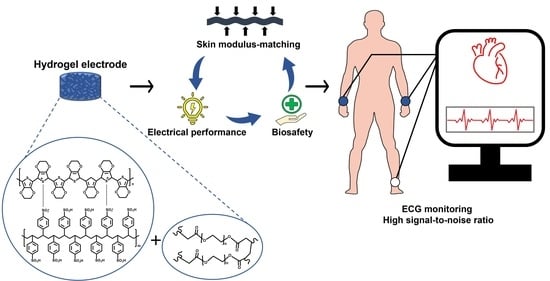
Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteriazation of PEGDA–PEDOT:PSS Hydrogel
2.2. Electrical Characterization of PEGDA–PEDOT:PSS Hydrogel
2.3. Biological Safety and Swelling Behavior of PEGDA–PEDOT:PSS Hydrogel
2.4. ECG Monitoring Using PEGDA–PEDOT:PSS Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of PEGDA Hydrogel, PEGDA–PEDOT:PSS Hydrogel and Pure PEDOT:PSS Film
4.2.1. PEGDA Hydrogel
4.2.2. PEGDA–PEDOT:PSS Hydrogel
4.2.3. Pure PEDOT:PSS Film
4.3. Rheological Characterization of PEGDA–PEDOT:PSS Hydrogels
4.4. UV-Vis Characterization of PEGDA–PEDOT:PSS Hydrogels
4.5. Morphological Analysis of PEGDA Hydrogel and PEGDA–PEDOT:PSS Hydrogel
4.6. In Vitro Cytotoxicity Test
4.7. Skin Allergic Reaction Test of PEGDA–PEDOT:PSS Hydrogels
4.8. Swelling Studies of PEGDA–PEDOT:PSS Hydrogels
4.9. Electrical Characterization of PEGDA–PEDOT:PSS Hydrogels
4.9.1. Impedance
4.9.2. Resistance
4.9.3. LED Emission
4.10. ECG Signals Monitoring
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional conductive hydrogels for bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Hoffmann, K.P.; Ruff, R. Flexible dry surface-electrodes for ECG long-term monitoring. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Lyon, France, 22–26 August 2007; pp. 5739–5742. [Google Scholar]
- Lee, Y.; Yim, S.G.; Lee, G.W.; Kim, S.; Kim, H.S.; Hwang, D.Y.; An, B.S.; Lee, J.H.; Seo, S.; Yang, S.Y. Self-adherent biodegradable gelatin-based hydrogel electrodes for electrocardiography monitoring. Sensors 2020, 20, 5737. [Google Scholar] [CrossRef]
- Xu, C.; Lemon, W.; Liu, C. Design and fabrication of a high-density metal microelectrode array for neural recording. Sens. Actuators A Phys. 2002, 96, 78–85. [Google Scholar] [CrossRef]
- Park, S.; Song, Y.J.; Boo, H.; Chung, T.D. Nanoporous Pt microelectrode for neural stimulation and recording: In vitro characterization. J. Phys. Chem. C 2010, 114, 8721–8726. [Google Scholar] [CrossRef]
- Tam, H.; Webster, J.G. Minimizing electrode motion artifact by skin abrasion. IEEE Trans. Biomed. Eng. 1977, BME-24, 134–139. [Google Scholar] [CrossRef]
- Yamashita, S.; Mizutani, T.; Shimizu, T.; Sakakura, Y.; Tanaka, M. BIS pediatric sensor can cause blisters in small children. J. Anesth. 2010, 24, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Noriko, T.; Shijima, T.; Syoichiro, I.; Ito, H. Contact dermatitis associated with the Bispectral index™ sensor: A case report. JA Clin. Rep. 2020, 6, 87. [Google Scholar]
- Chi, M.; Zhao, J.; Dong, Y.; Wang, X. Flexible carbon nanotube-based polymer electrode for long-term electrocardiographic recording. Materials 2019, 12, 971. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, G.; Zhou, H.; Qian, K.; Hu, J. Preparation and property evaluation of conductive hydrogel using poly (vinyl alcohol)/polyethylene glycol/graphene oxide for human electrocardiogram acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef]
- Park, H.H.; Seong, M.; Sun, K.; Ko, H.; Kim, S.M.; Jeong, H.E. Flexible and shape-reconfigurable hydrogel interlocking adhesives for high adhesion in wet environments based on anisotropic swelling of hydrogel microstructures. ACS Macro Lett. 2017, 6, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Kim, J.; Wang, J.; Kim, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Cai, S.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible hybrid electronics for digital healthcare. Adv. Mater. 2020, 32, 1902062. [Google Scholar] [CrossRef]
- Green, R.A.; Baek, S.; Poole-Warren, L.A.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11, 014107. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable hydrogel electronics and devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Patrick, P.; Mercier Wang, J. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive hydrogels—A novel material: Recent advances and future perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene nanocomposite organohydrogel for flexible, healable, low-temperature tolerant strain sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Pan, L.; Shi, Y.; Yu, G. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, A.; Yang, Y.; Zhu, Y.; Song, Y.; Li, Y.; Li, J. Highly stretchable and antimicrobial strain sensors based on mussel-inspired super-adhesive self-healing hydrogels for human motion monitoring. Int. J. Biol. Macromol. 2023, 245, 125469. [Google Scholar]
- Liu, Y.J.; Cao, W.T.; Ma, M.G.; Wan, P. Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, T.; Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Wang, C.; Yokota, T.; Someya, T. Natural biopolymer-based biocompatible conductors for stretchable bioelectronics. Chem. Rev. 2021, 121, 2109–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, X.; Wang, S.; Jin, X.; Li, J.; Wei, Y.; Wang, Y. Natural polymers-enhanced double-network hydrogel as wearable flexible sensor with high mechanical strength and strain sensitivity. Chin. Chem. Lett. 2023, 34, 107892. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Y.; Gui, Y.; Wang, C.; Wu, Y.; Yao, J.; Wang, J. Ultrastretchable E-Skin Based on Conductive Hydrogel Microfibers for Wearable Sensors. Small 2023, e2305951. [Google Scholar] [CrossRef]
- Tan JM, R.; Farraj, Y.; Kamyshny, A.; Magdassi, S. Fabrication Approaches of Soft Electronics. ACS Appl. Electron. Mater. 2023, 5, 1376–1393. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, transparent, ionic conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Synthesis of a novel pH responsive phyllosilicate loaded polymeric hydrogel based on poly (acrylic acid-co-N-vinylpyrrolidone) and polyethylene glycol for drug delivery: Modelling and kinetics study for the sustained release of an antibiotic drug. RSC Adv. 2015, 5, 18312–18327. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, T.; Mondal, S.; Das, P.; Das, N.C. Starch functionalized biodegradable semi-IPN as a pH-tunable controlled release platform for memantine. Int. J. Biol. Macromol. 2017, 95, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Zhang, R.; Liu, H.; Chen, H.; Zhang, X.; Li, C.; Zeng, Q.; Chen, Y.; Huang, G. Conductive GelMA/PEDOT: PSS Hybrid Hydrogel as a Neural Stem Cell Niche for Treating Cerebral Ischemia-Reperfusion Injury. Front. Mater. 2022, 9, 914994. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT: PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and characterization of poly (ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Peyton, S.R.; Raub, C.B.; Keschrumrus, V.P.; Putnam, A.J. The use of poly (ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 2006, 27, 4881–4893. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.D. Thermal considerations for the design of an implanted cortical brain–machine interface (BMI). In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 33–38. [Google Scholar]
- Castagnola, E.; Ansaldo, A.; Maggiolini, E.; Ius, T.; Skrap, M.; Ricci, D.; Fadiga, L. Smaller, softer, lower-impedance electrodes for human neuroprosthesis: A pragmatic approach. Front. Neuroeng. 2014, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Fernández, E. Biocompatibility of intracortical microelectrodes: Current status and future prospects. Front. Neuroeng. 2010, 3, 1212. [Google Scholar] [CrossRef] [PubMed]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
- Holt, B.; Tripathi, A.; Morgan, J. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J. Biomech. 2008, 41, 2689–2695. [Google Scholar] [CrossRef]
- Testore, D.; Zoso, A.; Kortaberria, G.; Sangermano, M.; Chiono, V. Electroconductive photo-curable PEGDA-gelatin/PEDOT: PSS hydrogels for prospective cardiac tissue engineering application. Front. Bioeng. Biotechnol. 2022, 10, 897575. [Google Scholar] [CrossRef]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.-H.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Okuda, S.; Takamatsu, S.; Itoh, T. Combination of Micro-Corrugation Process and Pre-Stretched Method for Highly Stretchable Vertical Wavy Structured Metal Interconnects. Micromachines 2022, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Chortos, A.; Bao, Z. Skin-inspired electronic devices. Mater. Today 2014, 17, 321–331. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.; Son, D.; Shin, M. Conductive and adhesive granular alginate hydrogels for on-tissue writable bioelectronics. Gels 2023, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, N.; Zhang, T.; Sun, Q.; Han, B.; Yu, T. A tetra-PEG hydrogel based aspirin sustained release system exerts beneficial effects on periodontal ligament stem cells mediated bone regeneration. Front. Chem. 2019, 7, 682. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, M.; Krukiewicz, K.; Zhussupbekova, A.; Fleischer, K.; Biggs, M.J.; Monaghan, M.G. PEDOT: PSS interfaces stabilized using a PEGylated crosslinker yield improved conductivity and biocompatibility. J. Mater. Chem. B 2019, 7, 4811–4820. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Hou, J.; Yang, M.; Wu, G.; Sun, P. A bio-inspired, ultra-tough, high-sensitivity, and anti-swelling conductive hydrogel strain sensor for motion detection and information transmission. Mater. Horiz. 2022, 9, 3057–3069. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.I.; Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Ng, T.Y.; Sharma, P. The effect of water content on the elastic modulus and fracture energy of hydrogel. Extreme Mech. Lett. 2020, 35, 100617. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT: PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Song, J.; Kim, J.; Lee, J.; Son, D.; Shin, M. Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring. Gels 2023, 9, 957. https://doi.org/10.3390/gels9120957
Lee D, Song J, Kim J, Lee J, Son D, Shin M. Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring. Gels. 2023; 9(12):957. https://doi.org/10.3390/gels9120957
Chicago/Turabian StyleLee, Dongik, Jihyang Song, Jungwoo Kim, Jaebeom Lee, Donghee Son, and Mikyung Shin. 2023. "Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring" Gels 9, no. 12: 957. https://doi.org/10.3390/gels9120957