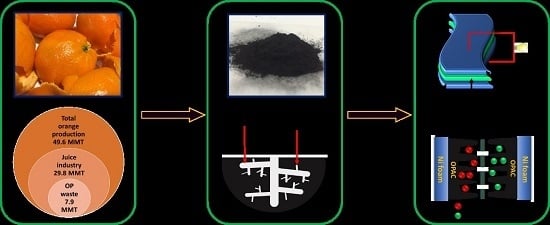
Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes
Abstract
:1. Introduction
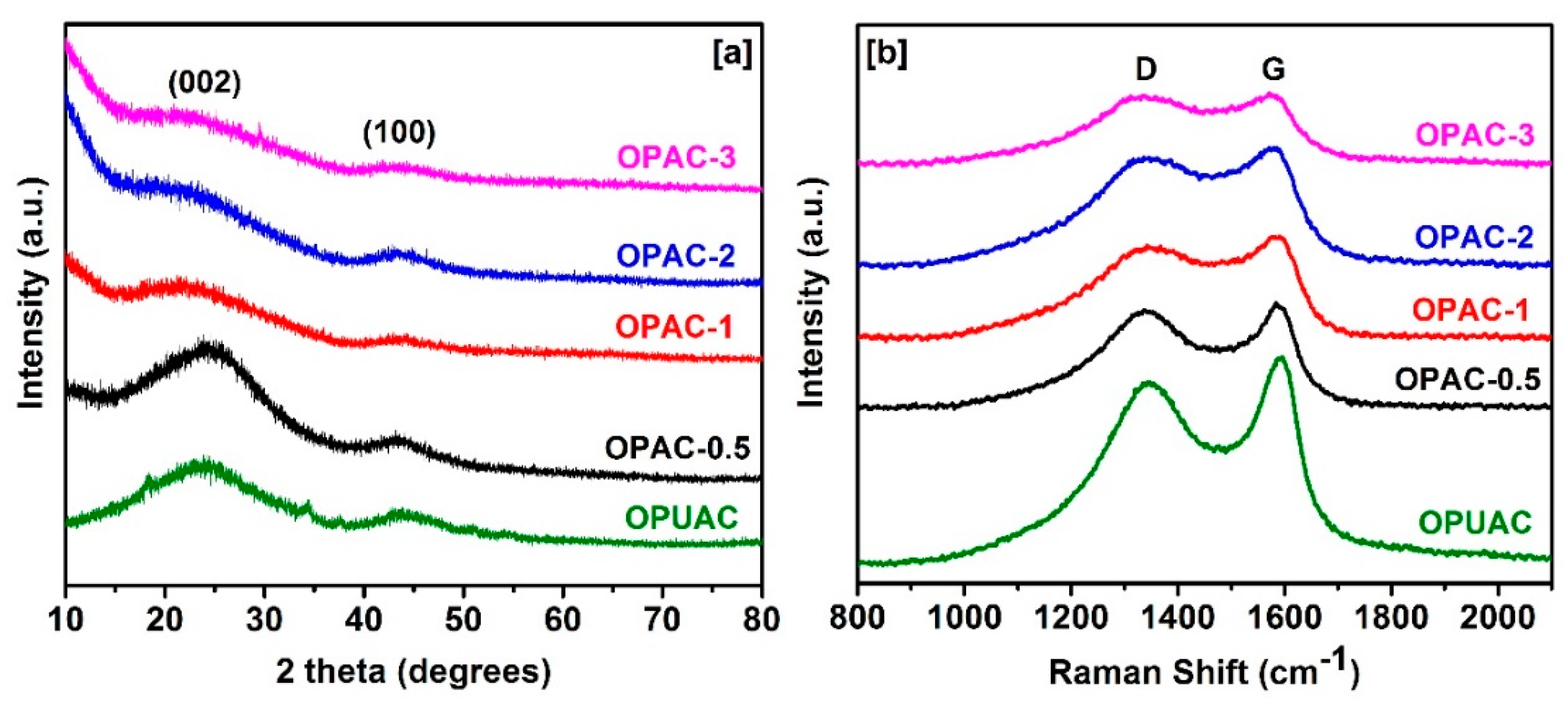
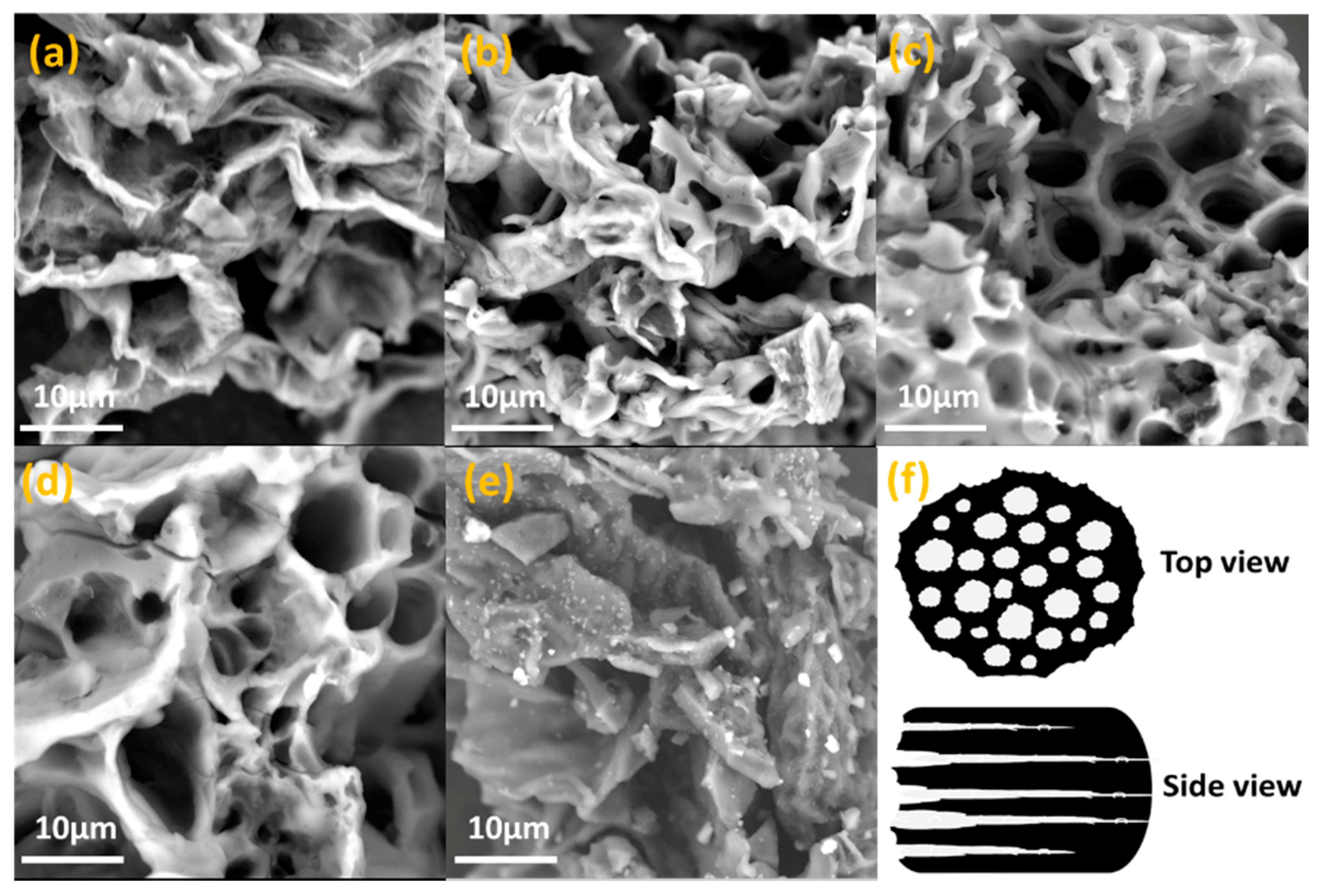
2. Results and Discussion
3. Experimental
3.1. Preparation of Orange-Peel-Derived Porous Carbon
3.2. Structural Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beidaghi, M.; Gogotsi, Y.; Wang, Z.L.; Akyildiz, I.F.; Su, W.; Sankarasubramaniam, Y.; Cayirci, E.; Wang, Z.L.; Wu, W.; Wang, Z.L.; et al. Capacitive Energy Storage in Micro-Scale Devices: Recent Advances in Design and Fabrication of Micro-Supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the Molecular Origin of Supercapacitance in Nanoporous Carbon Electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Dyatkin, B.; Presser, V.; Heon, M.; Lukatskaya, M.R.; Beidaghi, M.; Gogotsi, Y. Development of a Green Supercapacitor Composed Entirely of Environmentally Friendly Materials. ChemSusChem 2013, 6, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhou, W.; Chen, J.; Feng, G.; Li, H.; Ma, W.; Li, J.; Dong, H.; Ren, Y.; Zhao, D.; et al. Compact-Designed Supercapacitors Using Free-Standing Single-Walled Carbon Nanotube Films. Energy Environ. Sci. 2011, 4, 1440–1446. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, R.; Cao, G.; Chen, S.; Yang, Y. Mesoporous Activated Carbon Fiber as Electrode Material for High-Performance Electrochemical Double Layer Capacitors with Ionic Liquid Electrolyte. J. Power Sources 2010, 195, 2118–2124. [Google Scholar] [CrossRef]
- Dai, Y.; Jiang, H.; Hu, Y.; Fu, Y.; Li, C. Controlled Synthesis of Ultrathin Hollow Mesoporous Carbon Nanospheres for Supercapacitor Applications. Ind. Eng. Chem. Res. 2014, 53, 3125–3130. [Google Scholar] [CrossRef]
- Zequine, C.; Ranaweera, C.K.; Wang, Z.; Singh, S.; Tripathi, P.; Srivastava, O.N.; Gupta, B.K.; Ramasamy, K.; Kahol, P.K.; Dvornic, P.R.; et al. High per Formance and Flexible Supercapacitors Based on Carbonized Bamboo Fibers for Wide Temperature Applications. Sci. Rep. 2016, 6, 31740. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.H.; Xu, Y.Y.; Lu, A.H.; Zhang, X.Q.; Li, W.C. Converting Biowaste Corncob Residue into High Value Added Porous Carbon for Supercapacitor Electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Puthusseri, D.; Phase, D.; Ogale, S. Enhanced Capacitance Retention in a Supercapacitor Made of Carbon from Sugarcane Bagasse by Hydrothermal Pretreatment. Energy Fuels 2014, 28, 4233–4240. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising Activated Carbons Derived from Waste Tea-Leaves and Their Application in High Performance Supercapacitors Electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Ma, X.; Idrees, F.; Xu, B.; Hao, X.; Lin, W. From Rice Bran to High Energy Density Supercapacitors: A New Route to Control Porous Structure of 3D Carbon. Sci. Rep. 2014, 4, 7260. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S.; Wang, G.; Zhang, L.; Zhang, J.; Frackowiaka, E.; Béguin, F.; Dai, L.; et al. From Dead Leaves to High Energy Density Supercapacitors. Energy Environ. Sci. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A Self-Template Synthesis of Hierarchical Porous Carbon Foams Based on Banana Peel for Supercapacitor Electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Hu, C.-C.; Wang, C.-C. Effects of Pore Structure and Electrolyte on the Capacitive Characteristics of Steam- and KOH-Activated Carbons for Supercapacitors. J. Power Sources 2005, 144, 302–309. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W.H.; McKay, G. Preparation of Activated Carbon Using Low Temperature Carbonisation and Physical Activation of High Ash Raw Bagasse for Acid Dye Adsorption. Chemosphere 2004, 56, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, H.; Tao, N.; Liu, Y.; Qi, J.; Wang, Z.; Xu, H. Adsorption of Malachite Green and Iodine on Rice Husk-Based Porous Carbon. Mater. Chem. Phys. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, J.; Jiang, Y.; Yang, S.; Wang, Z.; Xu, H. Performance of Electrical Double Layer Capacitors with Porous Carbons Derived from Rice Husk. Mater. Chem. Phys. 2003, 80, 704–709. [Google Scholar] [CrossRef]
- Girgis, B.S.; El-Hendawy, A.-N.A. Porosity Development in Activated Carbons Obtained from Date Pits under Chemical Activation with Phosphoric Acid. Microporous Mesoporous Mater. 2002, 52, 105–117. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of Activated Carbon by Chemical Activation with ZnCl2. Carbon N. Y. 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Maciá-Agulló, J.A.; Moore, B.C.; Cazorla-Amorós, D.; Linares-Solano, A. Activation of Coal Tar Pitch Carbon Fibres: Physical Activation vs. Chemical Activation. Carbon N. Y. 2004, 42, 1367–1370. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Leroux, F.; Béguin, F. A High-Performance Carbon for Supercapacitors Obtained by Carbonization of a Seaweed Biopolymer. Adv. Mater. 2006, 18, 1877–1882. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M.; Choi, N.-S.; Frackowiak, E.; Beguin, F.; Beguin, F.; Presser, V.; Balducci, A.; Frackowiak, E.; et al. From Soybean Residue to Advanced Supercapacitors. Sci. Rep. 2015, 5, 16618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and Sulfur Co-Doped Porous Carbon Nanosheets Derived from Willow Catkin for Supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Foreign Agricultural Service/USDA. Citrus: World Markets and Trade; USDA: Washington, DC, USA, 2017.
- Grohmann, K.; Baldwin, E.A. Hydrolysis of Orange Peel with Pectinase and Cellulase Enzymes. Biotechnol. Lett. 1992, 14, 1169–1174. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment Effects on Orange Processing Waste for Making Ethanol by Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of Heavy Metals from Aqueous Solutions by Chemically Modified Orange Peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.; El Nemr, A.; El-Sikaily, A.; Abdelwahab, O. Removal of Direct N Blue-106 from Artificial Textile Dye Effluent Using Activated Carbon from Orange Peel: Adsorption Isotherm and Kinetic Studies. J. Hazard. Mater. 2009, 165, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of Orange Peel Waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, C.K.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.K.; Gupta, R.K. Biobased Polyols Using Thiol-Ene Chemistry for Rigid Polyurethane Foams with Enhanced Flame-Retardant Properties. J. Renew. Mater. 2017. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous Carbon Made from Rice Husk as Electrode Material for Electrochemical Double Layer Capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Cheng, P.; Li, T.; Yu, H.; Zhi, L.; Liu, Z.; Lei, Z. Biomass-Derived Carbon Fiber Aerogel as a Binder-Free Electrode for High-Rate Supercapacitors. J. Phys. Chem. C 2016, 120, 2079–2086. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Paris, O.; Zollfrank, C.; Zickler, G.A. Decomposition and Carbonisation of Wood Biopolymers—A Microstructural Study of Softwood Pyrolysis. Carbon N. Y. 2005, 43, 53–66. [Google Scholar] [CrossRef]
- Kastanaki, E.; Vamvuka, D.; Grammelis, P.; Kakaras, E. Thermogravimetric Studies of the Behavior of Lignite–biomass Blends during Devolatilization. Fuel Process. Technol. 2002, 77, 159–166. [Google Scholar] [CrossRef]
- Mi, J.; Wang, X.; Fan, R.; Qu, W.; Li, W. Coconut-Shell-Based Porous Carbons with a Tunable Micro/Mesopore Ratio for High-Performance Supercapacitors. Energy Fuels 2012, 26, 5321–5329. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Shen, P.K. Simultaneous Formation of Ultrahigh Surface Area and Three-Dimensional Hierarchical Porous Graphene-Like Networks for Fast and Highly Stable Supercapacitors. Adv. Mater. 2013, 25, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Müllen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of Capacitor’s Electrode from Cassava Peel Waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Gao, S.; Zang, P.; Yang, X.; Bai, Y.; Xu, H.; Liu, Z.; Lei, Z. Hierarchically Porous Carbon by Activation of Shiitake Mushroom for Capacitive Energy Storage. Carbon N. Y. 2015, 93, 315–324. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J.; Burke, A.; Simon, P.; Gogotsi, Y.; Naoi, K.; et al. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical Properties of Carbon from Oil Palm Kernel Shell for High Performance Supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-Inhibited 3D Reduced Graphene Oxide for High Performance Supercapacitor Electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Li, J.; Lai, Y.; Zhang, Z.; Liu, Y.; Zhang, G.; Huang, H. Polyaniline Nanowire Array Encapsulated in Titania Nanotubes as a Superior Electrode for Supercapacitors. Nanoscale 2011, 3, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gao, Z.; Wang, X.; Wu, D.; Xu, F.; Wang, X.; Guo, Y.; Jiang, K. Activated Porous Carbon Prepared from Paulownia Flower for High Performance Supercapacitor Electrodes. Electrochim. Acta 2015, 157, 290–298. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Bhise, S.C.; Patil, S.K.; Kolekar, S.S.; Chang, J.-Y.; Ghule, A.V. Comparative Study of Individual and Mixed Aqueous Electrolytes with ZnFe2O4 Nano-Flakes Thin Film as an Electrode for Supercapacitor Application. ChemistrySelect 2016, 1, 959–966. [Google Scholar] [CrossRef]
- Dubal, D.P.; Lee, S.H.; Kim, J.G.; Kim, W.B.; Lokhande, C.D. Porous Polypyrrole Clusters Prepared by Electropolymerization for a High Performance Supercapacitor. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human Hair-Derived Carbon Flakes for Electrochemical Supercapacitors. Energy Environ. Sci. 2013, 7, 379–386. [Google Scholar] [CrossRef]
- Divyashree, A.; Hegde, G. Activated Carbon Nanospheres Derived from Bio-Waste Materials for Supercapacitor Applications—A Review. RSC Adv. 2015, 5, 88339–88352. [Google Scholar]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-Based Nanostructured Materials and Their Composites as Supercapacitor Electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Lee, Y.-H.; Chang, K.-H.; Li, J.-M.; Simon, P.; Tang, J.; Torad, N.L.; Hu, C.-C.; Yamauchi, Y. Nanoarchitectured Graphene-Based Supercapacitors for Next-Generation Energy-Storage Applications. Chem. A Eur. J. 2014, 20, 13838–13852. [Google Scholar] [CrossRef] [PubMed]
- Obreja, V.V.N. On the Performance of Supercapacitors with Electrodes Based on Carbon Nanotubes and Carbon Activated material—A Review. Phys. E Low-dimens. Syst. Nanostruct. 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, Z.; Chen, M.; Wang, C. Hierarchical Porous Carbon Derived from Sulfonated Pitch for Electrical Double Layer Capacitors. J. Power Sources 2014, 252, 235–243. [Google Scholar] [CrossRef]
- Du, S.; Wang, L.; Fu, X.; Chen, M.; Wang, C. Hierarchical Porous Carbon Microspheres Derived from Porous Starch for Use in High-Rate Electrochemical Double-Layer Capacitors. Bioresour. Technol. 2013, 139, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising Porous Carbon Derived from Celtuce Leaves with Outstanding Supercapacitance and CO2 Capture Performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Hegde, G.; Abdul Manaf, S.A.; Kumar, A.; Ali, G.A.M.; Chong, K.F.; Ngaini, Z.; Sharma, K.V. Biowaste Sago Bark Based Catalyst Free Carbon Nanospheres: Waste to Wealth Approach. ACS Sustain. Chem. Eng. 2015, 3, 2247–2253. [Google Scholar] [CrossRef]
- Xie, Q.; Bao, R.; Zheng, A.; Zhang, Y.; Wu, S.; Xie, C.; Zhao, P. Sustainable Low-Cost Green Electrodes with High Volumetric Capacitance for Aqueous Symmetric Supercapacitors with High Energy Density. ACS Sustain. Chem. Eng. 2016, 4, 1422–1430. [Google Scholar] [CrossRef]
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-Based Activated Carbon for Supercapacitor Applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High Surface Area Activated Carbon from Rice Husk as a High Performance Supercapacitor Electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Du, X.; Zhao, W.; Wang, Y.; Wang, C.; Chen, M.; Qi, T.; Hua, C.; Ma, M. Preparation of Activated Carbon Hollow Fibers from Ramie at Low Temperature for Electric Double-Layer Capacitor Applications. Bioresour. Technol. 2013, 149, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, L.; Sun, K.; Jiang, J.; Zhang, X. Preparation of Activated Carbon from Waste Camellia Oleifera Shell for Supercapacitor Application. J. Solid State Electrochem. 2012, 16, 2179–2186. [Google Scholar] [CrossRef]
- Yang, Y.; Ruan, G.; Xiang, C.; Wang, G.; Tour, J.M. Flexible Three-Dimensional Nanoporous Metal-Based Energy Devices. J. Am. Chem. Soc. 2014, 136, 6187–6190. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, G.; Liu, X.; Liu, F.; Xie, Y.; Yang, C.; Lin, T.; Gu, H.; Huang, F. Niobium Nitride Nb4N5 as a New High-Performance Electrode Material for Supercapacitors. Adv. Sci. 2015, 2, 1500126. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, W.; Pei, S.; Gong, K.; Wang, L.; Meng, L.; Huang, Y.; Smith, J.P.; Booksh, K.S.; Li, Q.; et al. Omnidirectionally Stretchable High-Performance Supercapacitor Based on Isotropic Buckled Carbon Nanotube Films. ACS Nano 2016, 10, 5204–5211. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; Chen, J.; Li, X.; Walsh, F.C.; Ouyang, J.-H.; Jia, D.; Zhou, Y. Three-Dimensional Graphene Oxide/polypyrrole Composite Electrodes Fabricated by One-Step Electrodeposition for High Performance Supercapacitors. J. Mater. Chem. A 2015, 3, 14445–14457. [Google Scholar] [CrossRef]
- Liu, N.; Ma, W.; Tao, J.; Zhang, X.; Su, J.; Li, L.; Yang, C.; Gao, Y.; Golberg, D.; Bando, Y. Cable-Type Supercapacitors of Three-Dimensional Cotton Thread Based Multi-Grade Nanostructures for Wearable Energy Storage. Adv. Mater. 2013, 25, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Kim, H.; Ghosh, A.; Kim, J.; Chang, J.; Vu, Q.A.; Pham, D.T.; Lee, J.-H.; Kim, S.-W.; Lee, Y.H. Coaxial Fiber Supercapacitor Using All-Carbon Material Electrodes. ACS Nano 2013, 7, 5940–5947. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Su, Y.; Wang, D.-W.; Li, F.; Du, J.; Cheng, H.-M. Graphene-Cellulose Paper Flexible Supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Masarapu, C.; Zeng, H.F.; Hung, K.H.; Wei, B. Effect of Temperature on the Capacitance of Carbon Nanotube Supercapacitors. ACS Nano 2009, 3, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
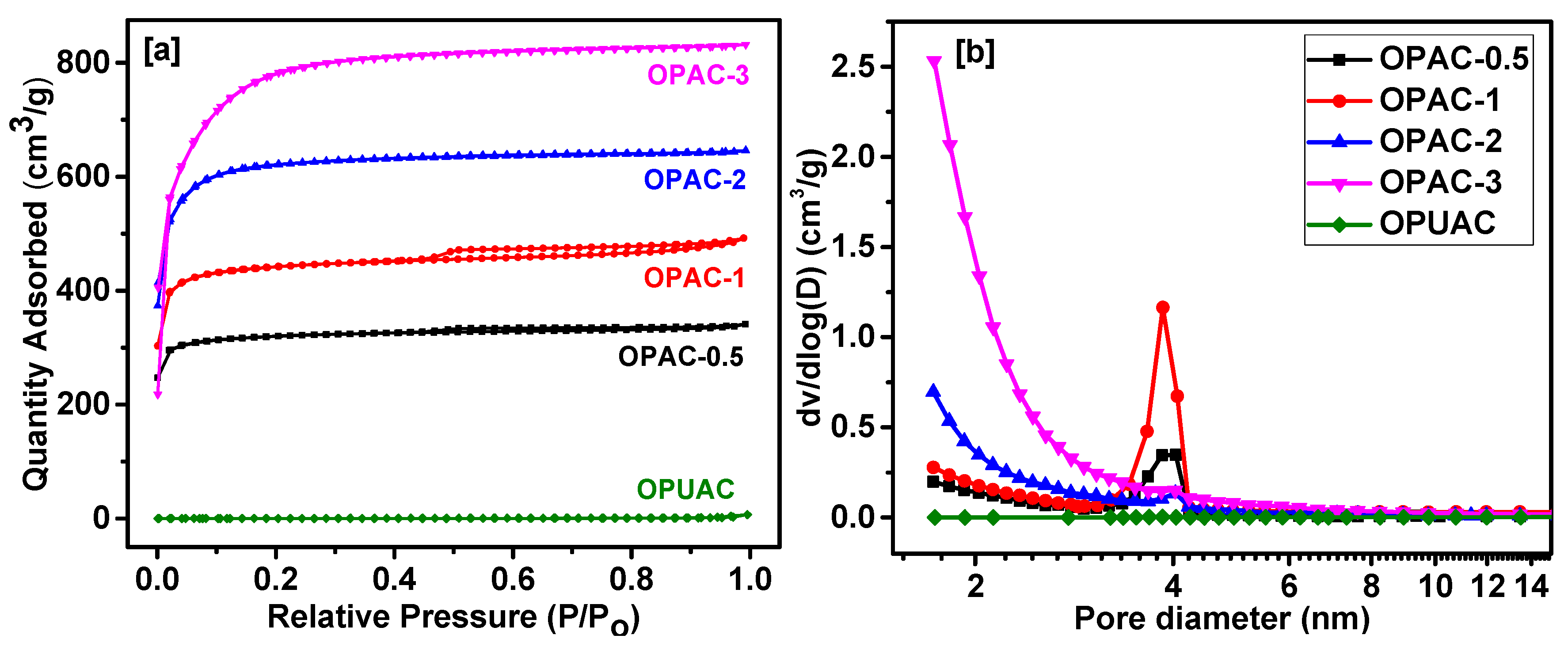
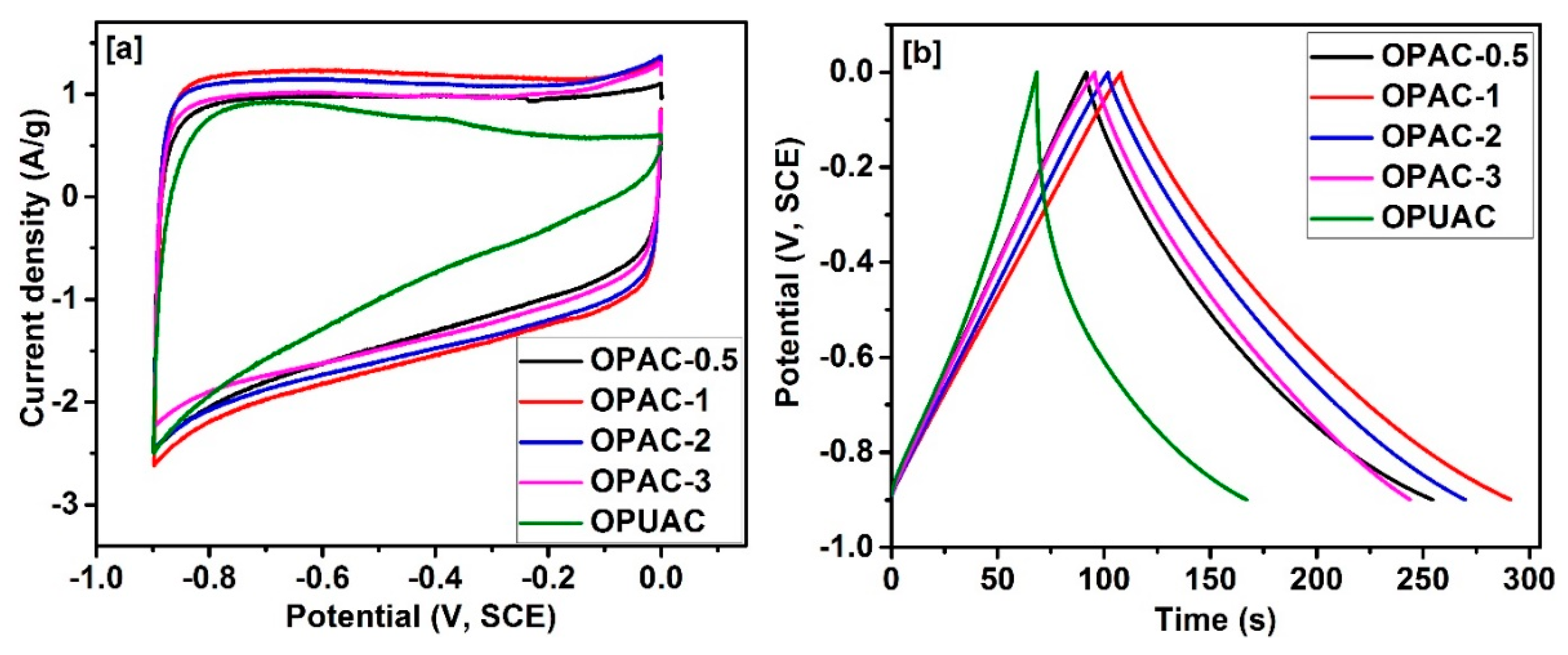
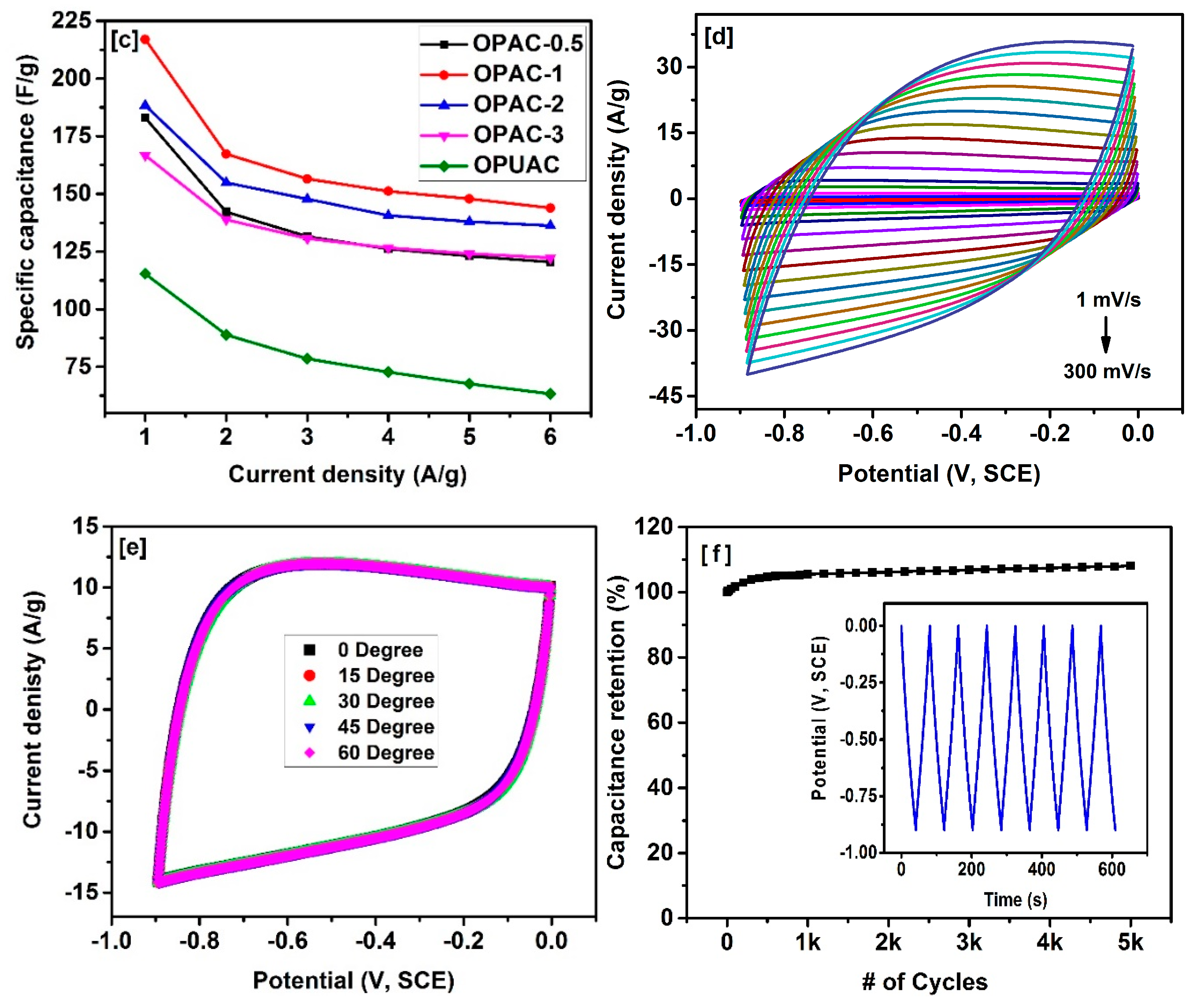
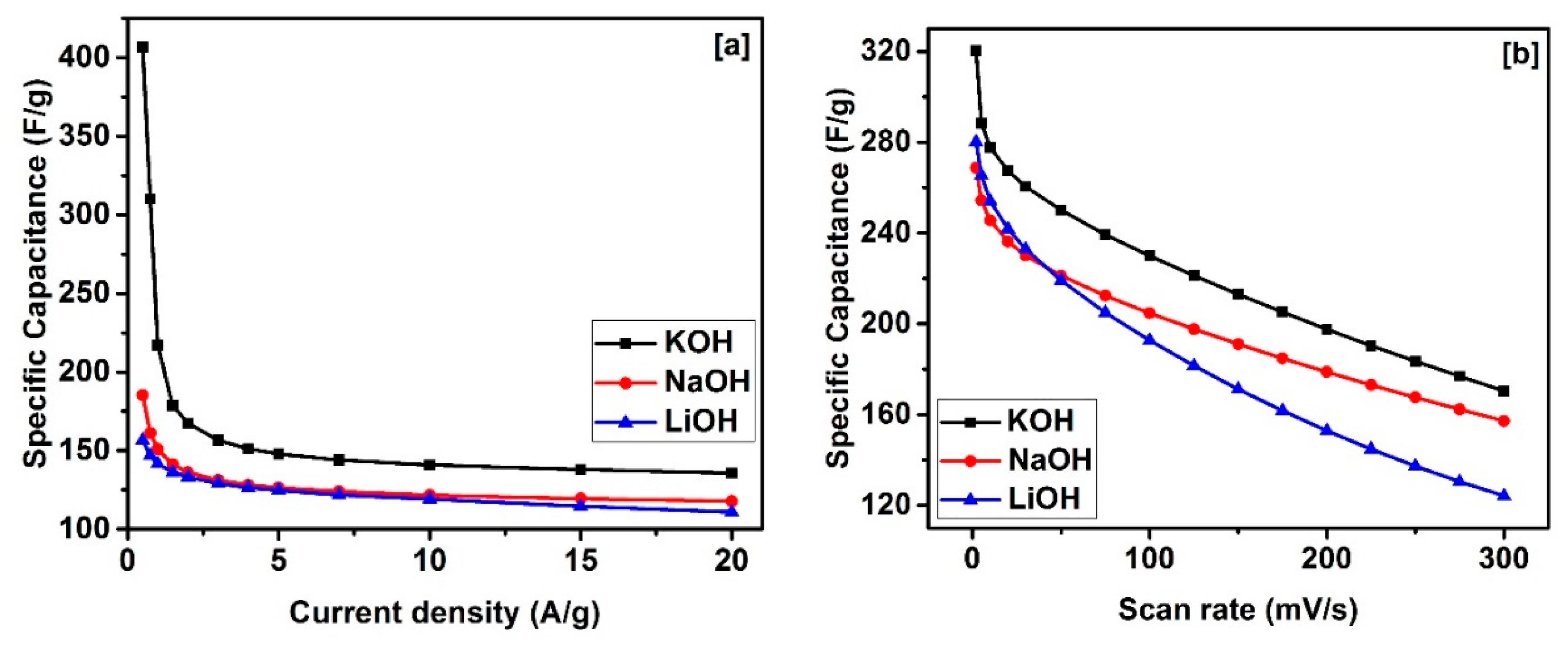
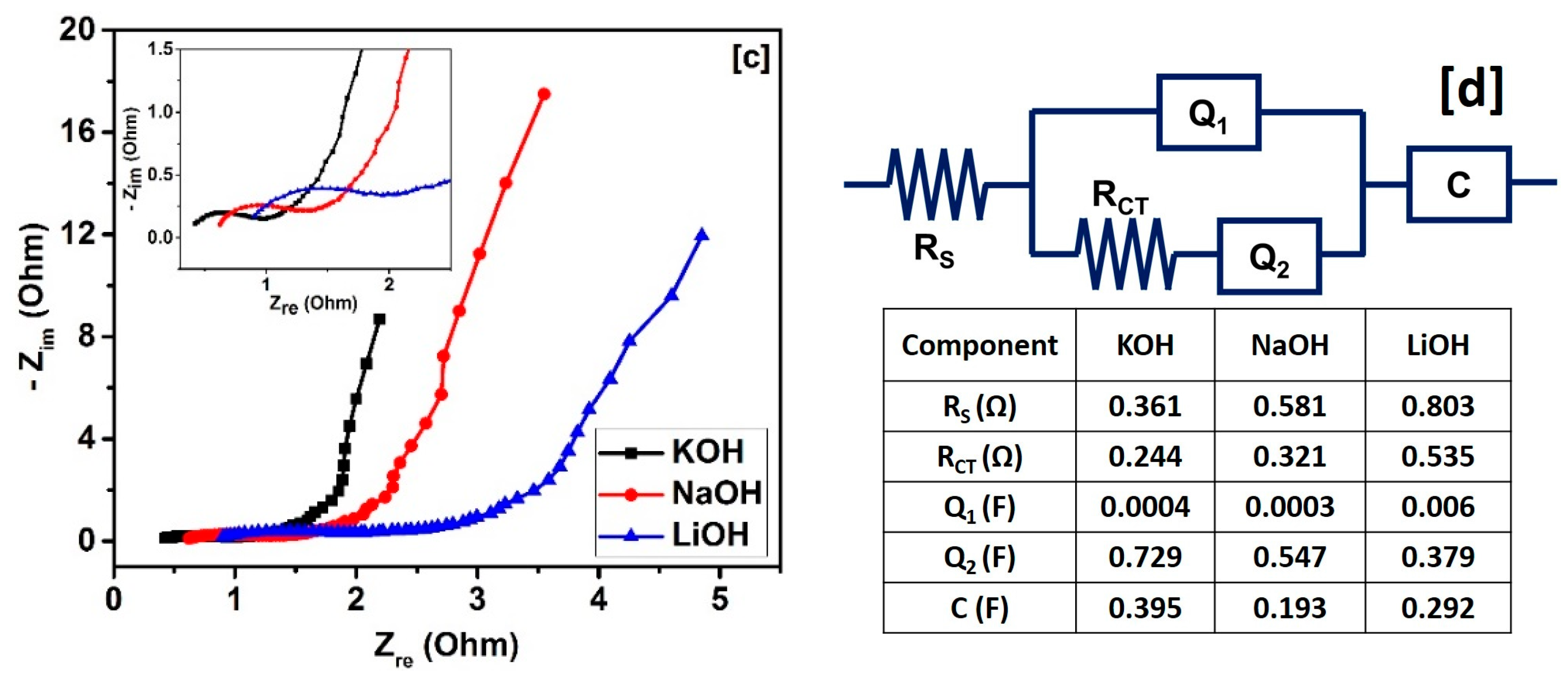
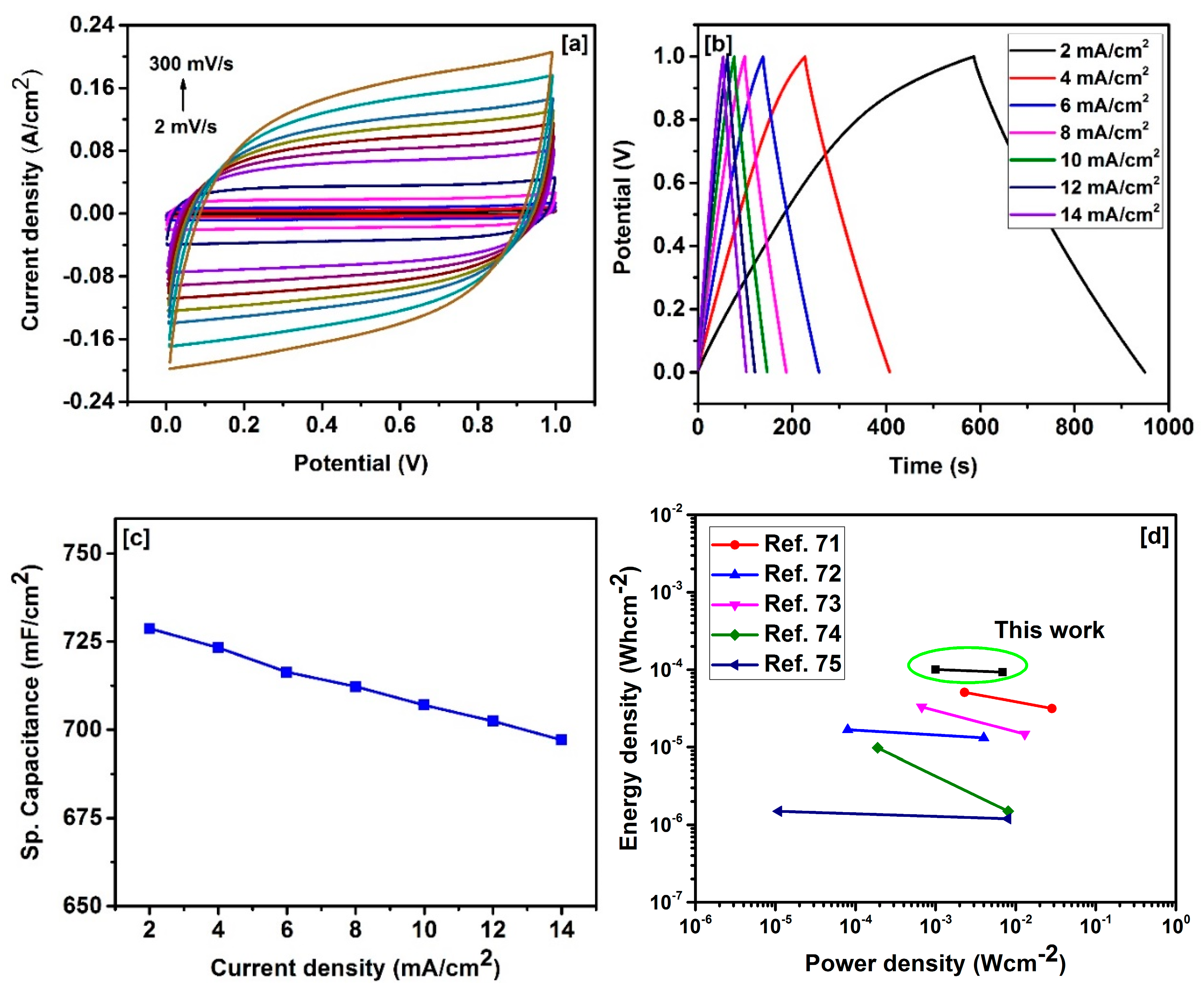
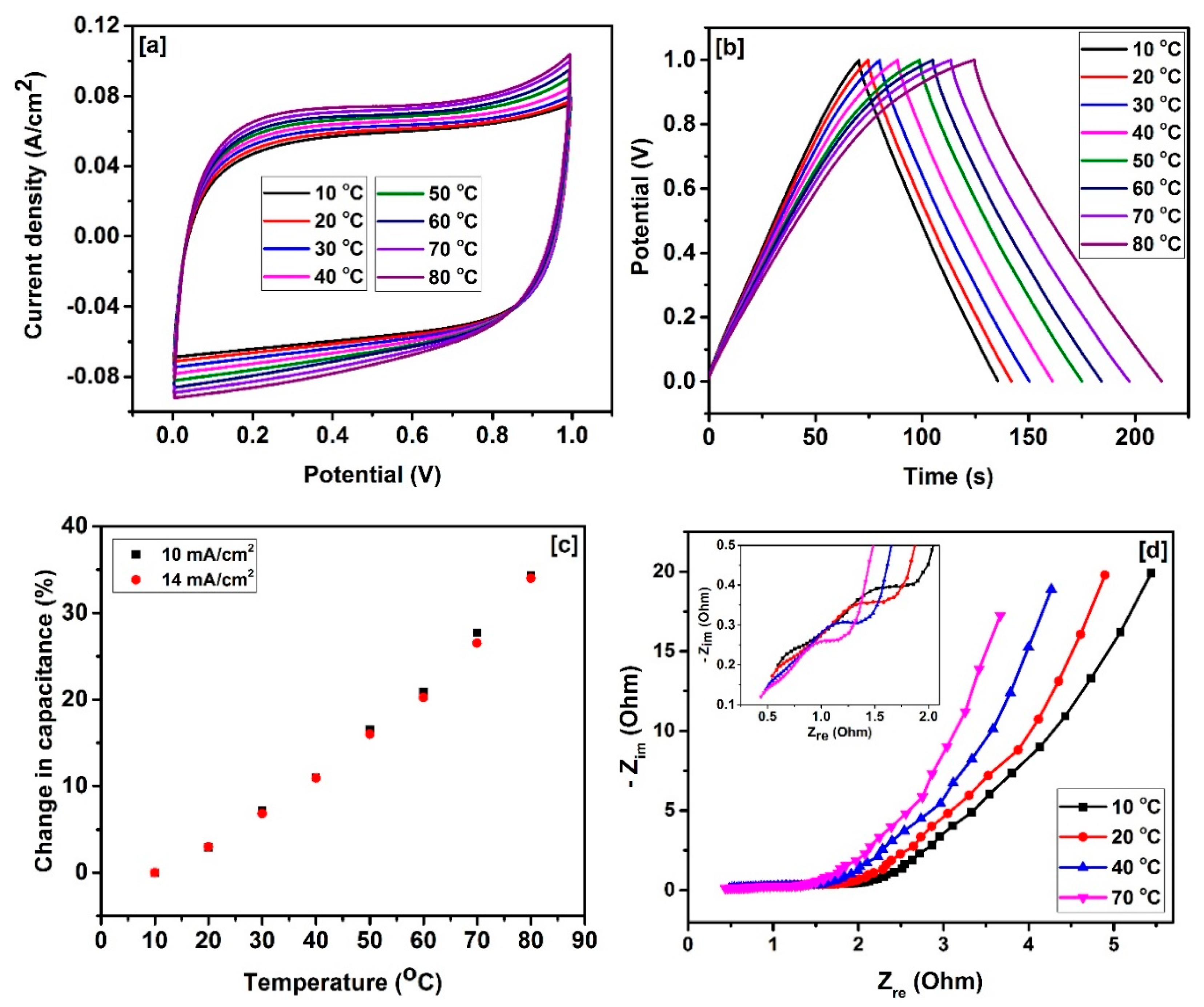
| Sample | SBET a (m2/g) | Vtotal b (cm3/g) | Dave c (nm) |
|---|---|---|---|
| OPAC-0.5 | 1004 | 0.52 | 1.69 |
| OPAC-1 | 1391 | 0.72 | 1.59 |
| OPAC-2 | 1960 | 1.01 | 1.45 |
| OPAC-3 | 2521 | 1.30 | 1.04 |
| OPUAC | 0.852 | 0.0004 | 1.16 |
| Carbon Source | BET Surface Area (m2/g) | Specific Capacitance (F/g) | Current Density (A/g) | Electrolyte | Reference |
|---|---|---|---|---|---|
| Pitch | 2602 | 263 | 0.05 | 6 M KOH | [59] |
| Porous starch | 3251 | 304 | 0.05 | 6 M KOH | [60] |
| Celtuce leaves | 3404 | 421 | 0.5 | 2 M KOH | [61] |
| Sago bark | 58 | 113 | 0.02 | 5 M KOH | [62] |
| Corn straw | 1413 | 379 | 0.05 | 6 M KOH | [63] |
| Bamboo | 3061 | 258 | 0.1 | 6 M KOH | [64] |
| Oil palm kernel shell | 462 | 210 | 0.5 | 1 M KOH | [48] |
| Rice husk | 2696 | 147 | 0.1 | 6 M KOH | [65] |
| Ramie | 1616 | 287 | 0.05 | 6 M KOH | [66] |
| Camellia oleifera shell | 1935 | 266 | 0.2 | 6 M KOH | [67] |
| Soybean residue | 1950 | 261 | 0.2 | 1 M H2SO4 | [25] |
| Neem dead leaves | 1230 | 400 | 0.5 | 1 M H2SO4 | [14] |
| Orange peel | 1391 | 407 | 0.5 | 3 M KOH | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. C 2017, 3, 25. https://doi.org/10.3390/c3030025
Ranaweera CK, Kahol PK, Ghimire M, Mishra SR, Gupta RK. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. C. 2017; 3(3):25. https://doi.org/10.3390/c3030025
Chicago/Turabian StyleRanaweera, C. K., P. K. Kahol, M. Ghimire, S. R. Mishra, and Ram K. Gupta. 2017. "Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes" C 3, no. 3: 25. https://doi.org/10.3390/c3030025