Waxy Oleogels for Partial Substitution of Solid Fat in Margarines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fat Component Analysis
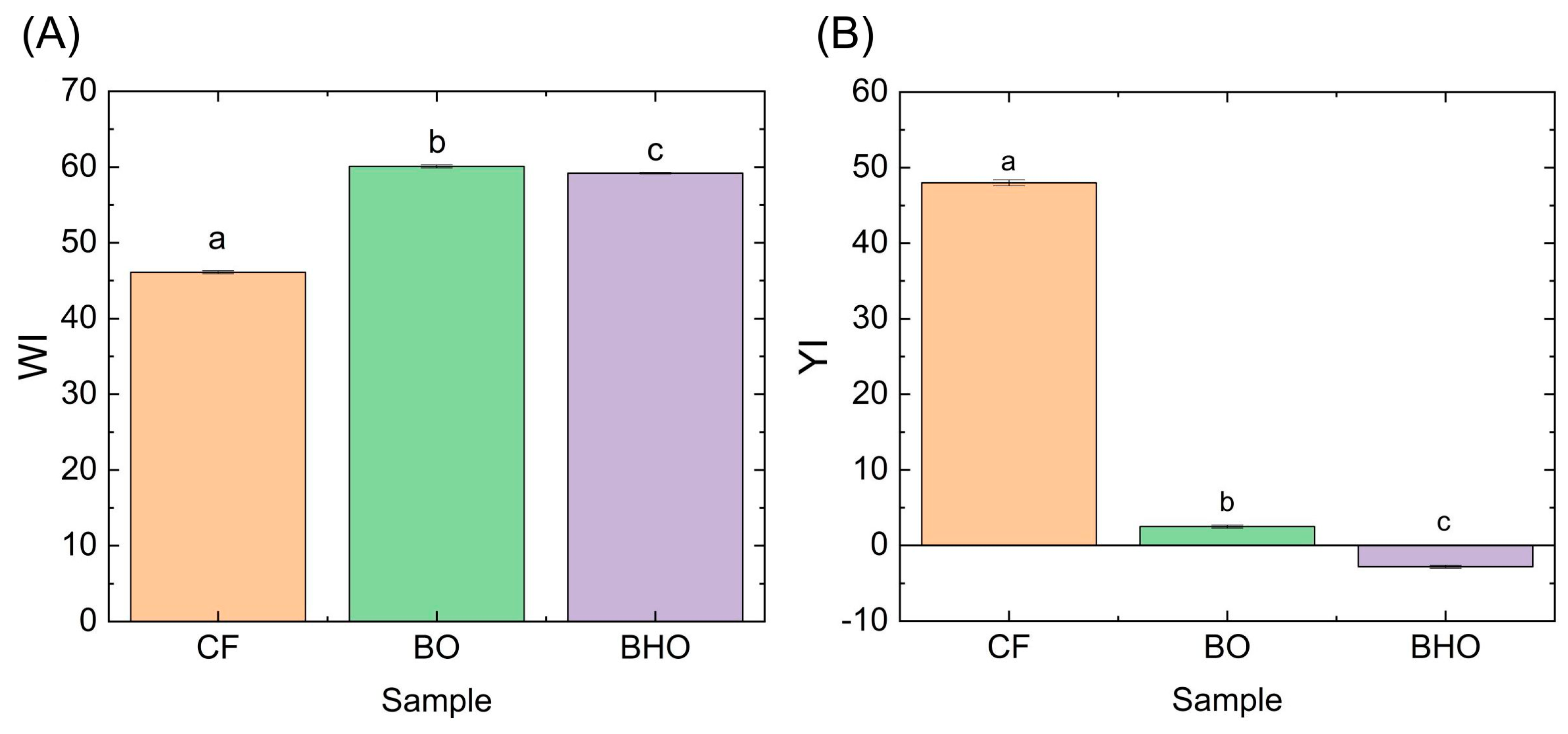
2.1.1. Appearance and Color Characteristics
2.1.2. Fatty Acid Composition
2.1.3. Texture Properties
2.2. Preparation and Analysis of Margarines
2.2.1. Development of Fat Product Technology
2.2.2. Comparison of Fatty Acid Composition (FAC) of Margarines
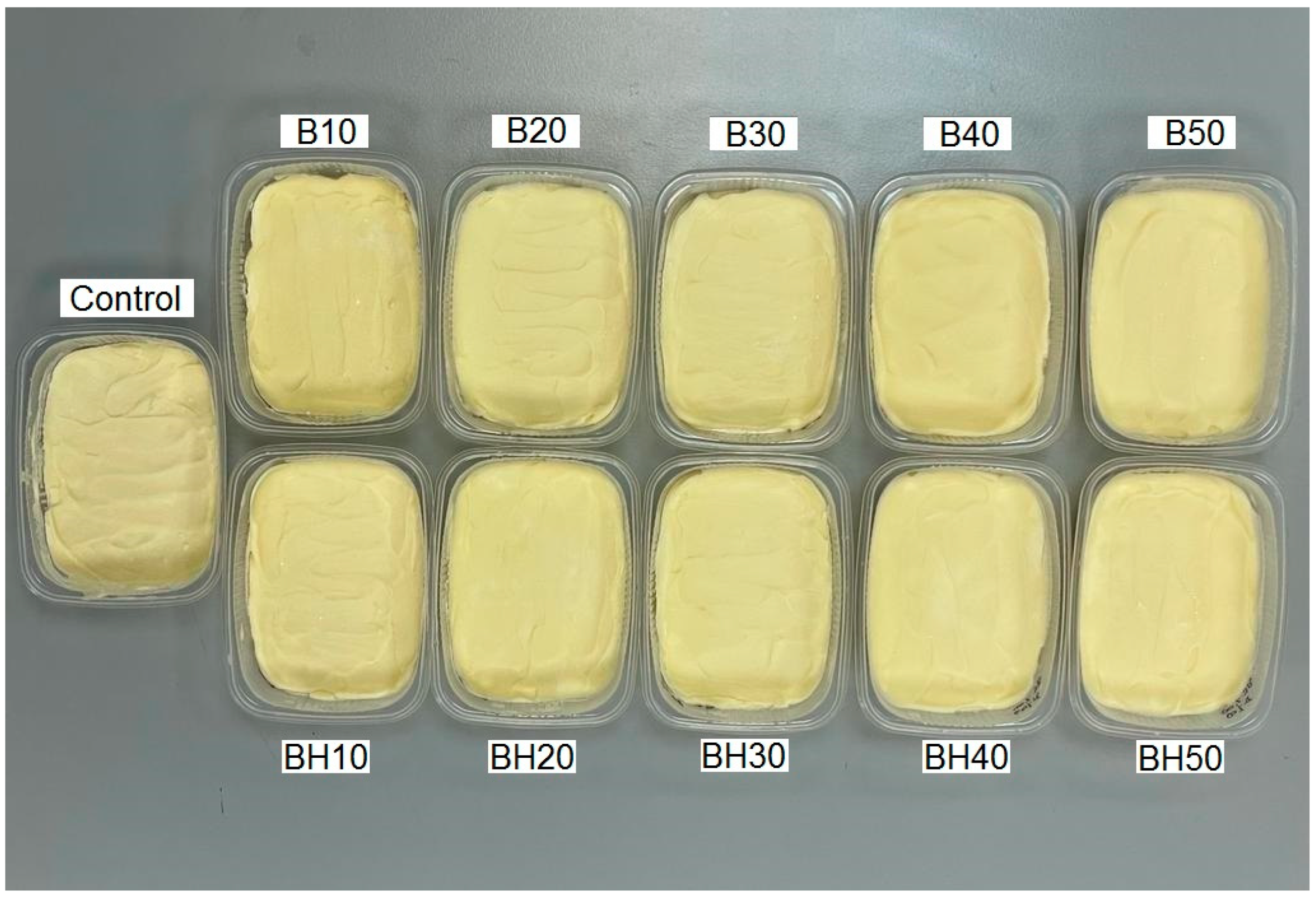
2.2.3. Visual Assessment
2.2.4. Color Analysis
2.2.5. Texture Analysis
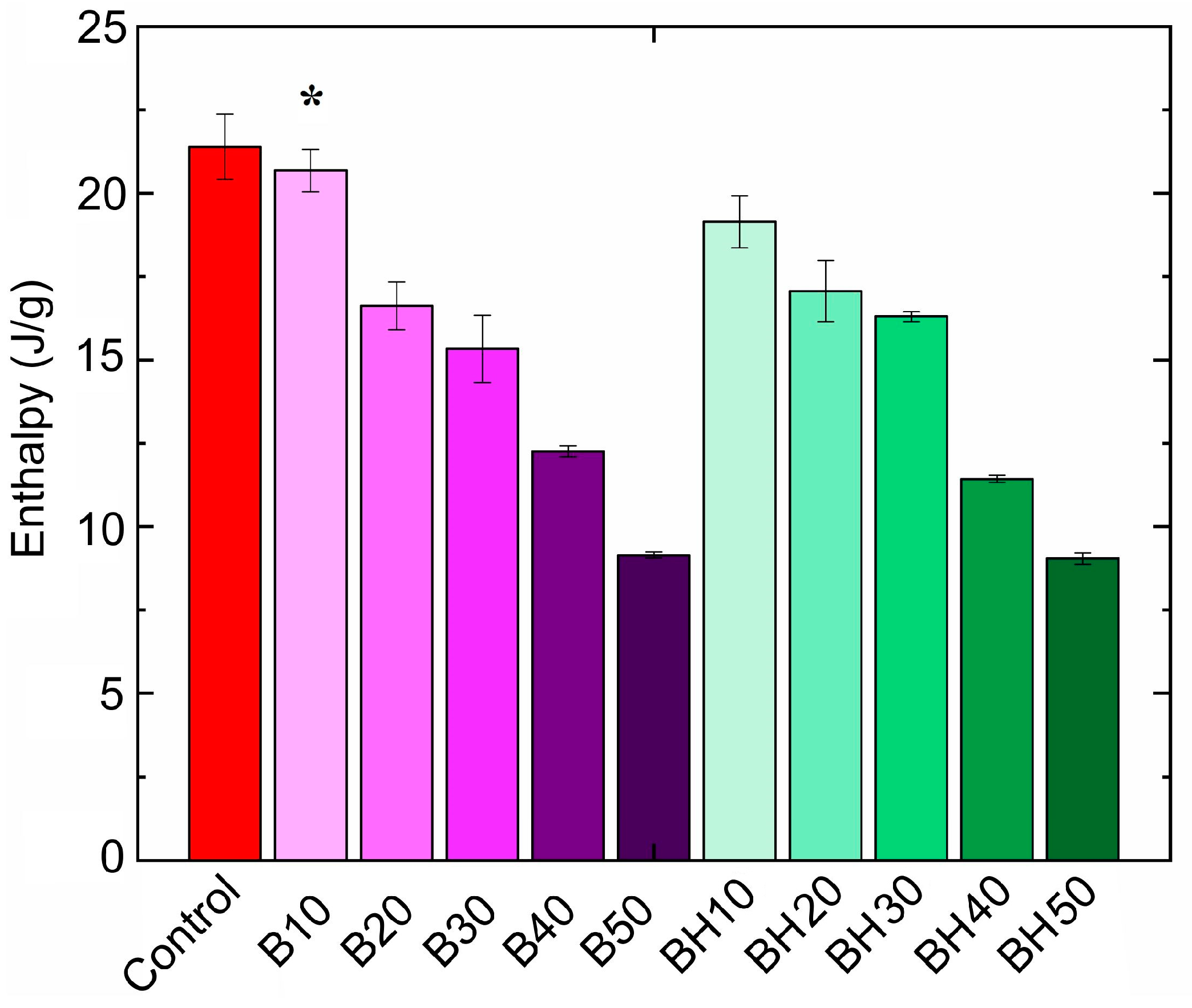
2.2.6. Thermal Properties
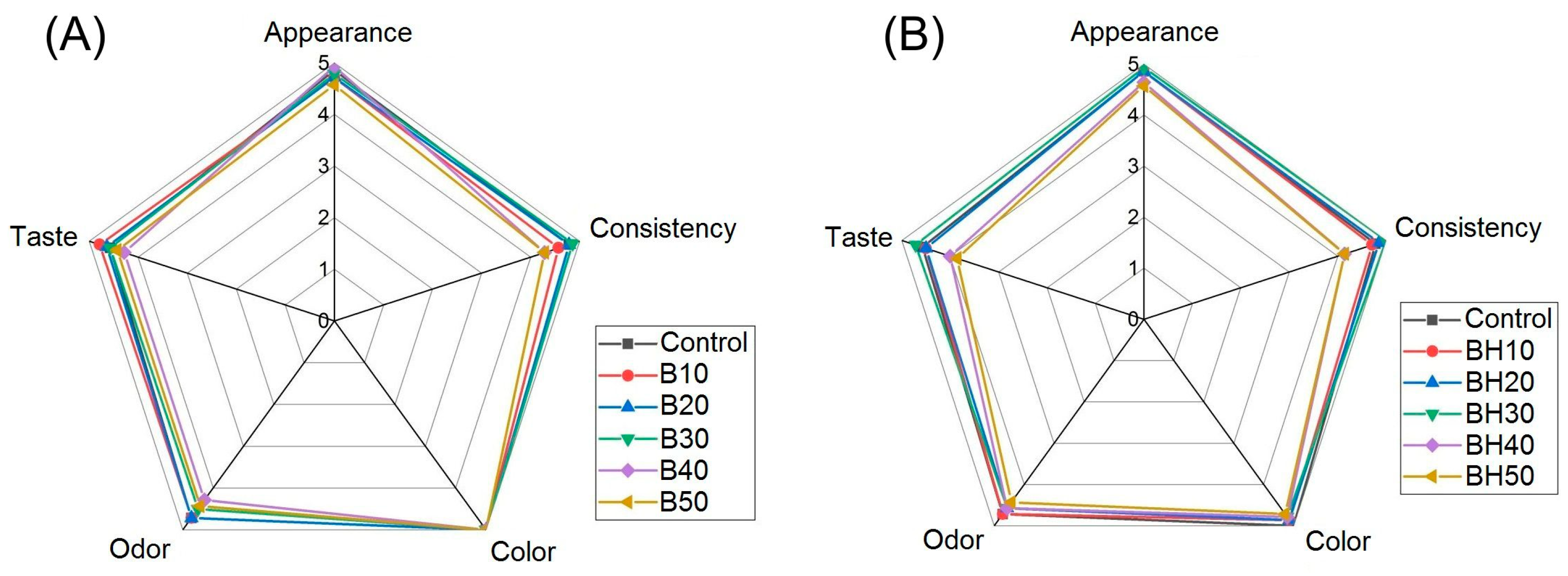
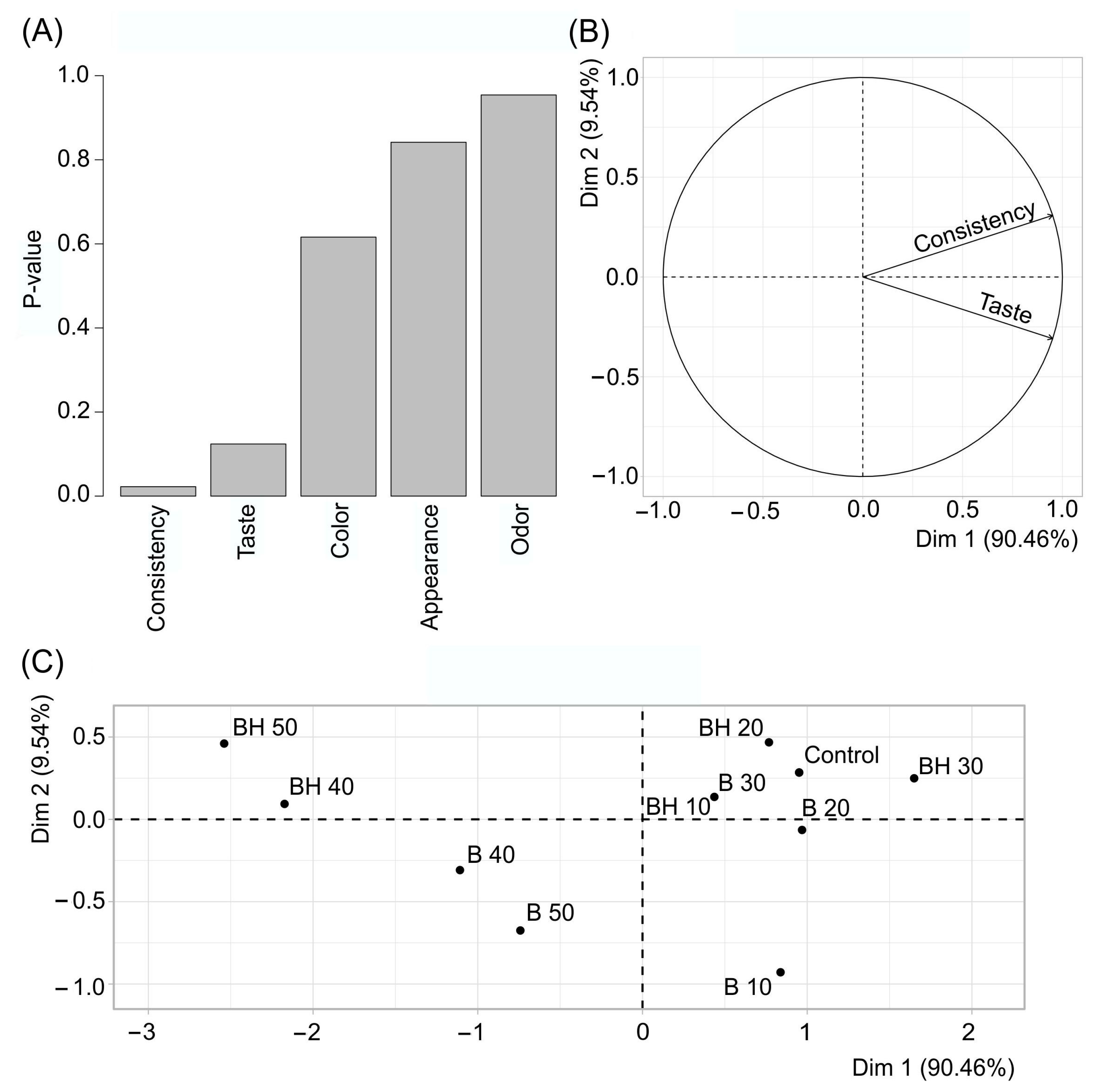
2.2.7. Sensory Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oleogel Preparation
4.3. Analysis of Fat Components
4.3.1. Color Analysis
4.3.2. Analysis of Fatty Acid Composition
4.3.3. Texture Analysis
4.4. Preparation and Analysis of Margarines
4.4.1. Color Analysis
4.4.2. Texture Analysis
4.4.3. Differential Scanning Calorimetry (DSC)
4.4.4. Sensory Evaluation
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salama, H.H.; Hashim, A.F. A functional spreadable canola and milk proteins oleogels as a healthy system for candy gummies. Sci. Rep. 2022, 12, 12619. [Google Scholar] [CrossRef]
- Perna, M.; Hewlings, S. Saturated fatty acid chain length and risk of cardiovascular disease: A systematic review. Nutrients 2022, 15, 30. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Toth, P.P.; Judd, S.; Blaha, M.J.; Hernandez, A.V.; Penson, P.E.; Banach, M.; International Lipid Expert Panel (ILEP) & Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Association of types of dietary fats and all-cause and cause-specific mortality: A prospective cohort study and meta-analysis of prospective studies with 1,164,029 participants. Clin. Nutr. 2020, 39, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Nicholson, R.A.; Marangoni, A.G. Overview of the Structure-Property Relationship in Fat Mimetics. Fat Mimetics Food Appl. 2023, 7–19. [Google Scholar] [CrossRef]
- Frolova, Y.V.; Kochetkova, A.A.; Sobolev, R.V.; Vorobyeva, V.M.; Kodentsova, V.M. Oleogels as prospective nutritional ingredients of lipid nature. Vopr. Pitan. 2021, 90, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Winkler-Moser, J.K. Properties of margarines prepared from soybean oil oleogels with mixtures of candelilla wax and beeswax. J. Food Sci. 2020, 85, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Cerqueira, M.A.; Purriños, L.; Pastrana, L.M.; Vicente, A.A.; Zapata, C.; Lorenzo, J.M. Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages. J. Sci. Food Agric. 2020, 100, 218–224. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of antioxidant-fortified oleogel and its application as a solid fat replacer to muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Pateiro, M.; Domínguez, R.; Munekata, P.E.S.; Pastrana, L.M.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Characterization of enriched meat-based pâté manufactured with oleogels as fat substitutes. Gels 2020, 6, 17. [Google Scholar] [CrossRef]
- Frolova, Y.V.; Sobolev, R.V.; Kochetkova, A.A. Comparative analysis of the properties of cookies containing oleogel based on beeswax and its fractions. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 941, 12033. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on food-grade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 7659–7676. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, Y.; Wu, Y.; Liang, H.; Li, J.; Pei, Y.; Li, Y.; Li, B.; Luo, X.; Liu, S. Beeswax: A potential self-emulsifying agent for the construction of thermal-sensitive food W/O emulsion. Food Chem. 2021, 349, 129203. [Google Scholar] [CrossRef] [PubMed]
- Penagos, I.A.; Murillo Moreno, J.S.; Dewettinck, K.; Van Bockstaele, F. Carnauba Wax and Beeswax as Structuring Agents for Water-in-Oleogel Emulsions without Added Emulsifiers. Foods 2023, 12, 1850. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, Y.; Shi, Y.; Liu, Y. Crystallization and Structural Properties of Oleogel-Based Margarine. Molecules 2022, 27, 8952. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, S.; Winkler-Moser, J.K.; Lee, S.; Liu, S.X. Feasibility of hemp seed oil oleogels structured with natural wax as solid fat replacement in margarine. J. Am. Oil Chem. Soc. 2022, 99, 1055–1070. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Zheng, Z.; Chai, X.; Han, W.; Liu, Y. Gelation behavior and crystal network of natural waxes and corresponding binary blends in high-oleic sunflower oil. J. Food Sci. 2021, 86, 3987–4000. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Malinkin, A.; Makarenko, M.; Kochetkova, A. Beeswax Fractions Used as Potential Oil Gelling Agents. J. Am. Oil Chem. Soc. 2021, 98, 281–296. [Google Scholar] [CrossRef]
- Starowicz, M.; Hanus Pawełand Lamparski, G.; Sawicki, T. Characterizing the volatile and sensory profiles, and sugar content of beeswax, beebread, bee pollen, and honey. Molecules 2021, 26, 3410. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Frolova, Y.; Sobolev, R.; Kochetkova, A. On the Role of Beeswax Components in the Regulation of Sunflower Oil Oleogel Properties. Food Biophys. 2023, 18, 262–272. [Google Scholar] [CrossRef]
- Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Makarenko, M.; Kochetkova, A. Effect of beeswax and combinations of its fractions on the oxidative stability of oleogels. Food Biosci. 2022, 48, 101744. [Google Scholar] [CrossRef]
- Mao, L.; Miao, S. Structuring food emulsions to improve nutrient delivery during digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Felker, F.C.; Hwang, H.S. Physical Properties of Beeswax, Sunflower Wax, and Candelilla Wax Mixtures and Oleogels. J. Am. Oil Chem. Soc. 2019, 96, 1125–1142. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2006. [Google Scholar]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The influence of edible oils’ composition on the properties of beeswax-based oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Miele, N.A.; Masi, P.; Aiello, A.; Cavella, S. Effect of fatty acid composition of vegetable oils on crystallization and gelation kinetics of oleogels based on natural wax. Food Chem. 2022, 375, 131805. [Google Scholar] [CrossRef]
- Frolova, Y.; Sobolev, R.; Kochetkova, A. Influence of oil combinations on the structural properties of oleogels. In Proceedings of the International Conference on Advances in Agrobusiness and Biotechnology Research (ABR 2021), Moscow, Russia, 6 July 2021; Volume 285. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO: Geneve, Switzerland, 2010. [Google Scholar]
- da Silva, T.L.T.; Chaves, K.F.; Fernandes, G.D.; Rodrigues, J.B.; Bolini, H.M.A.; Arellano, D.B. Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. J. Am. Oil Chem. Soc. 2018, 95, 673–685. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in microstructural and physicochemical properties of candelilla wax/rice bran oil—Derived oleogels using sunflower lecithin and soya lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Muste, S. Application of analytical methods for the comprehensive analysis of oleogels—A review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef]
- Ayuningtias, A.; Nurhasanah, S.; Nurhadi, B.; Subroto, E. Textural Characteristic of Margarine Enriched with Pectin Fiber by Using Blending Method. Int. J. Adv. Sci Eng. Inf. Tech 2013, 3, 268–272. [Google Scholar] [CrossRef]
- Patel, A.R.; Schatteman, D.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Preparation and rheological characterization of shellac oleogels and oleogel-based emulsions. J. Colloid Interface Sci. 2013, 411, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Cuspinera, V.; de Sousa, J.; Knoop, M. Sensory and analytical characterization of the “cool-melting” perception of commercial spreads. J. Texture Stud. 2017, 48, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Espert, M.; Hernández, M.J.; Sanz, T.; Salvador, A. Reduction of saturated fat in chocolate by using sunflower oil-hydroxypropyl methylcellulose based oleogels. Food Hydrocoll. 2021, 120, 106917. [Google Scholar] [CrossRef]
- Doan, C.D.; Patel, A.R.; Tavernier, I.; De Clercq, N.; Van Raemdonck, K.; de Walle, D.; Delbaere, C.; Dewettinck, K. The feasibility of wax-based oleogel as a potential co-structurant with palm oil in low-saturated fat confectionery fillings. Eur. J. Lipid Sci. Technol. 2016, 118, 1903–1914. [Google Scholar] [CrossRef]
- Tarté, R.; Paulus, J.S.; Acevedo, N.C.; Prusa, K.J.; Lee, S.L. High-oleic and conventional soybean oil oleogels structured with rice bran wax as alternatives to pork fat in mechanically separated chicken-based bologna sausage. LWT 2020, 131, 109659. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Flöter, E. Wax based oleogels and their application in sponge cakes. Food Funct. 2022, 13, 9419–9433. [Google Scholar] [CrossRef]
- Rutkowska, J.; Żbikowska, A. Effects of fatty acid composition of liquid margarines on sensory quality of cakes. Acta Aliment. 2010, 39, 136–148. [Google Scholar] [CrossRef]
- Pușcaș, A.; Murecșan, V. The Feasibility of Shellac Wax Emulsion Oleogels as Low-Fat Spreads Analyzed by Means of Multidimensional Statistical Analysis. Gels 2022, 8, 749. [Google Scholar] [CrossRef]
- Sena, B.; Dhal, S.; Sahu, D.; Sarkar, P.; Mohanty, B.; Jarzębski, M.; Wieruszewski, M.; Behera, H.; Pal, K. Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin. Polymers 2022, 14, 3928. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of Fatty Acid Composition, Tocols Profile, and Oxidative Stability of Some Fully Refined Edible Oils. Int. J. Food Prop. 2015, 18, 2064–2076. [Google Scholar] [CrossRef]
- Sato, K. Crystallization behaviour of fats and lipids—A review. Chem. Eng. Sci. 2001, 56, 2255–2265. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneve, Switzerland, 2012.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneve, Switzerland, 2007.
- Husson, F.; Le, S.; Cadoret, M. SensoMineR: A package for sensory data analysis with R. J. Sens. Stud. 2008, 23, 14. [Google Scholar]
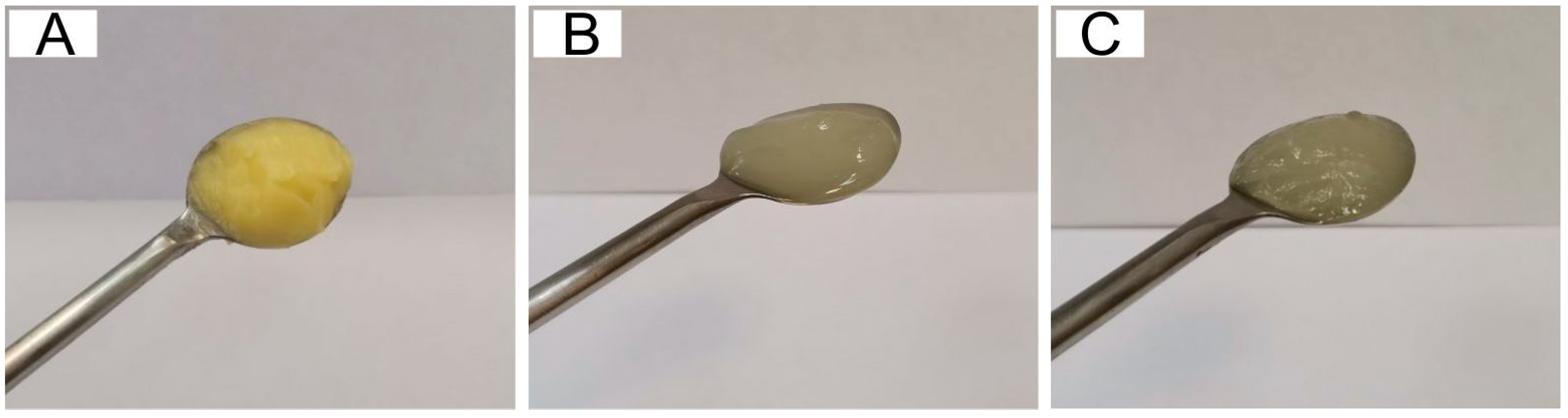

| Sample | Firmness (N) | Elastic Modulus, N/mm2 |
|---|---|---|
| CF | 2.01 ± 0.07 a | 81.72 ± 1.55 a |
| BO | 0.04 ± 0.01 b | 2.05 ± 1.30 b |
| BHO | 0.31 ± 0.06 b | 6.39 ± 1.95 c |
| Sample | Fat Phase 82.5% | Aqueous Phase 17.5% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | CF Replacement | MG | SL | Water | SMP | Flavoring | Salt | SA | CA | |||
| SO | BW | HC | ||||||||||
| Control | 81.90 | - | - | - | 0.50 | 0.10 | 14.96 | 2.00 | 0.30 | 0.15 | 0.07 | 0.02 |
| B10 | 73.71 | 7.94 | 0.25 | - | ||||||||
| B20 | 65.52 | 15.89 | 0.49 | - | ||||||||
| B30 | 57.33 | 23.83 | 0.74 | - | ||||||||
| B40 | 49.14 | 31.78 | 0.98 | - | ||||||||
| B50 | 40.95 | 39.72 | 1.23 | - | ||||||||
| BH10 | 73.71 | 7.94 | 0.22 | 0.03 | ||||||||
| BH20 | 65.52 | 15.89 | 0.44 | 0.05 | ||||||||
| BH30 | 57.33 | 23.83 | 0.67 | 0.07 | ||||||||
| BH40 | 49.14 | 31.78 | 0.88 | 0.10 | ||||||||
| BH50 | 40.95 | 39.72 | 1.11 | 0.12 | ||||||||
| Type of Fatty Acid | Sample | |||||
|---|---|---|---|---|---|---|
| Control | B10/BH10 | B20/BH20 | B30/BH30 | B40/BH40 | B50/BH50 | |
| SFAs, % Difference from control, % | 35.3 | 32.8 | 30.3 | 27.8 | 25.3 | 22.8 |
| - | −7.1 | −14.2 | −21.3 | −28.4 | −35.5 | |
| PUFAs, % Difference from control, % | 19.1 | 22.7 | 26.2 | 29.7 | 33.2 | 36.8 |
| - | 18,4 | 36.9 | 55.3 | 73.7 | 92.2 | |
| MUFAs, % Difference from control, % | 28.0 | 27.0 | 26.0 | 24.9 | 23.9 | 22.9 |
| - | −3.7 | −7.4 | −11.1 | −14.8 | −18.4 | |
| PUFAs/SFAs | 0.5 | 0.7 | 0.9 | 1.1 | 1.3 | 1.6 |
| MUFAs/SFAs | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| Sample | L* | a* | b* | WI | YI |
|---|---|---|---|---|---|
| Control | 85.17 ± 0.67 | 2.77 ± 0.06 | 21.83 ± 0.49 | 26.54 ± 0.58 | 36.62 ± 0.91 |
| B10 | 83.73 ± 0.45 * | 2.60 ± 0.10 | 21.70 ± 0.50 | 27.24 ± 0.60 | 37.02 ± 0.97 |
| B20 | 84.50 ± 0.36 | 2.30 ± 0.00 * | 23.67 ± 0.40 * | 28.38 ± 0.24 * | 40.01 ± 0.57 * |
| B30 | 83.03 ± 0.42 * | 2.45 ± 0.06 * | 23.37 ± 0.12 * | 28.98 ± 0.16 * | 40.20 ± 0.05 * |
| B40 | 82.43 ± 0.31 * | 2.13 ± 0.06 * | 22.53 ± 0.06 | 28.65 ± 0.24 * | 39.05 ± 0.24 * |
| B50 | 81.67 ± 0.06 * | 1.43 ± 0.06 * | 21.13 ± 0.06 | 28.01 ± 0.04 * | 36.97 ± 0.09 |
| BH10 | 85.80 ± 0.26 | 2.77 ± 0.15 | 23.73 ± 0.35 * | 27.80 ± 0.22 * | 39.52 ± 0.49 * |
| BH20 | 84.30 ± 0.46 | 2.30 ± 0.17 * | 22.23 ± 0.38 | 27.31 ± 0.53 | 37.68 ± 0.79 |
| BH30 | 83.47 ± 0.06 * | 2.23 ± 0.06 * | 23.27 ± 0.06 * | 28.63 ± 0.07 * | 39.82 ± 0.12 * |
| BH40 | 82.60 ± 0.26 * | 1.90 ± 0.00 * | 22.47 ± 0.21 | 28.48 ± 0.33 * | 38.86 ± 0.49 * |
| BH50 | 80.23 ± 0.06 * | 1.30 ± 0.00 * | 21.13 ± 0.06 | 28.97 ± 0.07 * | 37.63 ± 0.12 |
| Sample | Firmness (N) | Elastic Modulus (N/mm2) |
|---|---|---|
| Control | 30.84 ± 9.65 | 1.12 ± 0.21 |
| B10 | 27.74 ± 8.25 | 1.75 ± 0.01 * |
| B20 | 28.07 ± 10.32 | 2.68 ± 0.26 * |
| B30 | 20.65 ± 6.12 | 1.55 ± 0.04 * |
| B40 | 10.50 ± 1.94 * | 0.36 ± 0.01 * |
| B50 | 9.33 ± 2.73 * | 0.43 ± 0.05 * |
| BH10 | 33.71 ± 10.10 | 1.86 ± 0.12 * |
| BH20 | 26.34 ± 9.45 | 2.81 ± 0.25 * |
| BH30 | 20.49 ± 5.30 | 1.23 ± 0.16 |
| BH40 | 11.80 ± 4.56 * | 0.58 ± 0.05 * |
| BH50 | 8.32 ± 1.33 * | 0.68 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Kochetkova, A. Waxy Oleogels for Partial Substitution of Solid Fat in Margarines. Gels 2023, 9, 683. https://doi.org/10.3390/gels9090683
Sobolev R, Frolova Y, Sarkisyan V, Kochetkova A. Waxy Oleogels for Partial Substitution of Solid Fat in Margarines. Gels. 2023; 9(9):683. https://doi.org/10.3390/gels9090683
Chicago/Turabian StyleSobolev, Roman, Yuliya Frolova, Varuzhan Sarkisyan, and Alla Kochetkova. 2023. "Waxy Oleogels for Partial Substitution of Solid Fat in Margarines" Gels 9, no. 9: 683. https://doi.org/10.3390/gels9090683






