Effect of GelMA Hydrogel Properties on Long-Term Encapsulation and Myogenic Differentiation of C2C12 Spheroids
Abstract
:1. Introduction
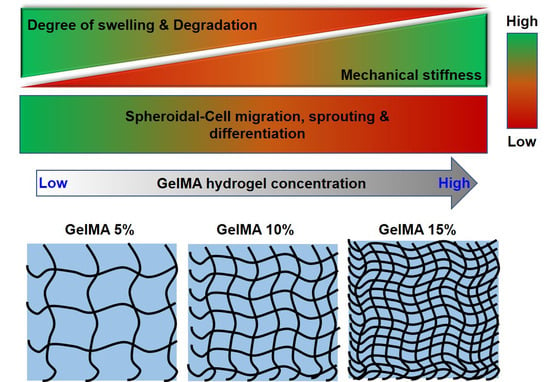
2. Results and Discussion
2.1. GelMA Hydrogel Fabrication and Characterization
2.2. C2C12 Spheroid Formation and Encapsulation in GelMA Hydrogels
2.3. Myogenic Differentiation of C2C12 Spheroid in GelMA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. GelMA Hydrogel Fabrication
4.3. GelMA Hydrogel Characterization
4.4. Cell Culture
4.5. Spheroid Formation and Encapsulation
4.6. Characterization of Spheroids Encapsulated within Hydrogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. Biomed Res. Int. 2018, 2018, 1984879. [Google Scholar] [CrossRef]
- Smoak, M.M.; Mikos, A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio 2020, 7, 100069. [Google Scholar] [CrossRef] [PubMed]
- Alarcin, E.; Bal-Öztürk, A.; Avci, H.; Ghorbanpoor, H.; Dogan Guzel, F.; Akpek, A.; Yesiltas, G.; Canak-Ipek, T.; Avci-Adali, M. Current Strategies for the Regeneration of Skeletal Muscle Tissue. Int. J. Mol. Sci. 2021, 22, 5929. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.W.; Capel, A.J.; Rimington, R.P.; Wheeler, P.; Leonard, A.N.; Bishop, N.C.; Davies, O.G.; Lewis, M.P. Bioengineered human skeletal muscle capable of functional regeneration. BMC Biol. 2020, 18, 145. [Google Scholar] [CrossRef]
- Rizzi, R.; Bearzi, C.; Mauretti, A.; Bernardini, S.; Cannata, S.; Gargioli, C. Tissue engineering for skeletal muscle regeneration. Muscles Ligaments Tendons J. 2012, 2, 230–234. [Google Scholar] [PubMed]
- Genovese, P.; Patel, A.; Ziemkiewicz, N.; Paoli, A.; Bruns, J.; Case, N.; Zustiak, S.P.; Garg, K. Co-delivery of fibrin-laminin hydrogel with mesenchymal stem cell spheroids supports skeletal muscle regeneration following trauma. J. Tissue Eng. Regen. Med. 2021, 15, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Gwon, Y.; Park, S.; Kim, H.; Kim, J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact. Mater. 2023, 19, 50–74. [Google Scholar] [CrossRef]
- Griffin, K.H.; Fok, S.W.; Kent Leach, J. Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling. NPJ Regen. Med. 2022, 7, 70. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Hu, W.; Lazar, M.A. Modelling metabolic diseases and drug response using stem cells and organoids. Nat. Rev. Endocrinol. 2022, 18, 744–759. [Google Scholar] [CrossRef]
- Decarli, M.C.; Amaral, R.; Santos, D.P.D.; Tofani, L.B.; Katayama, E.; Rezende, R.A.; Silva, J.; Swiech, K.; Suazo, C.A.T.; Mota, C.; et al. Cell spheroids as a versatile research platform: Formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021, 13, 032002. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Chae, S.; Hong, J.; Hwangbo, H.; Kim, G. The utility of biomedical scaffolds laden with spheroids in various tissue engineering applications. Theranostics 2021, 11, 6818–6832. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-j.; Byun, H.; Lee, S.; Kim, E.; Lee, G.M.; Huh, S.J.; Joo, J.; Shin, H. Spatially arranged encapsulation of stem cell spheroids within hydrogels for the regulation of spheroid fusion and cell migration. Acta Biomater. 2022, 142, 60–72. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Yang, L. Hydrogel Encapsulation: Taking the Therapy of Mesenchymal Stem Cells and Their Derived Secretome to the Next Level. Front. Bioeng. Biotechnol. 2022, 10, 859927. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef]
- Qazi, T.H.; Blatchley, M.R.; Davidson, M.D.; Yavitt, F.M.; Cooke, M.E.; Anseth, K.S.; Burdick, J.A. Programming hydrogels to probe spatiotemporal cell biology. Cell Stem Cell 2022, 29, 678–691. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, M.H.; Lee, J.H.; Seliktar, D.; Cho, N.J.; Tan, L.P. Modulation of Huh7.5 spheroid formation and functionality using modified PEG-based hydrogels of different stiffness. PLoS ONE 2015, 10, e0118123. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Martin, M.; El Tahchi, M.R.; Balme, S.; Faour, W.H.; Varga, B.; Cloitre, T.; Páll, O.; Cuisinier, F.J.G.; Gergely, C.; et al. Influence of Hydrolyzed Polyacrylamide Hydrogel Stiffness on Podocyte Morphology, Phenotype, and Mechanical Properties. ACS Appl. Mater. Interfaces 2019, 11, 32623–32632. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, H.; Wang, Y.; Du, B.; Yang, J.; Liu, L.; Zhang, Q. Hyaluronic Acid Hydrogel with Adjustable Stiffness for Mesenchymal Stem Cell 3D Culture via Related Molecular Mechanisms to Maintain Stemness and Induce Cartilage Differentiation. ACS Appl. Bio Mater. 2021, 4, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, S.; Li, Y.; Li, M.; Sun, X.; An, J.; Xu, Q.; Chen, Z.; Wang, Y. Impact of hydrogel stiffness on the induced neural stem cells modulation. Ann. Transl. Med. 2021, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Chae, S.; Kim, G. An in vitro model using spheroids-laden nanofibrous structures for attaining high degree of myoblast alignment and differentiation. Theranostics 2021, 11, 3331–3347. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Ehret, A.E. Mechanical biocompatibility of highly deformable biomedical materials. J. Mech. Behav. Biomed. Mater. 2015, 48, 100–124. [Google Scholar] [CrossRef]
- Cao, S.; Wei, Y.; Bo, R.; Yun, X.; Xu, S.; Guan, Y.; Zhao, J.; Lan, Y.; Zhang, B.; Xiong, Y.; et al. Inversely engineered biomimetic flexible network scaffolds for soft tissue regeneration. Sci. Adv. 2023, 9, eadi8606. [Google Scholar] [CrossRef]
- Johnston, A.; Callanan, A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics 2023, 8, 205. [Google Scholar] [CrossRef]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef]
- Elvitigala, K.C.M.L.; Mubarok, W.; Sakai, S. Tuning the crosslinking and degradation of hyaluronic acid/gelatin hydrogels using hydrogen peroxide for muscle cell sheet fabrication. Soft Matter 2023, 19, 5880–5887. [Google Scholar] [CrossRef]
- Cai, G.; Li, X.; Lin, S.S.; Chen, S.; Koning, K.; Bi, D.; Liu, A.P. Matrix stiffness modulates 3D spheroid sorting and burst-like collective migration. bioRxiv 2023. [CrossRef]
- Quarta, A.; Gallo, N.; Vergara, D.; Salvatore, L.; Nobile, C.; Ragusa, A.; Gaballo, A. Agarose-collagen I hydrogels: Impact of the matrix stiffness on the growth of breast cancer cell lines spheroids and on drug penetration. Authorea 2021. [CrossRef]
- Sánchez-Cardona, Y.; Echeverri-Cuartas, C.E.; López, M.E.L.; Moreno-Castellanos, N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, Y.; Liu, Y.; Cai, H.; Zhang, W.; Tan, W.S. POSS-enhanced thermosensitive hybrid hydrogels for cell adhesion and detachment. RSC Adv. 2018, 8, 13813–13819. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel degradation promotes angiogenic and regenerative potential of cell spheroids for wound healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Stange, K.; Keric, A.; Friese, A.; Röntgen, M. Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential. Cells 2022, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Mori, Y.; Yamawaki, K.; Ishiguro, T.; Ohata, H.; Sato, A.; Sugino, K.; Yachida, N.; Yamaguchi, M.; Suda, K.; et al. Establishment of in vitro 3D spheroid cell cultivation from human gynecologic cancer tissues. STAR Protoc. 2021, 2, 100354. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Abbas, S.; Saxena, A.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Critical role of three-dimensional tumorsphere size on experimental outcome. Biotechniques 2020, 69, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Seyedmahmoud, R.; Çelebi-Saltik, B.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, M.R.; Ahadian, S. Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines 2019, 10, 679. [Google Scholar] [CrossRef]
- Demri, N.; Dumas, S.; Nguyen, M.-L.; Gropplero, G.; Abou-Hassan, A.; Descroix, S.; Wilhelm, C. Remote Magnetic Microengineering and Alignment of Spheroids into 3D Cellular Fibers. Adv. Funct. Mater. 2022, 32, 2204850. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthuramalingam, K.; Lee, H.J. Effect of GelMA Hydrogel Properties on Long-Term Encapsulation and Myogenic Differentiation of C2C12 Spheroids. Gels 2023, 9, 925. https://doi.org/10.3390/gels9120925
Muthuramalingam K, Lee HJ. Effect of GelMA Hydrogel Properties on Long-Term Encapsulation and Myogenic Differentiation of C2C12 Spheroids. Gels. 2023; 9(12):925. https://doi.org/10.3390/gels9120925
Chicago/Turabian StyleMuthuramalingam, Karthika, and Hyun Jong Lee. 2023. "Effect of GelMA Hydrogel Properties on Long-Term Encapsulation and Myogenic Differentiation of C2C12 Spheroids" Gels 9, no. 12: 925. https://doi.org/10.3390/gels9120925