Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide Suspensions
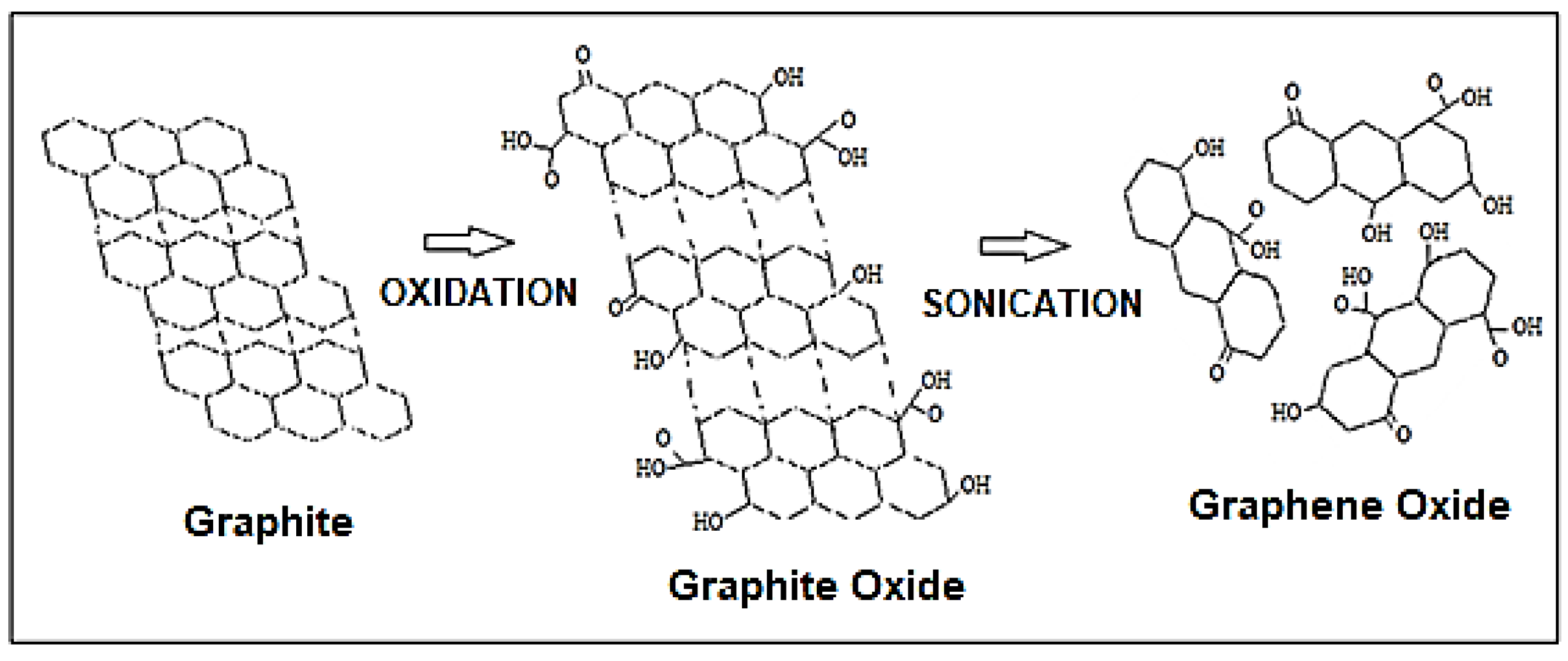
2.3. Characterization of Graphite, Graphite Oxide (GrO) and Graphene Oxide (GO)
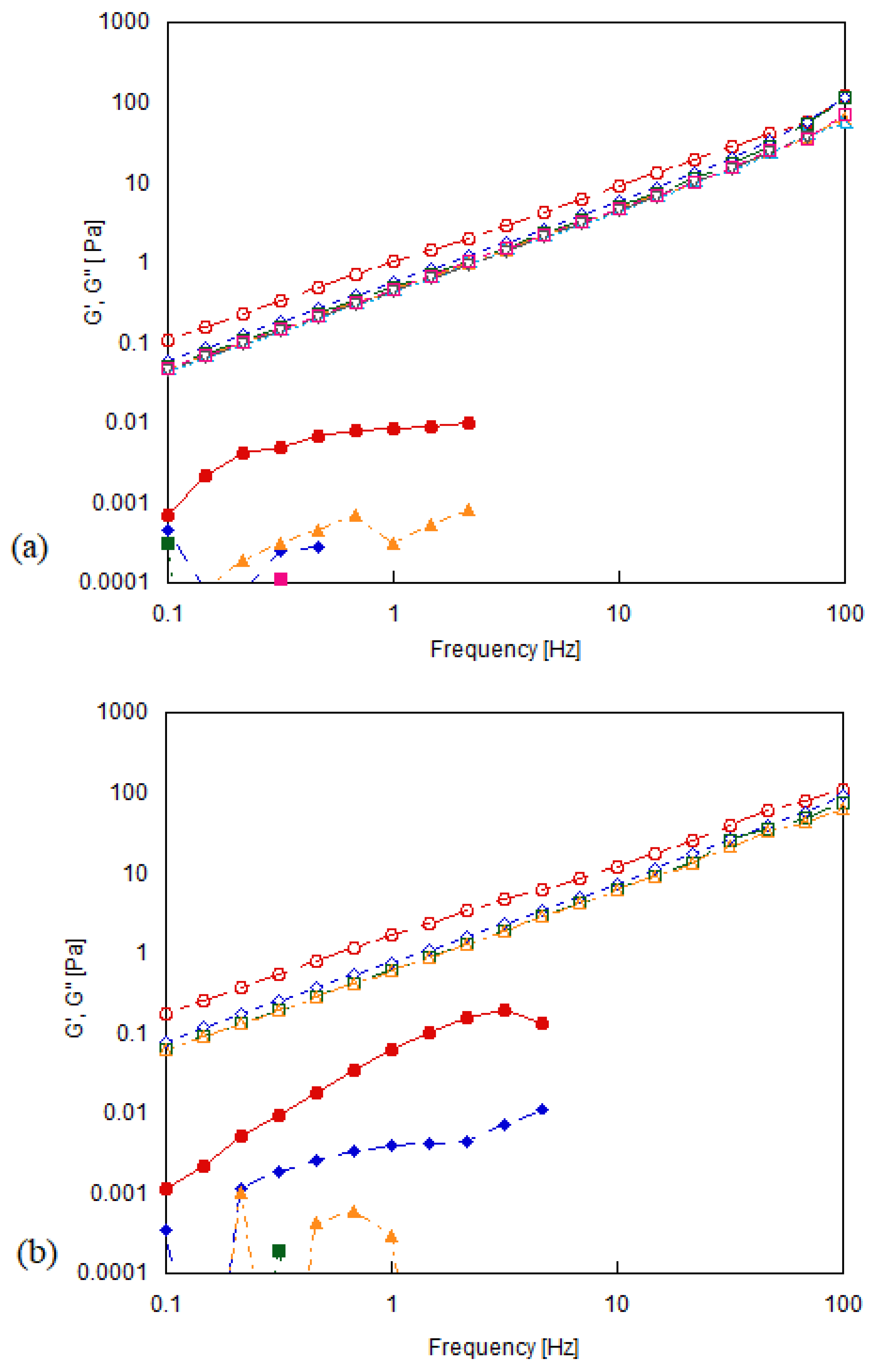
2.4. Rheological Measurements
3. Results and Discussion
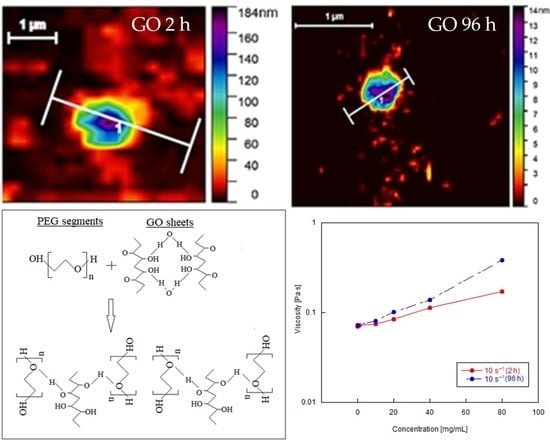
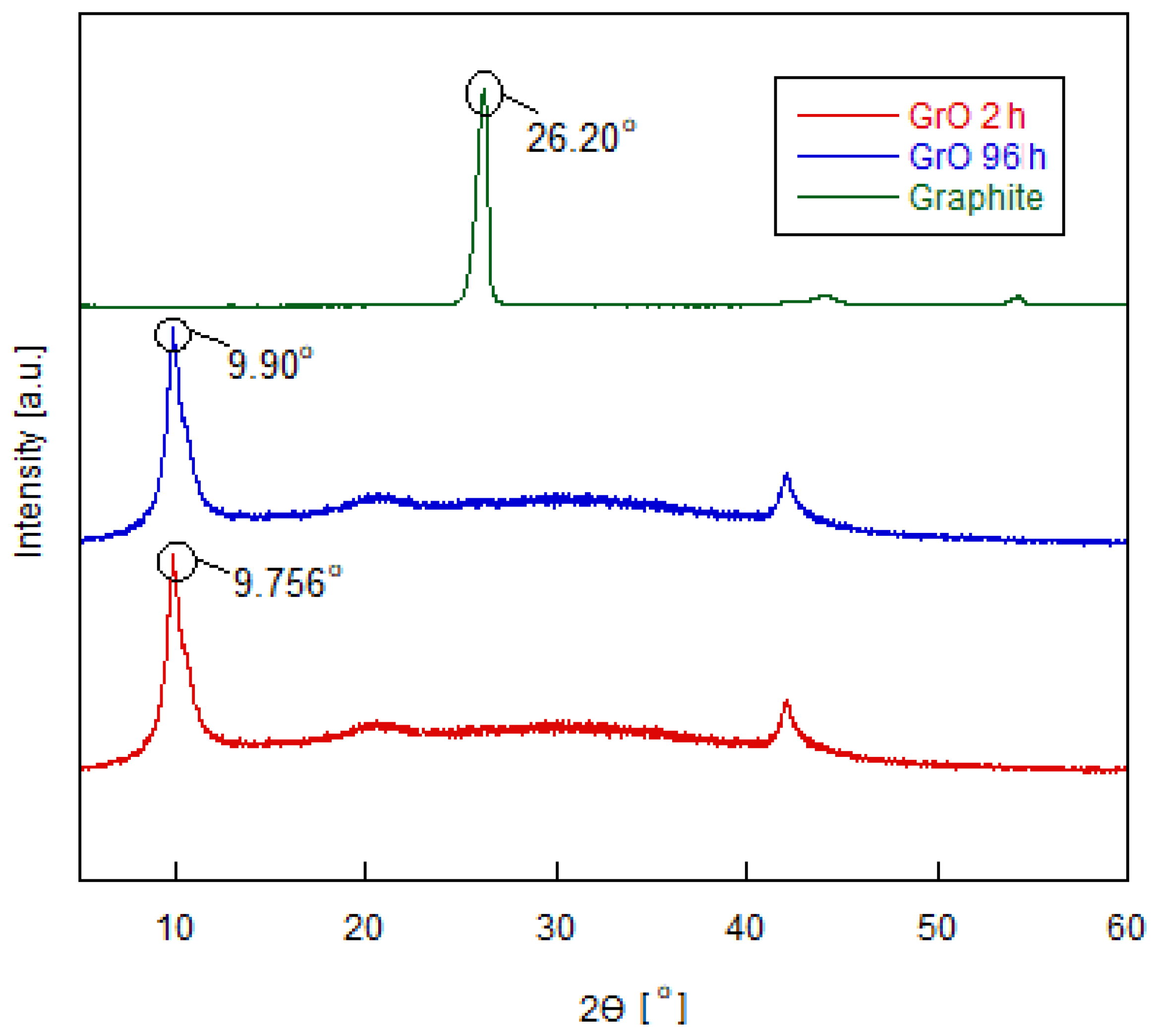
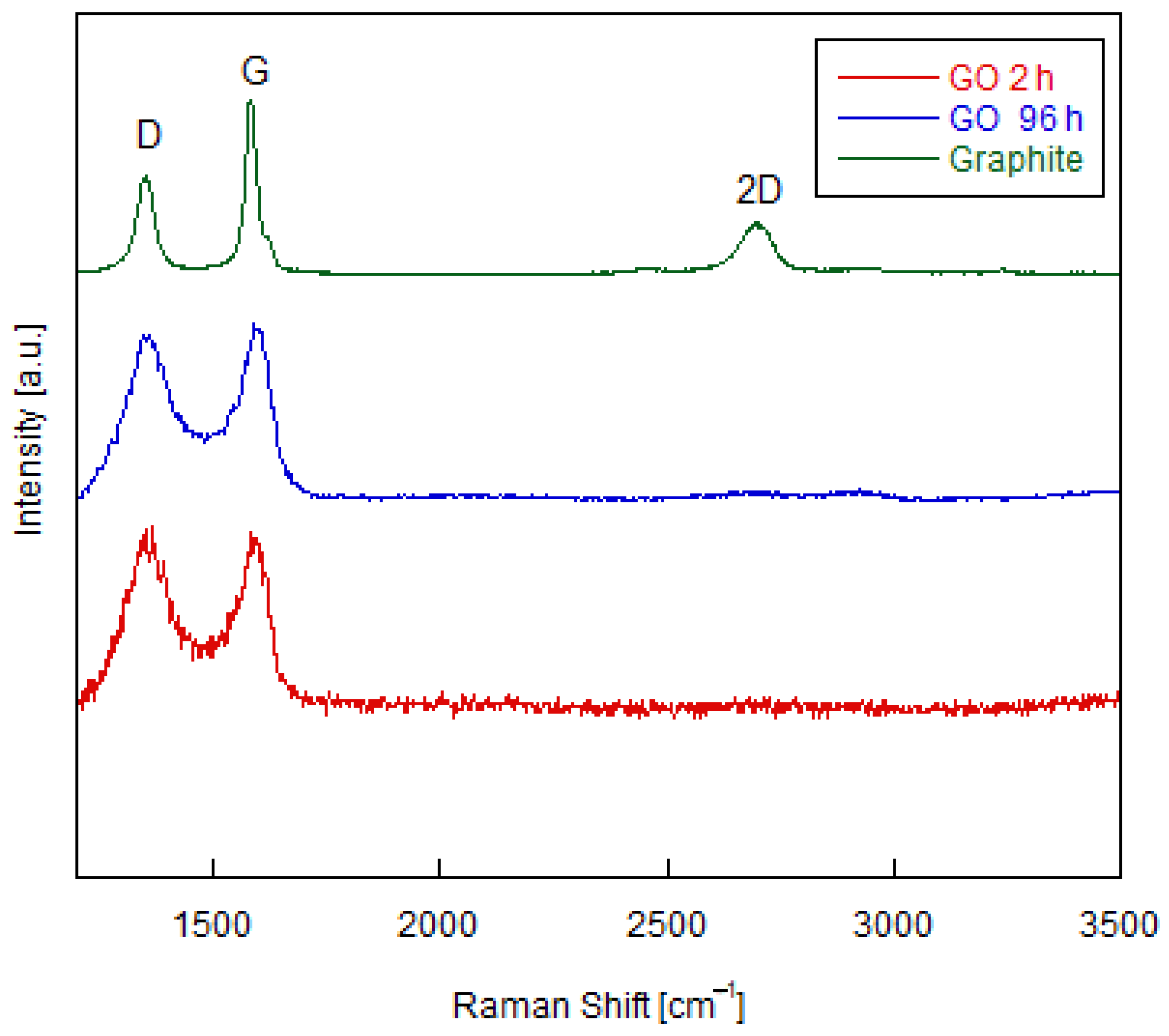
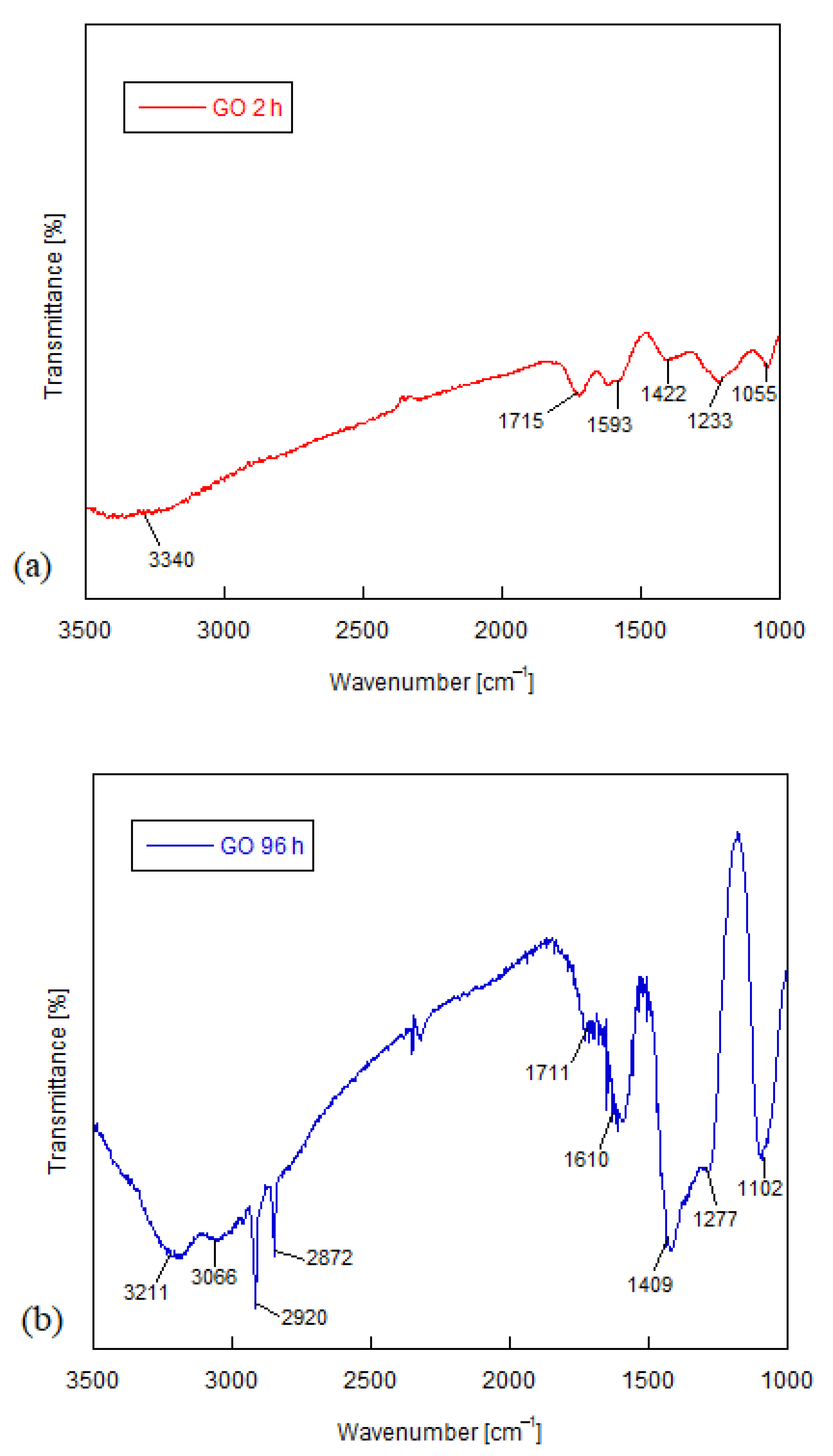
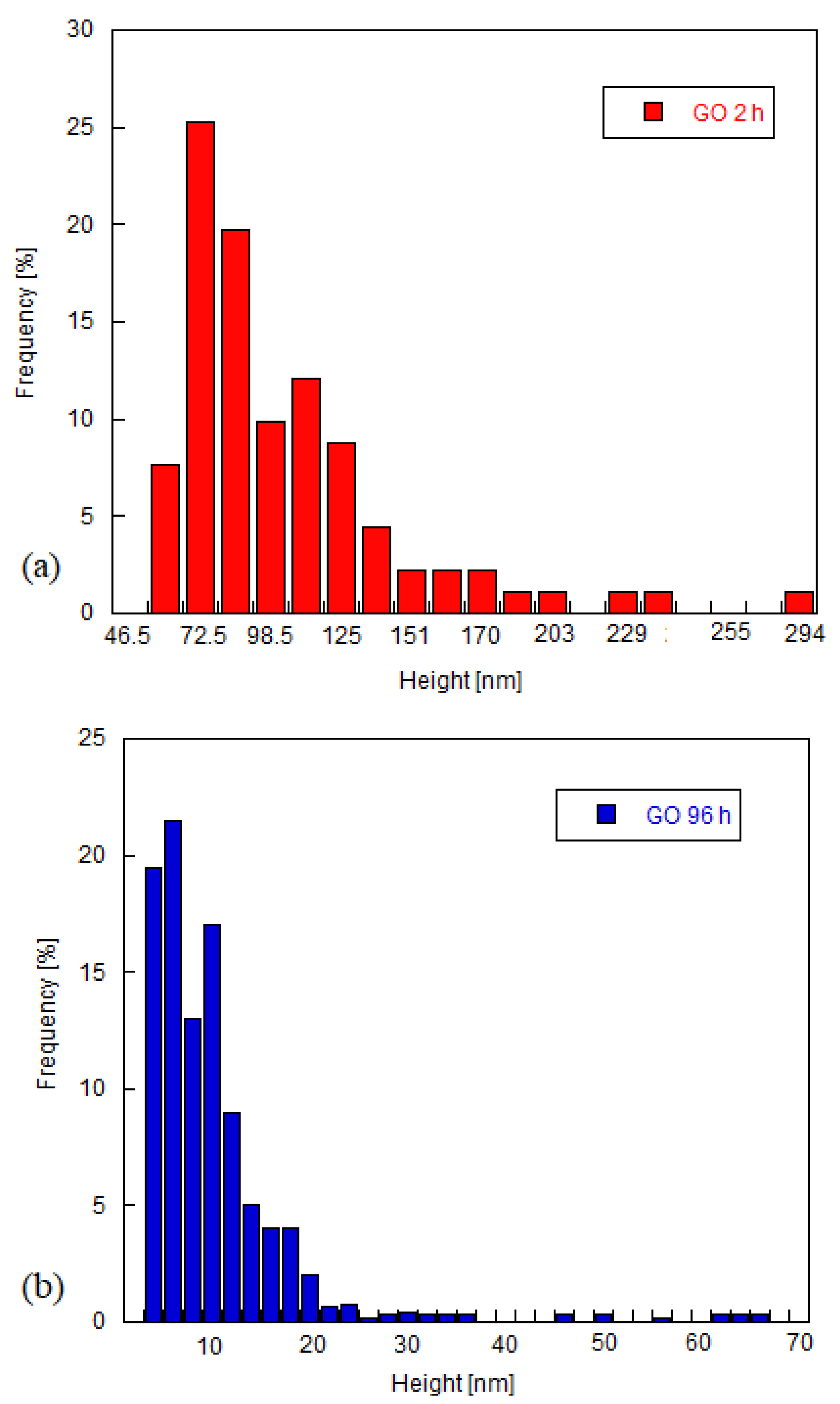
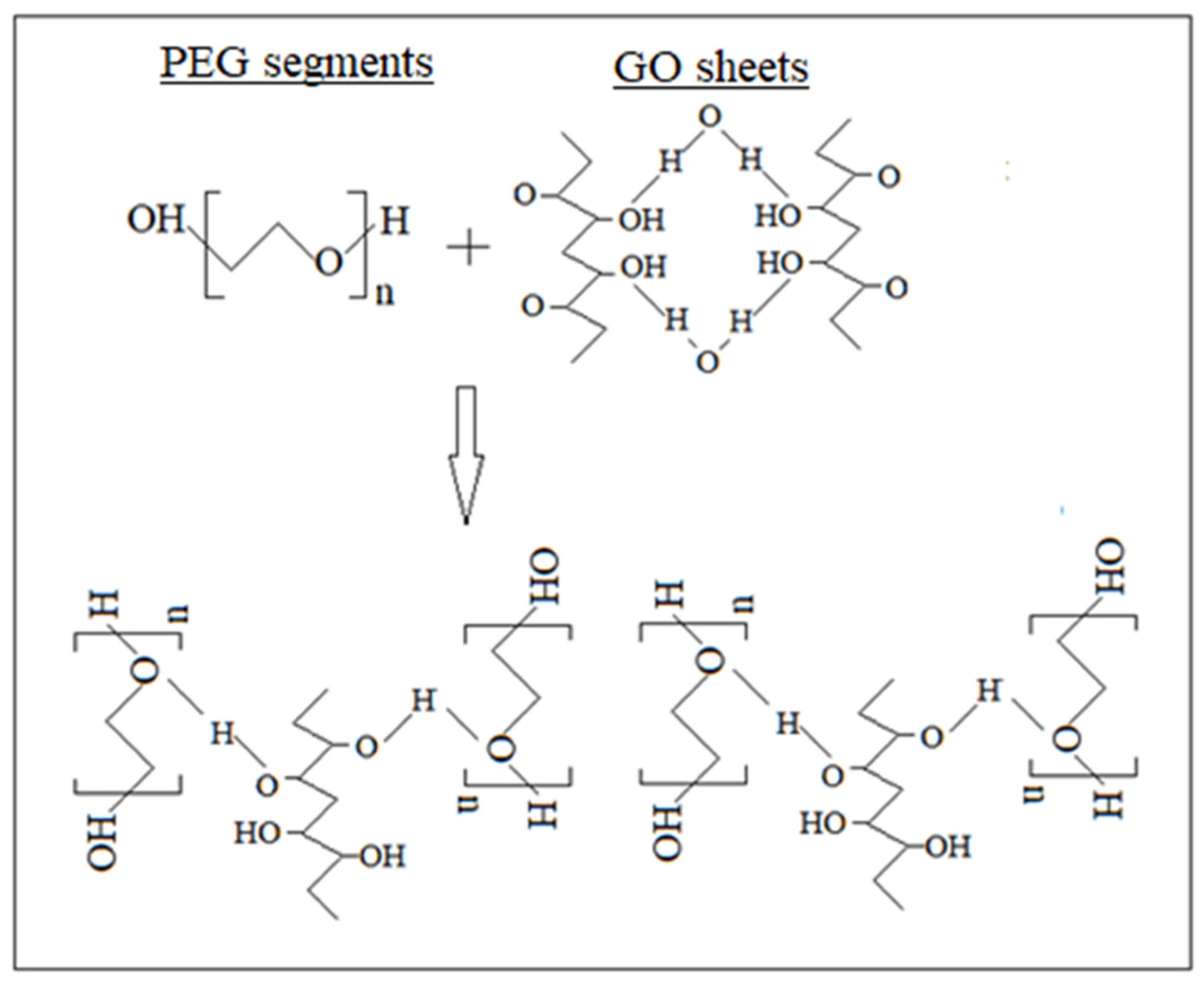
3.1. Structural Characterization of Graphene Oxide
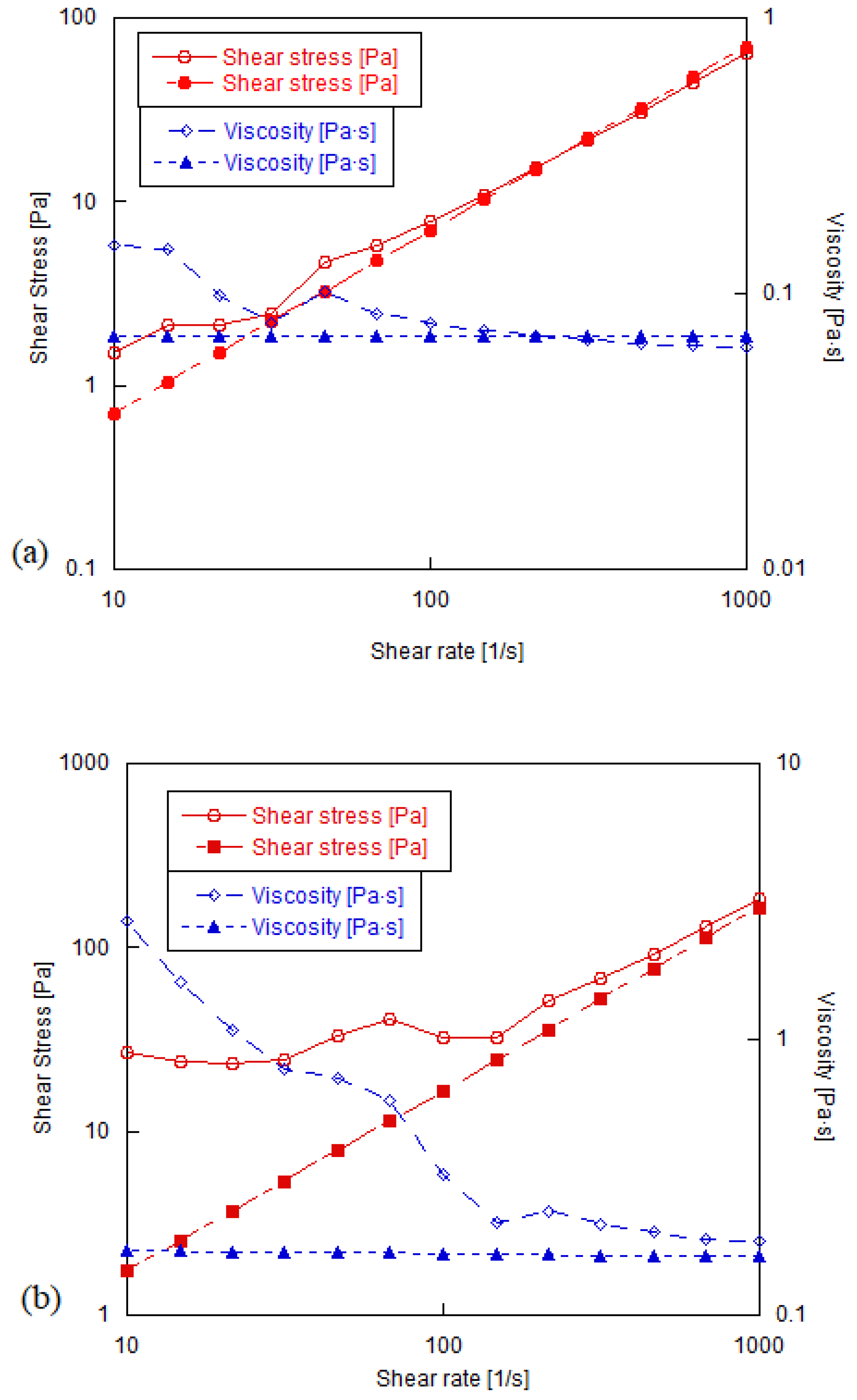
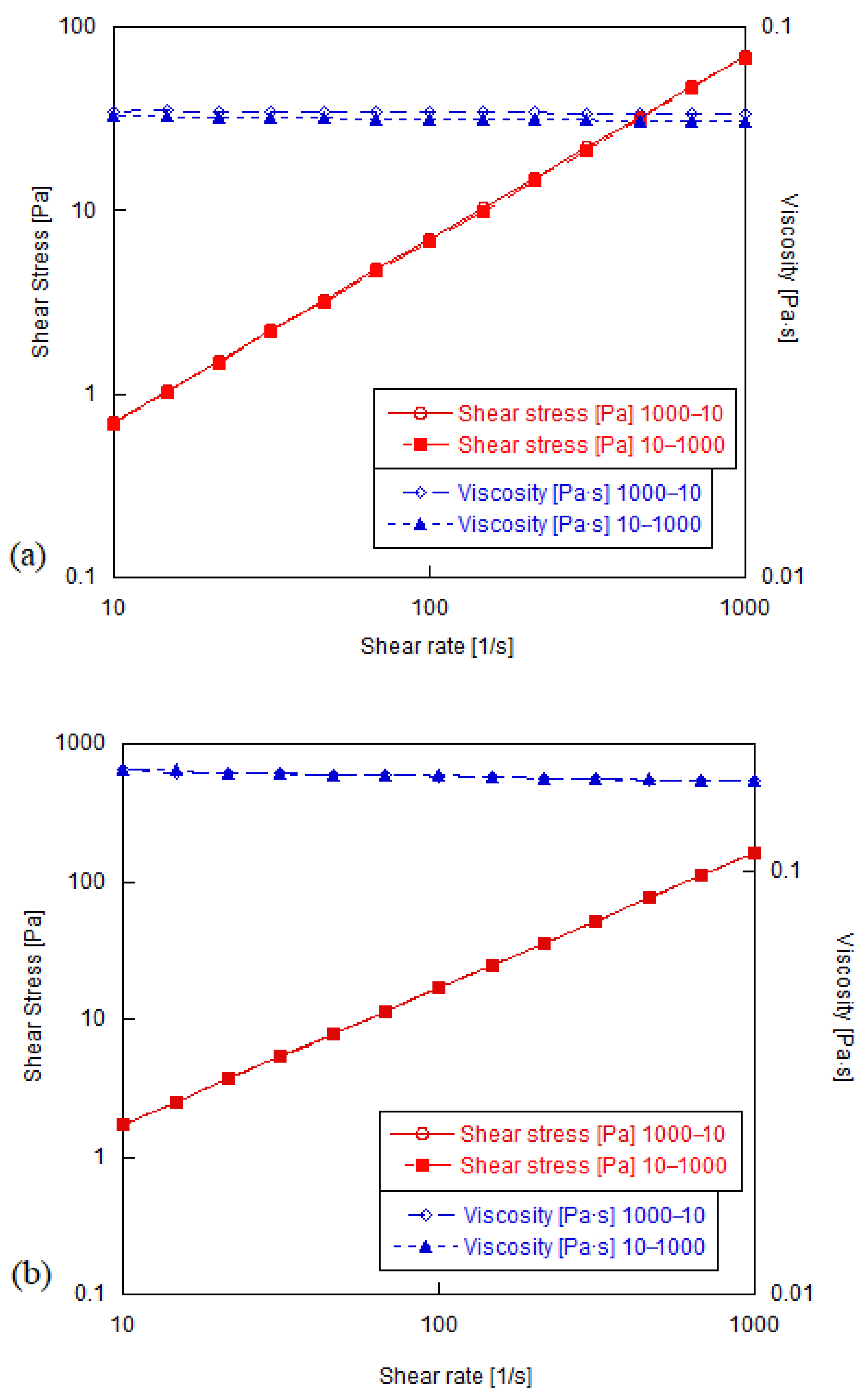
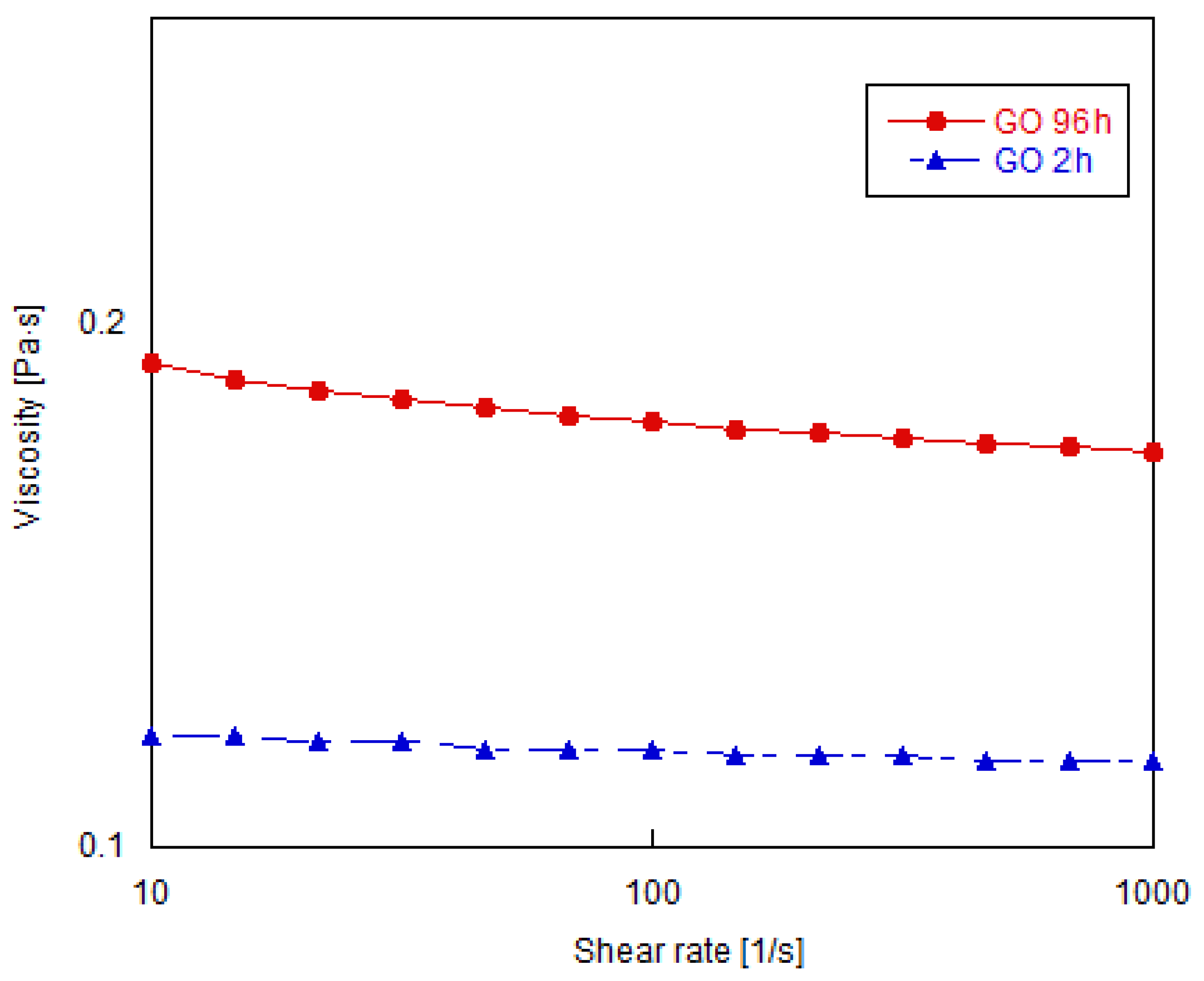
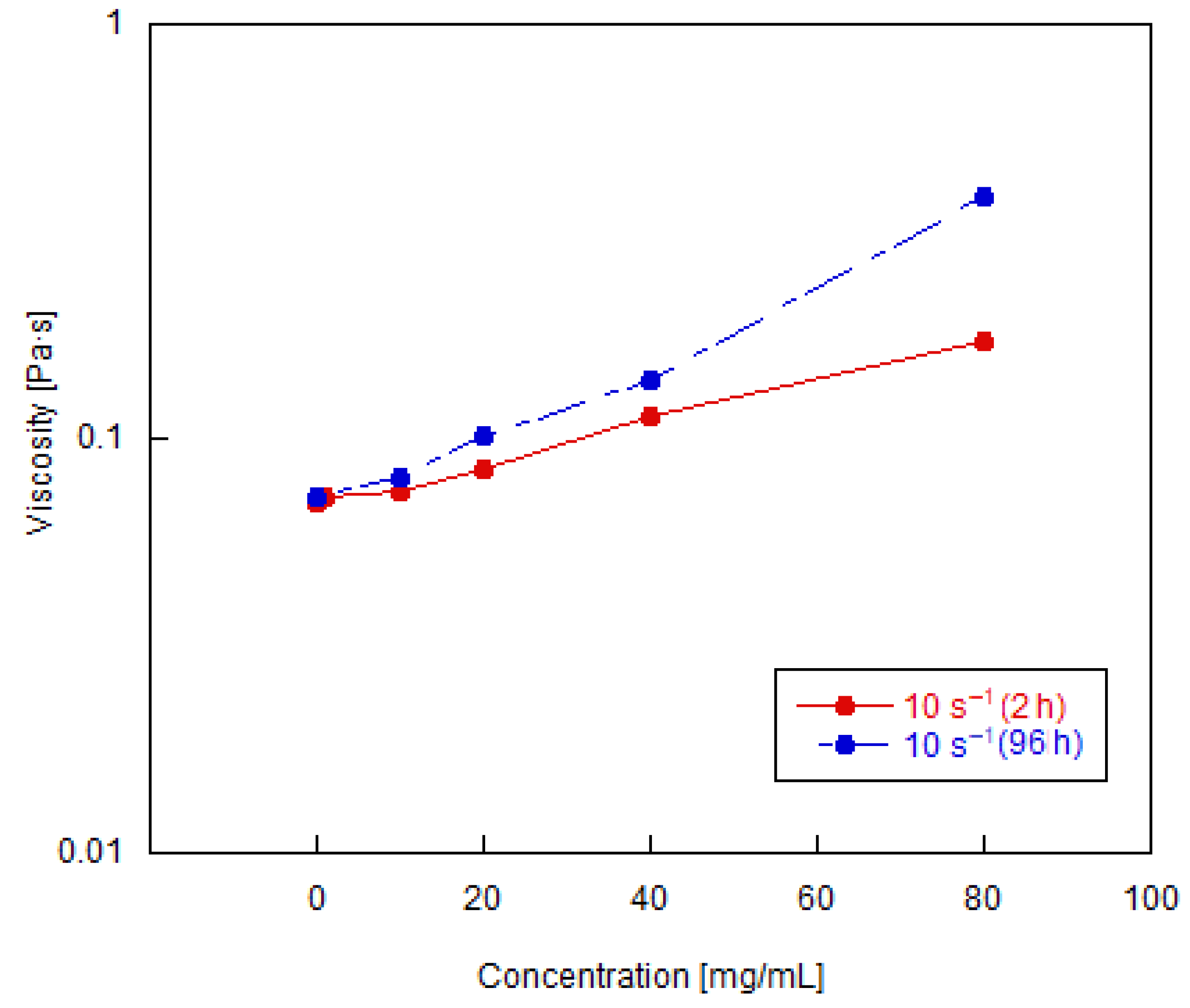
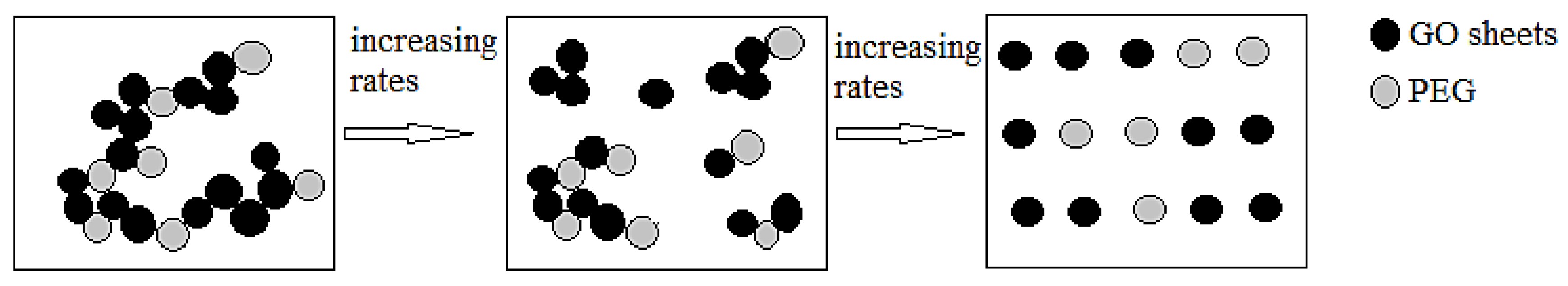
3.2. Shear Rheology of Graphene Oxide Suspensions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Neuberger, N.; Adidharma, H.; Fan, M. Graphene: A review of applications in the petroleum industry. J. Pet. Sci. Eng. 2018, 167, 152–159. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ridha, S.; Amer, A.; Shahari, R.; Ganat, T. Influence of Degree of Dispersion of Noncovalent Functionalized Graphene Nanoplatelets on Rheological Behaviour of Aqueous Drilling Fluids. Int. J. Chem. Eng. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Bao, D. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2009, 21, 055705. [Google Scholar] [CrossRef]
- Frank, I.W.; Tanenbaum, D.M.; Van Der Zande, A.M.; McEuen, P.L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B 2007, 25, 2558–2561. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.E.; Zhong, Y.L. Challenges of Industrial-Scale Graphene Oxide Production. In Graphene Oxide: Fundamentals and Applications; Dimiev, A.M., Eigler, S., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 410–431. [Google Scholar]
- Hongjuan, S.; Tongjiang, P.; Bo, L.; Caifeng, M.; Liming, L.; Quanjun, W.; Xiaoyi, L. Study of oxidation process occurring in natural graphite deposits. RSC Adv. 2017, 7, 51411–51418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Seyedin, S.; Gu, Z.; Salim, N.; Wang, X.; Razal, J.M. Liquid Crystals of Graphene Oxide: A Route Towards Solution-Based Processing and Applications; Wiley: Hoboken, NJ, USA, 2017; Volume 34. [Google Scholar]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Bianco, A. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]
- Imperiali, L.; Liao, K.H.; Clasen, C.; Fransaer, J.; Macosko, C.W.; Vermant, J. Interfacial rheology and structure of tiled graphene oxide sheets. Langmuir 2012, 28, 7990–8000. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Guo, Y.; Cheng, C.; Yang, L.; Jiang, L.; Zhu, D. Reduction of graphene oxide to highly conductive graphene by Lawesson’s reagent and its electrical applications. J. Mater. Chem. C 2013, 1, 3104–3109. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Hack, R.; Correia, C.H.G.; Zanon, R.A.D.S.; Pezzin, S.H. Characterization of graphene nanosheets obtained by a modified Hummer’s method. Matéria 2018, 23, e-11988. [Google Scholar] [CrossRef]
- Aladag, B.; Halelfadl, S.; Doner, N.; Maré, T.; Duret, S.; Estellé, P. Experimental investigations of the viscosity of nanofluids at low temperatures. Appl. Energy 2012, 97, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Sundén, B.; Wadsö, L. Aqueous carbon nanotube nanofluids and their thermal performance in a helical heat exchanger. Appl. Therm. Eng. 2016, 96, 364–371. [Google Scholar] [CrossRef]
- Jama, M.; Singh, T.; Gamaleldin, S.M.; Koc, M.; Samara, A.; Isaifan, R.J.; Atieh, M.A. Critical Review on Nanofluids: Preparation, Characterization, and Applications. J. Nanomater. 2016, 2016, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Rudyak, V.Y. Viscosity of Nanofluids—Why It Is Not Described by the Classical Theories. Adv. Nanoparticles 2013, 2, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Einstein, A. Eine Neue Bestimmung der Molekiildimensionen. Ann. Der. Phys. 1906, 19, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Utomo, A.T.; Poth, H.; Robbins, P.T.; Pacek, A.W. Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int. J. Heat Mass Transf. 2012, 55, 7772–7781. [Google Scholar] [CrossRef]
- Zhou, Z.; Scales, P.J.; Boger, D.V. Chemical and physical control of the rheology of concentrated metal oxide suspensions. Chem. Eng. Sci. 2001, 56, 2901–2920. [Google Scholar] [CrossRef]
- Thangavel, S.; Venugopal, G. Understanding the adsorption property of graphene-oxide with different degrees of oxidation levels. Powder Technol. 2014, 257, 141–148. [Google Scholar] [CrossRef]
- Naficy, S.; Jalili, R.; Aboutalebi, S.H.; Gorkin, R.A., III; Konstantinov, K.; Innis, P.C.; Wallace, G.G. Graphene oxide dispersions: Tuning rheology to enable fabrication. Mater. Horiz. 2014, 1, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Del Giudice, F.; Shen, A.Q. Shear rheology of graphene oxide dispersions. Curr. Opin. Chem. Eng. 2017, 16, 23–30. [Google Scholar] [CrossRef]
- Shu, R.; Yin, Q.; Xing, H.; Tan, D.; Gan, Y.; Xu, G. Colloidal and rheological behavior of aqueous graphene oxide dispersions in the presence of poly (ethylene glycol). Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 154–161. [Google Scholar] [CrossRef]
- Sadri, R.; Zangeneh Kamali, K.; Hosseini, M.; Zubir, N.; Kazi, S.N.; Ahmadi, G.; Golsheikh, A.M. Experimental study on thermo-physical and rheological properties of stable and green reduced graphene oxide nanofluids: Hydrothermal assisted technique. J. Dispers. Sci. Technol. 2017, 38, 1302–1310. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; de Souza Mendes, P.R. Rheological characterization of carbopol® dispersions in water and in water/glycerol solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.W.; Penha, F.G.; Braga, R.M.; Melo, D.M.D.A.; Pergher, S.B.; Petkowicz, D.I. Síntese e caracterização de argilas organofílicas contendo diferentes teores do surfactante catiônico brometo de hexadeciltrimetilamônio. Química Nova 2011, 34, 1152–1156. [Google Scholar] [CrossRef]
- De Oliveira, Y.D.; Amurin, L.G.; Valim, F.C.; Fechine, G.J.; Andrade, R.J. The role of physical structure and morphology on the photodegradation behaviour of polypropylene-graphene oxide nanocomposites. Polymer 2019, 176, 146–158. [Google Scholar] [CrossRef]
- Ferreira, E.H.; Andrade, R.J.; Fechine, G.J. The “Superlubricity State” of Carbonaceous Fillers on Polyethylene-Based Composites in a Molten State. Macromolecules 2019, 52, 9620–9631. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Jiang, W.; Nadeau, G.; Zaghib, K.; Kinoshita, K. Thermal analysis of the oxidation of natural graphite—Effect of particle size. Thermochim. Acta 2000, 351, 85–93. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Luceño-Sánchez, J.A.; Maties, G.; Gonzalez-Arellano, C.; Diez-Pascual, A.M. Synthesis and characterization of graphene oxide derivatives via functionalization reaction with hexamethylene diisocyanate. Nanomaterials 2018, 8, 870. [Google Scholar] [CrossRef] [Green Version]
- Taha-Tijerina, J.J. Thermal Transport and Challenges on Nanofluids Performance. Microfluid. Nanofluidics 2018, 1, 215–256. [Google Scholar]
- Wang, J.; Bai, R.Y.; Joseph, D.D. Nanoparticle-laden tubeless and open siphons. J. Fluid Mech. 2004, 516, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Kamibayashi, M.; Ogura, H.; Otsubo, Y. Shear-thickening flow of nanoparticle suspensions flocculated by polymer bridging. J. Colloid Interface Sci. 2008, 321, 294–301. [Google Scholar] [CrossRef]
- Mueller, S.; Llewellin, E.W.; Mader, H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 1201–1228. [Google Scholar] [CrossRef] [Green Version]
- Giannelis, E.P. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. Appl. Organomet. Chem. 1998, 12, 675–680. [Google Scholar] [CrossRef]
- Thomas, S.; Muller, R.; Abraham, J. Rheology and Processing of Polymer Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co KG: Hannover, Germany, 2006. [Google Scholar]



| Concentration mg/mL | Concentration wt.% |
|---|---|
| 0.1 | 0.01 |
| 1 | 0.1 |
| 10 | 1 |
| 20 | 2 |
| 40 | 4 |
| 80 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, Y.C.F.; Cargnin, E.; Naccache, M.F.; Andrade, R.J.E. Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions. Fluids 2020, 5, 41. https://doi.org/10.3390/fluids5020041
Soares YCF, Cargnin E, Naccache MF, Andrade RJE. Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions. Fluids. 2020; 5(2):41. https://doi.org/10.3390/fluids5020041
Chicago/Turabian StyleSoares, Yago Chamoun F., Elyff Cargnin, Mônica Feijó Naccache, and Ricardo Jorge E. Andrade. 2020. "Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions" Fluids 5, no. 2: 41. https://doi.org/10.3390/fluids5020041
APA StyleSoares, Y. C. F., Cargnin, E., Naccache, M. F., & Andrade, R. J. E. (2020). Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions. Fluids, 5(2), 41. https://doi.org/10.3390/fluids5020041