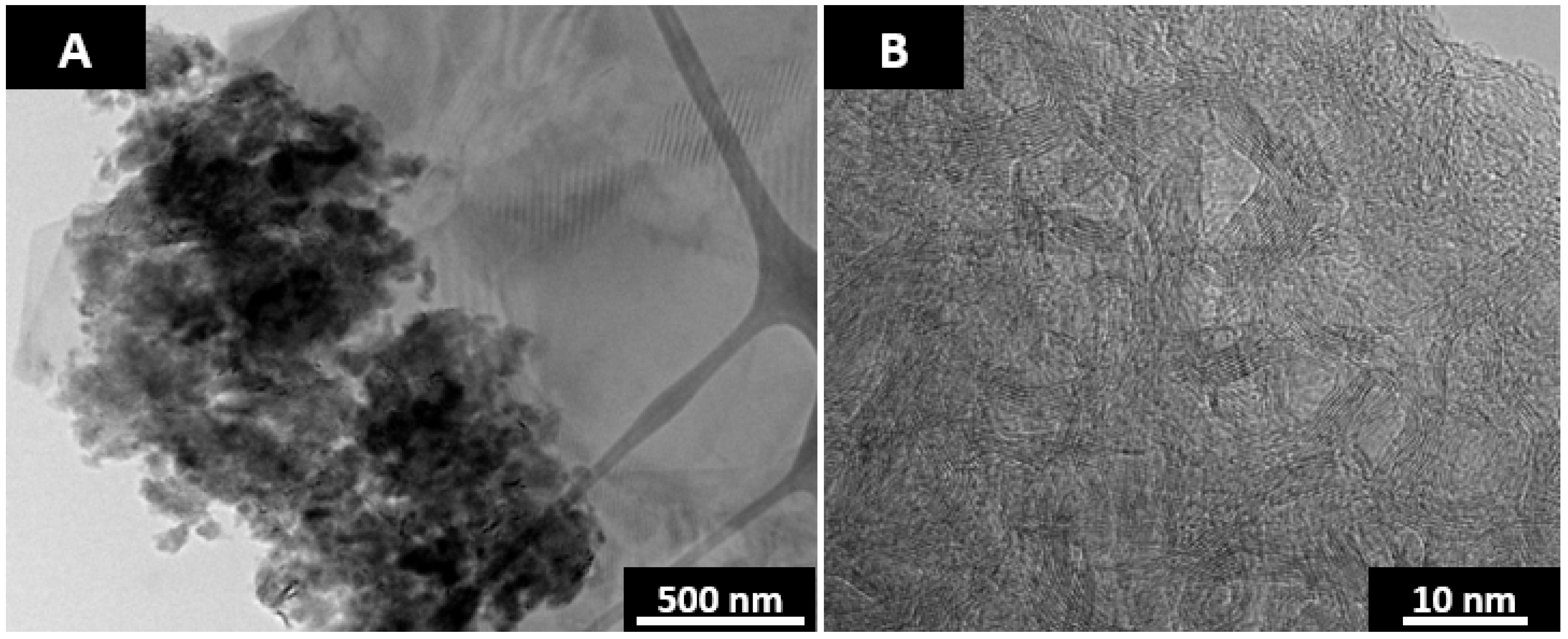
2 laser annealing vaporizes out the fluorene derived pitch while annealing the anthracene coke. The resulting structure is that of graphite with ~200 nm spherical pores as shown in
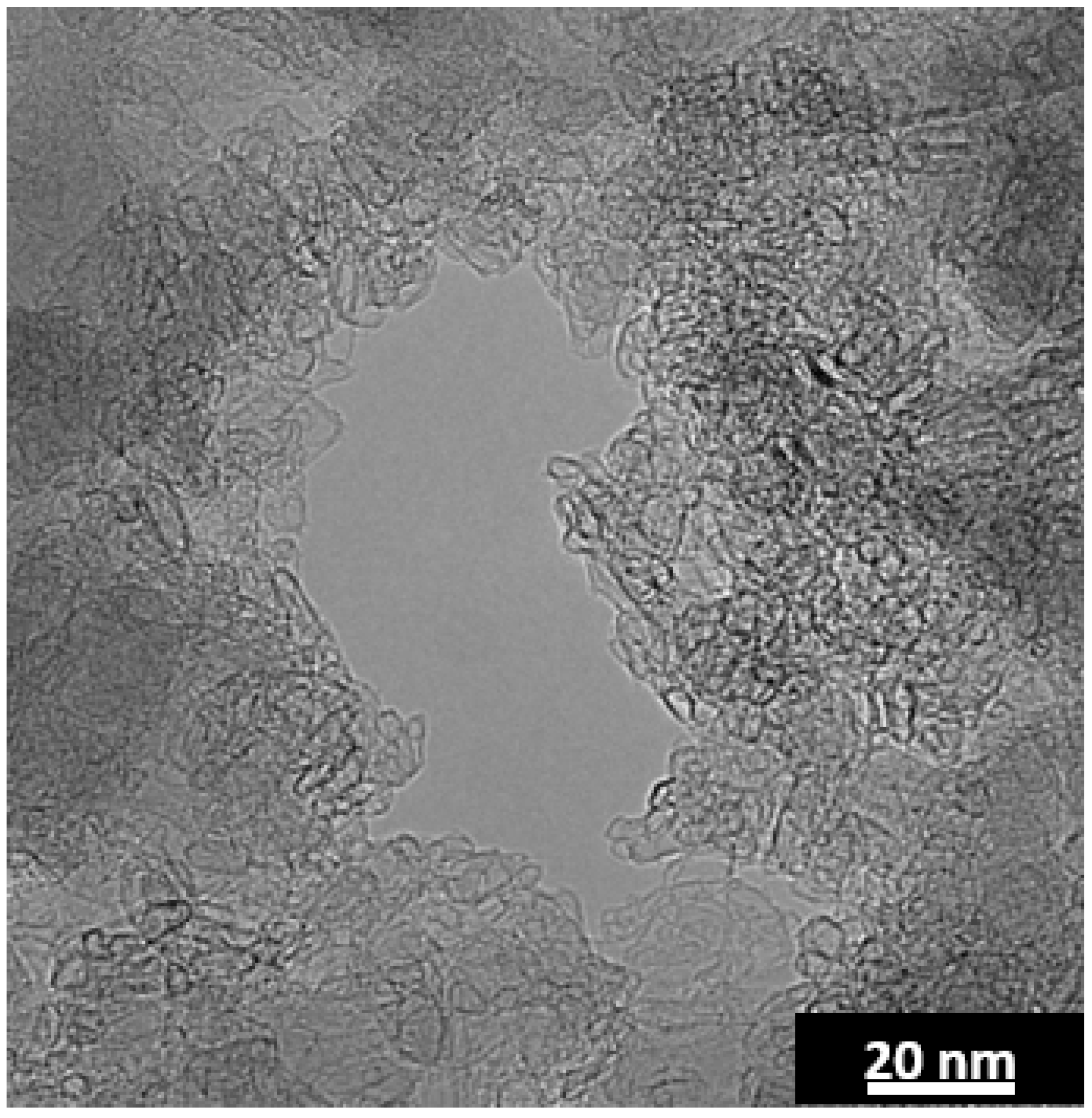
Figure 7. The Swiss cheese like structure in
Figure 7 was prepared by CO
2 laser annealing at 2600 °C for a duration of 1 min. Traditional furnace annealing of this material does not result in the porous structure as the heating rates are too slow to vaporize out the pitch. The heating rate of the CO
2 laser is 1.8 × 10
6 °C/s. The CO
2 laser heating rates and experimental setup have been reported elsewhere [
16].
The vaporization of the fluorene pitch is rapid and SCG is produced with very short laser pulses. Longer laser pulse widths are utilized to anneal the remaining anthracene coke. A thin carbon film from the deposition of the vaporized fluorene pitch was found around the annealed sample. A TEM grid was placed near the sample for the purpose of collecting the film. A TEM micrograph of the deposited carbon product is shown in
Figure 8. The film is in the form of soot and thus supports the assumption of vaporized pitch leaving behind the pores as the pyrolysis of pitch in aerosol will result in the formation of soot [
37,
38].
After 1 min of CO2 laser annealing the SCG has a d002 spacing of 3.40 Å, the same as pure anthracene coke subjected to the same heat treatment and thus the pores do not limit graphitizability. The laser synthesized SCG was heat treated at 2600 °C for a 1 h duration in a graphitization furnace. The layer plane spacing reduced to 3.36 Å, the same as pure anthracene coke.
A potential application of porous SCG is to use it as a lithiated anode. Graphite is the most common anode material for lithium ion batteries. However, the rate capacity of charging and discharging the lithiated graphite anode is a limiting factor. The porous SCG may provide improved rate capacity as the pores can increase Li
+ transport to the graphite gallery (edges), while improving their accessibility by increased exposure. Moreover, shorter interstitial distances (due to porosity) could also increase lithium intercalation and deintercalation rates, while retaining the desired properties of graphite (high energy density). The ideal porous graphite should contain pores covering the entire material and the graphite pore interface needs to be accessible to Li. However, as seen in the TEM micrograph in the right side of
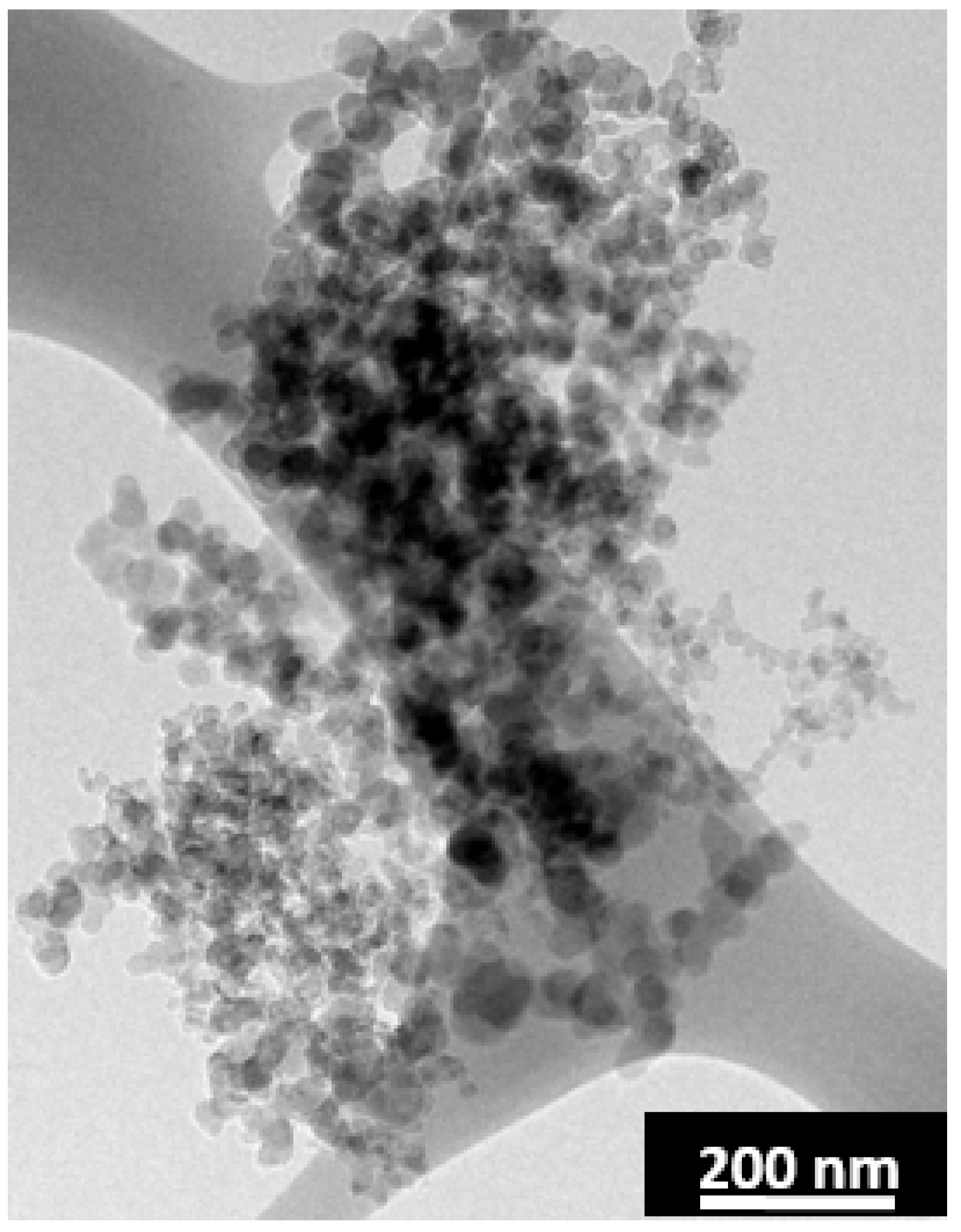
Figure 7, not all of the laser annealed anthracene-fluorene coke contains pores. The pores were found dispersed in only ~1/4 of the material. Additionally, the pore walls are comprised of closed shell nanoparticles and thus the graphitic layers are inaccessible to Li ions (
Figure 9).
In an attempt to optimize SCG, fluorene was replaced with pyrene as the fullerene-like structures around the pore walls may likely be due to the odd-membered ring. It has been repeatedly observed that oils with higher concentrations of pyrene result in extended fluidity of the carbonization medium [
4,
39,
40,
41]. Pyrene functions as a hydrogen shuttler and good solvent, extending fluidity and moderating the reactivity of the carbonizing medium. Co-carbonization of pyrene and anthracene yielded mostly tar after 5 h at 500 °C. The recovered solid product was 10% weight of that of the starting feed and did not yield SCG upon laser annealing as the pitch did not disperse throughout the solid. Co-carbonization of an equal part blend by weight of anthracene-fluorene-pyrene yielded 30% weight of solid carbon after carbonization at 500 °C for 5 h and resulted in SCG upon laser heating. The laser annealed product is shown in the TEM micrograph in
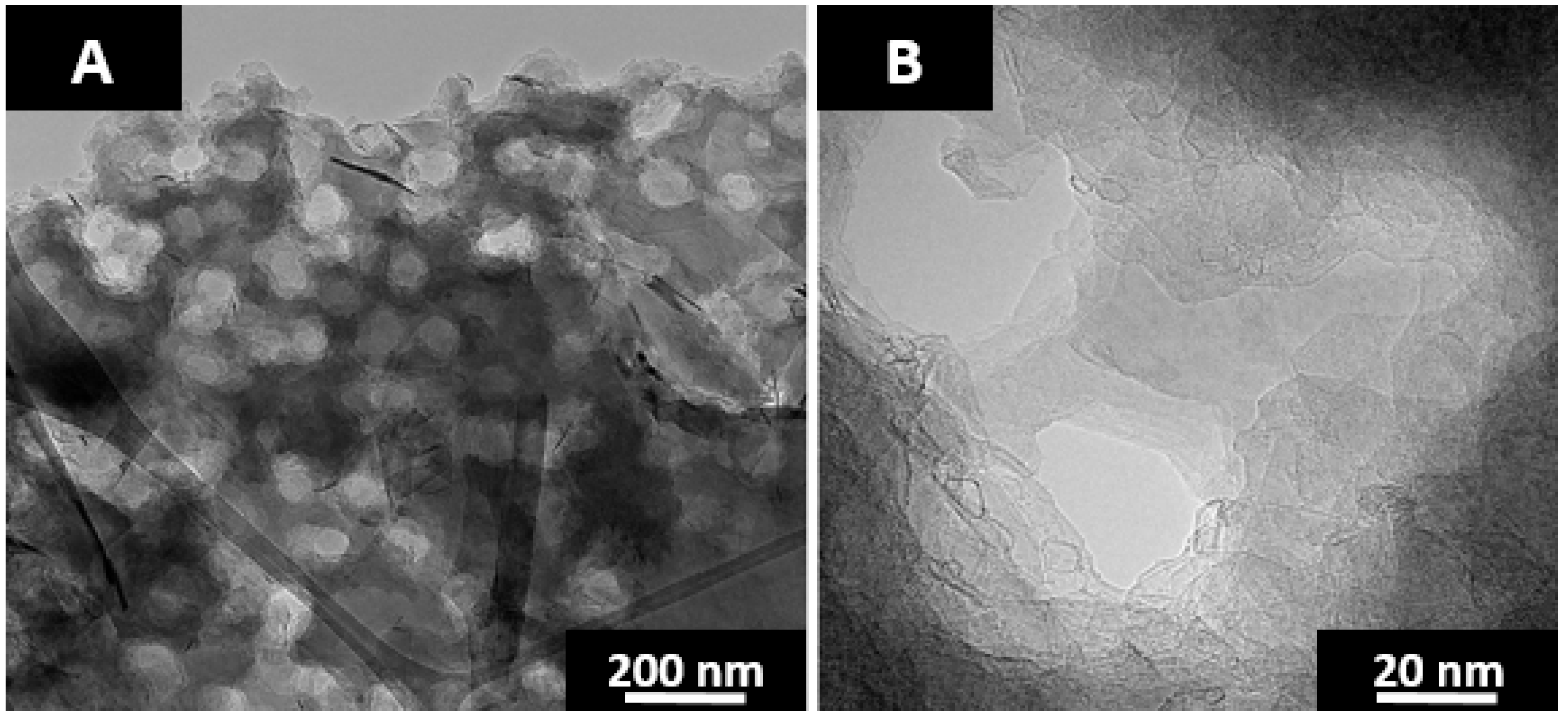
Figure 10. As seen in
Figure 10B, the graphitic layer planes are for the most part unimpeded with relatively few fullerene-like nanoparticles lining the pore walls. The reduction in fullerene-like particles may reflect the reduction in concentration of curvature inducing pentagonal ring systems. However, their nearly complete absence suggests otherwise. Pyrene mediated carbonization may create a boundary layer between the fluorene pitch and anthracene coke.
Pore coverage was increased to greater than half of the material by providing vertical agitation of the reactors at a frequency of 200 oscillations a minute and an amplitude of 2.5 cm. The pore diameter is ~100 nm with reactor agitation as shown in
Figure 10A, compared to ~200 nm w/out agitation and ~1/4 material coverage. Increasing the frequency beyond 200 oscillations a minute did not result in increased pore coverage. The crystal lattice dimensions of pure anthracene coke and SCG after furnace heat treatment at 2600 °C for 1 h are provided in
Table 1 as measured by XRD.
The carbon layer diameter (La) and stack height (Lc) are reduced due to the pores in SCG as compared to heat-treated anthracene coke. The lattice spacing and thus degree of graphitization are the same between the materials. Therefore, SCG is graphitized anthracene coke that contains quasi-spherical pores. Pore shape is assumed to be quasi-spherical based on the thickness contrast of the TEM micrographs in
Figure 10. The BET surface area was measured from both heat-treated anthracene coke and SCG after ball milling for 30 min. A TEM survey showed that both materials have an equivalent particle size of ~10 μm (micrographs provided in
Appendix B). The surface areas of SCG and heat-treated anthracene coke are 30 and 8 m
2/g, respectively. The nearly 4-fold greater surface area of SCG reflects the pores, considering the particle sizes of the 2 materials are equivalent. The total pore volume of SCG is 0.45 cm
3/g, the N
2 isotherm is provided in
Appendix C.
The optimized SCG was prelithiated using a short circuiting approach. The specific capacity of the SCG electrode was measured using half-cell measurements by delithiating the SCG electrode to 3 V.
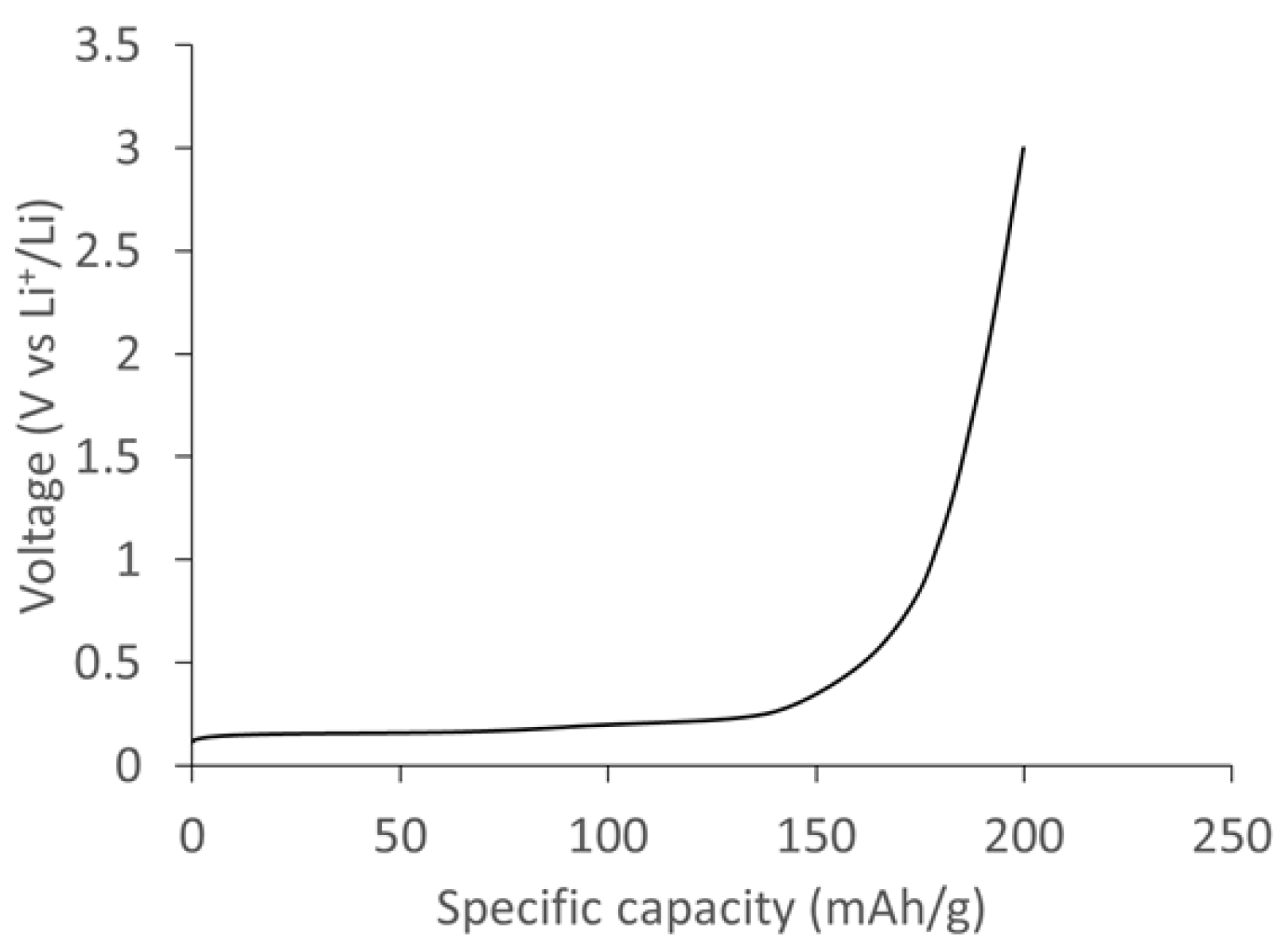
Figure 11 shows the specific capacity of the SCG electrode measured at a current density of 0.1 A/g. The delithiation curve shows a voltage plateau below 0.2 V vs. Li
+/Li corresponding to various stages of lithium deintercalation in SCG graphite. The total specific capacity measured at 0.1 A/g was ~235 mAh/g based on the active mass of SCG electrode.
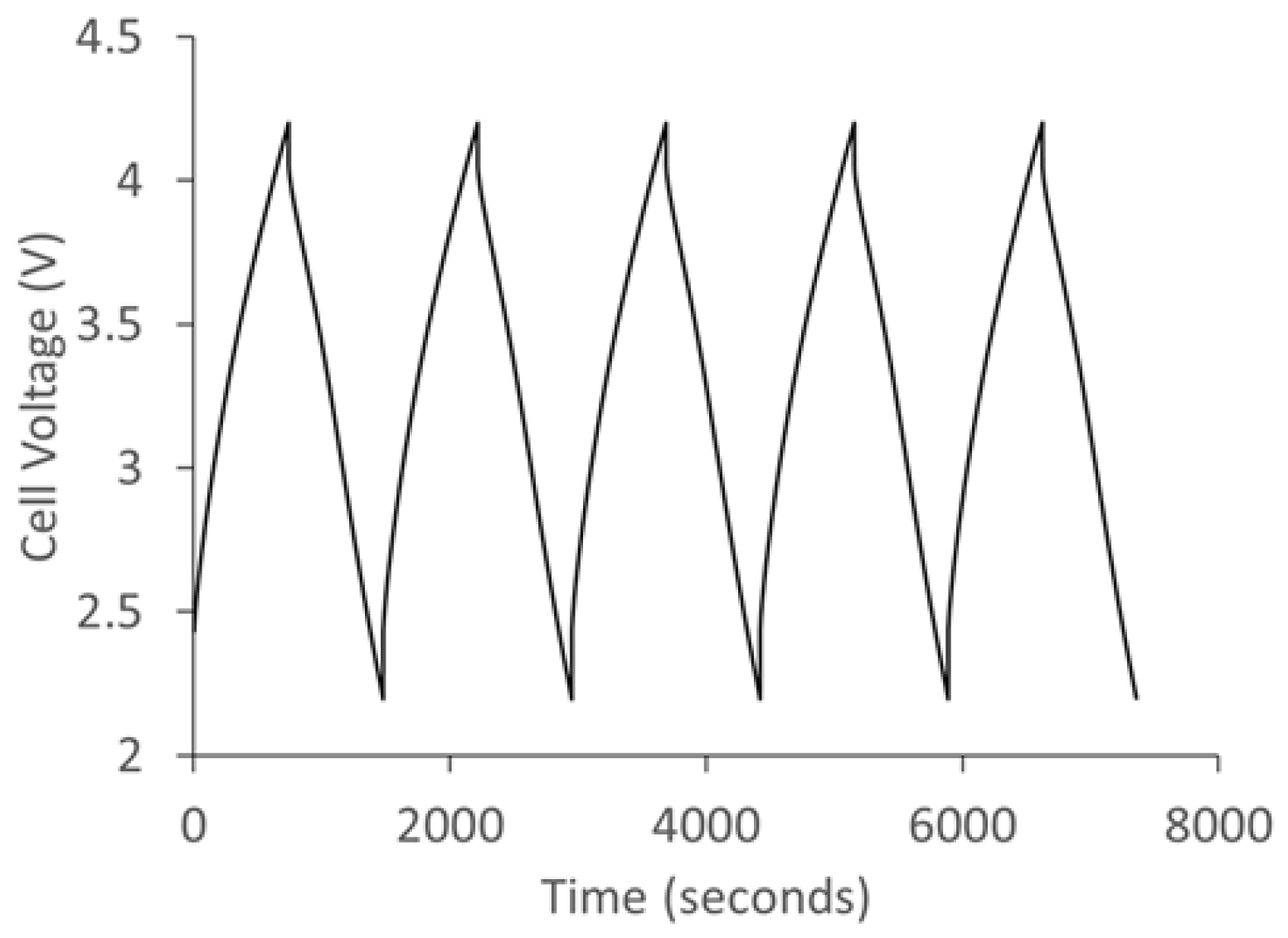
Figure 12 shows the charge/discharge curve of the lithium ion capacitor fabricated using polyfurfuryl alcohol/phloroglucinol carbon as cathode and prelithiated SCG as anode. The hybrid capacitor was cycled between 2.2 V and 4.2 V and exhibits a typical sawtooth profile.
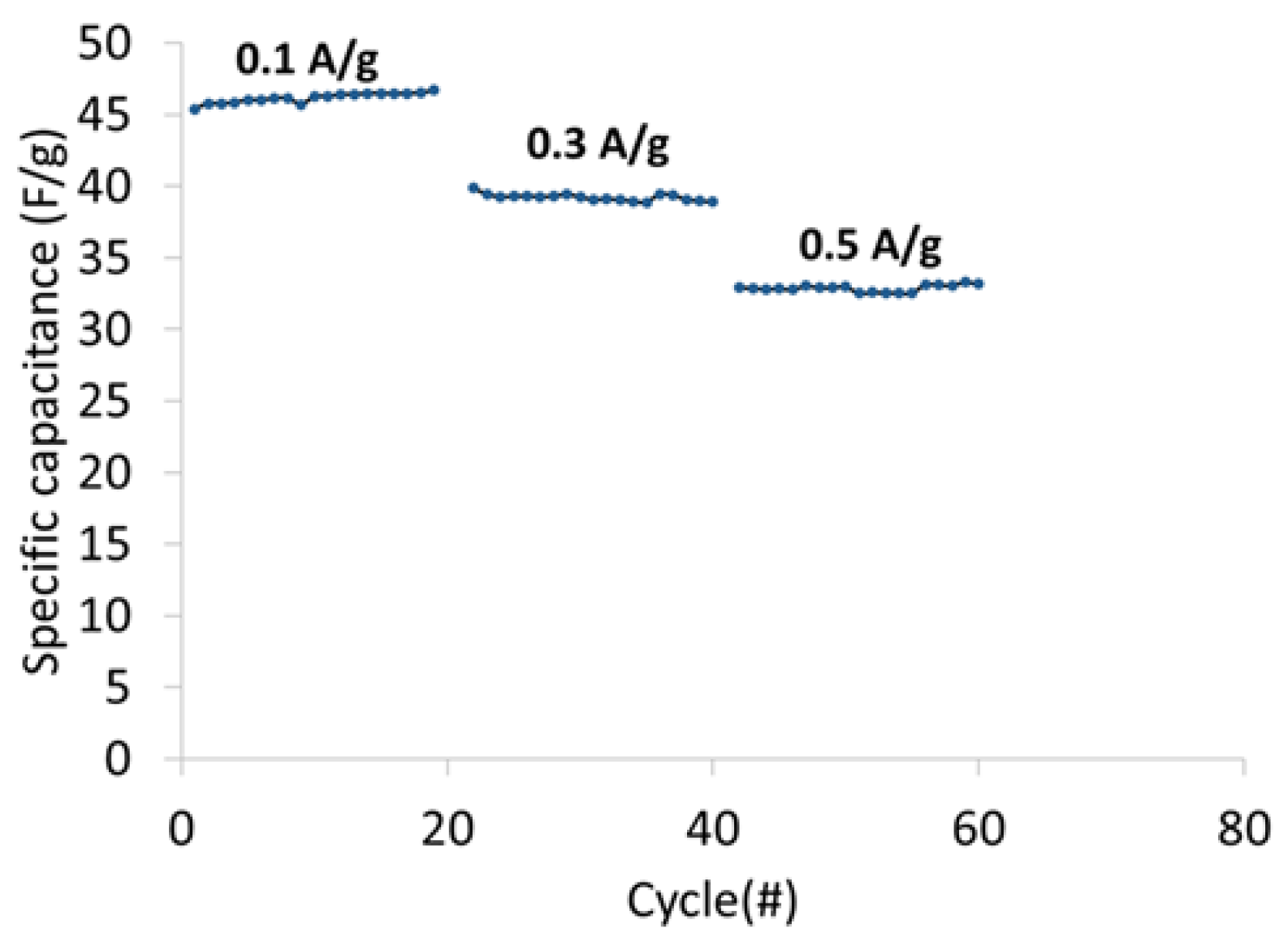
Figure 13 shows the specific cell capacitance plotted as a function of current density. The specific capacitance at 0.1 A/g, 0.3 A/g and 0.5 A/g was 46 F/g, 39 F/g, and 33 F/g, respectively.
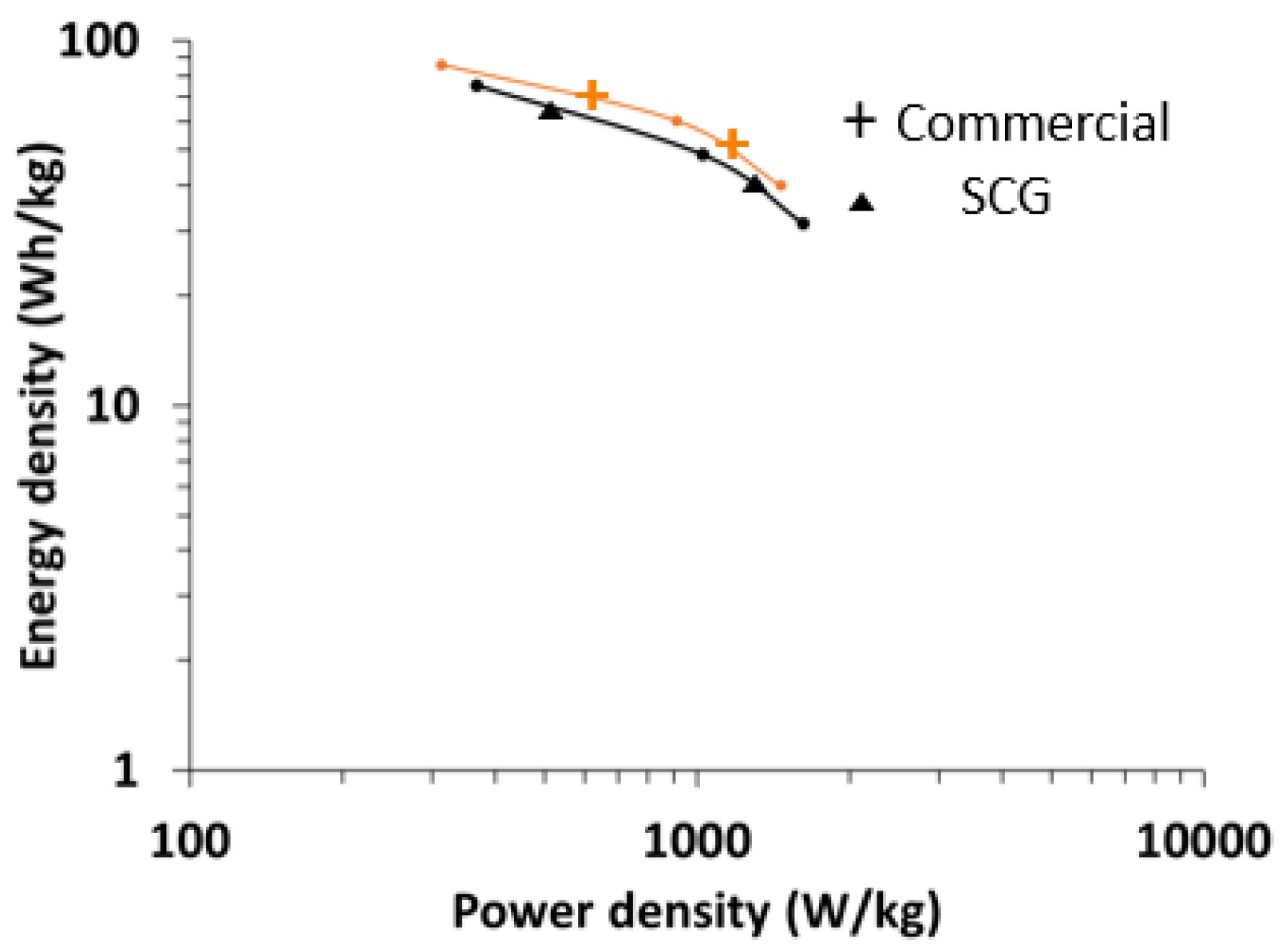
Figure 14 shows the comparison of the Ragone plot made using prelithiated SCG graphite and commercially available graphite derived from mesophase pitch. The energy density of the capacitor was ~75 Wh/kg at a power density of 370 W/kg. At 1 KW/kg, the energy density was about 48.5 Wh/kg.
In the current form, SCG does not provide improvement over commercial carbon anode materials. Although the relative surface area increase is large compared to pure anthracene derived graphite, 30 m2/g is low and likely a limiting factor. Further refinement of porosity and improvement in surface area, either pre-engineered by recipe and procedure or post partial activation, could result in increased rate capability and power characteristics. The low surface area of SCG is attributed to limited pore coverage. Approximately half of the material is non-porous. Pore coverage increased from ~1/4 to greater than half of the material by providing vertical agitation of the reactors. Increased vertical agitation beyond 200 oscillations a minute at an amplitude of 2.5 cm did not provide increased surface area. A potential method to increase surface area is to provide horizontal agitation of the reactor during carbonization. Horizontal agitation may result in the higher dispersion of the fluorene derived liquid pitch throughout the anthracene derived coke. Recipe modification is another avenue to explore, increasing the pitch precursor (fluorene) would decrease solid product yield, but may provide a more porous material with higher surface area. Other low carbonization reactivity compounds that yield pitch should be experimented with, in place and in combinations with fluorene and pyrene.
The electrical properties do demonstrate the graphitic nature of SCG. Thus, SCG is a porous graphite synthesized directly from tailored carbonization products via rapid thermal processing. Rapid thermal processing bypasses the need for templating and template removal. Laser annealing carbon is potentially of enormous technological importance as synthesis not possible via traditional annealing can be used in the development of novel materials like SCG. The pores in SCG provide access to the graphite gallery (edges), whereas templated carbons align normally to the template and thus block edge access.