Cysteine–Silver–Polymer Systems for the Preparation of Hydrogels and Films with Potential Applications in Regenerative Medicine
Abstract
:1. Introduction
2. Results and Discussion
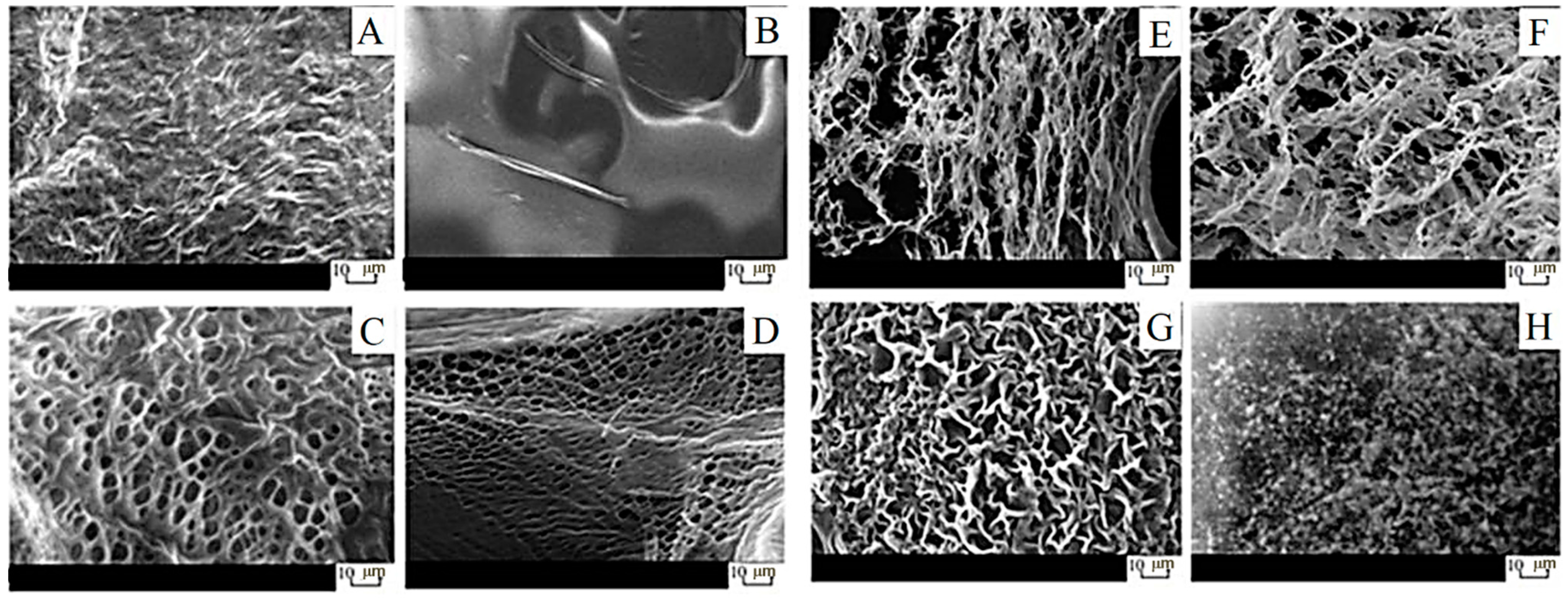
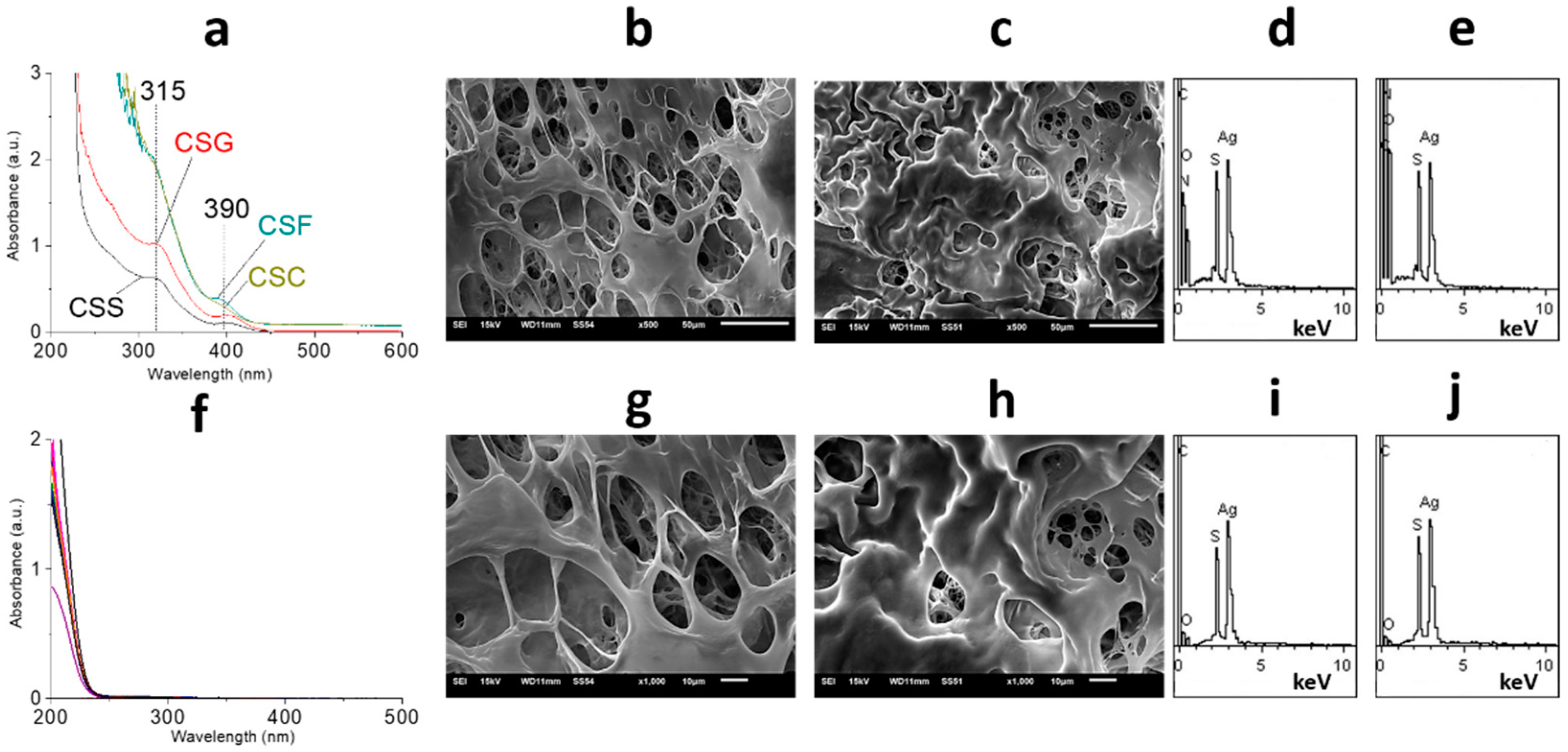
2.1. Characterization of Gels
2.2. Characterization of Films
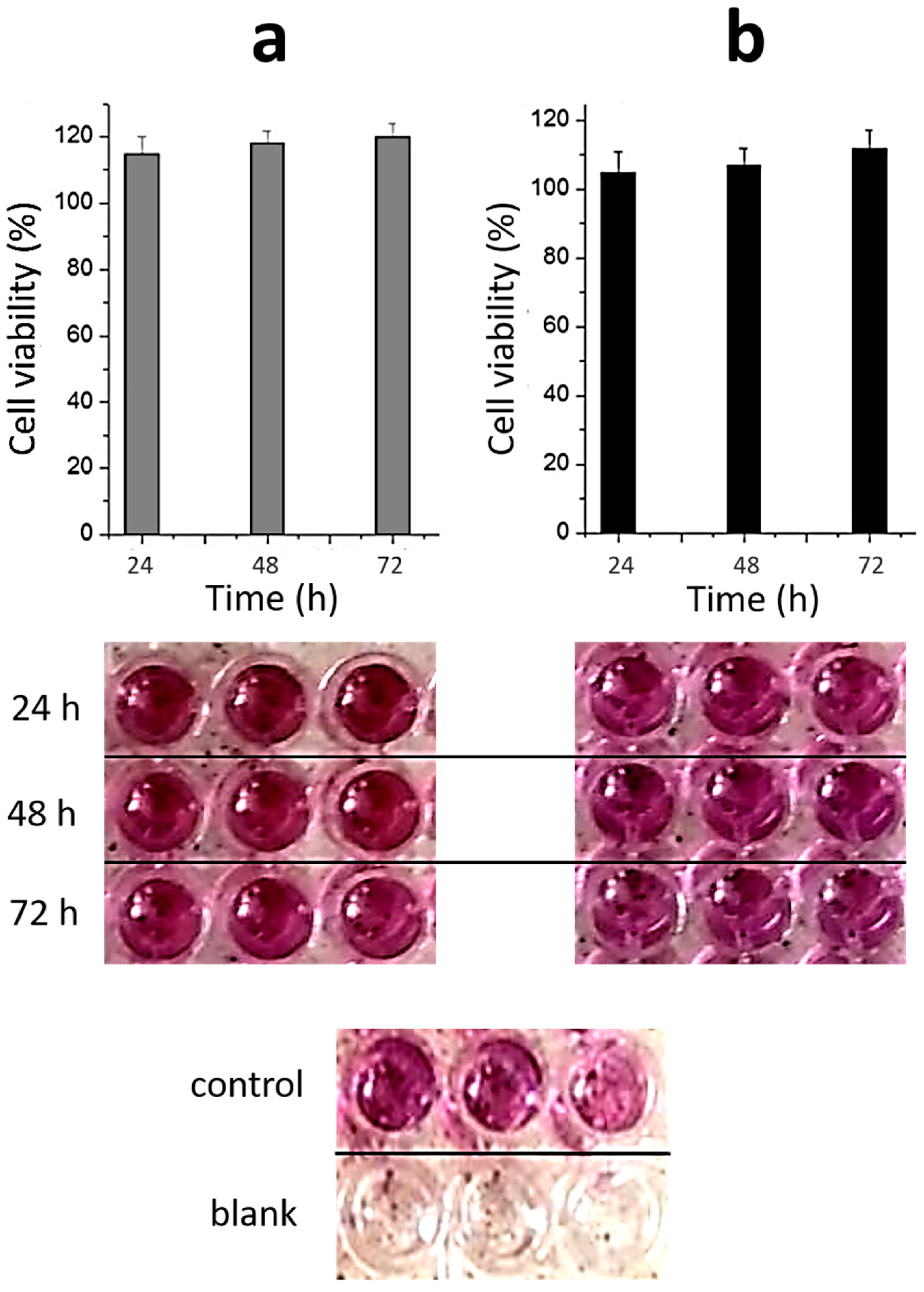
2.3. Toxicity of Materials
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. General Procedure for the Preparation of CSS, CSG, CSC and CSF
4.3. Rheological Test
4.4. SEM and EDS
4.5. UV Spectroscopy
4.6. DLS and Zeta Potential Measurements
4.7. pH Measurements
4.8. Cytotoxicity Evaluation of Hydrogels in Wi-38 Cells (MTT-TEST)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- FitzGerald, J.F.; Kumar, A.S. Biologic versus synthetic mesh reinforcement: What are the pros and cons? Clin. Colon. Rectal. Surg. 2014, 27, 140–148. [Google Scholar]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.S.; Adams, D.J. Multilayer 3D printed dipeptide-based on low molecular weight gels. Soft Matter 2022, 18, 5960–5965. [Google Scholar] [CrossRef]
- Chalard, A.; Mauduit, M.; Souleille, S.; Joseph, P.; Malaquin, L.; Fitremann, J. 3D printing of a biocompatible low molecular weight supramolecular hydrogel by dimethylsulfoxide water solvent exchange. Addit. Manuf. 2020, 33, 101162–101169. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels—combining polymers with low-molecular-weight gelators to yield modified self-assembling smart materials with hightech applications. Mater. Horiz. 2015, 2, 279–293. [Google Scholar] [CrossRef]
- Li, P.; Dou, X.Q.; Feng, C.L.; Zhang, D. Mechanical reinforcement of C 2-phenyl-derived hydrogels for controlled cell adhesion. Soft Matter 2013, 9, 3750–3757. [Google Scholar] [CrossRef]
- Kubiak, P.S.; Awhida, S.; Hotchen, C.; Deng, W.T.; Alston, B.; McDonald, T.O.; Adams, D.J.; Cameron, P.J. Polymerization of low molecular weight hydrogelators to form electrochromic polymers. Chem. Comm. 2015, 51, 10427–10430. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Controlling the Assembly and Properties of Low-Molecular-Weight Hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef]
- Xu, B.; Yan, H.; Sun, Y.; Chen, X.; Sun, Y.; Li, S.; Jing, Y.; Li, H. Organogels based on amino acid derivatives and their optimization for drug release using response surface methodology. Artif. Cells Nanomed. Biotechnol. 2020, 48, 266–275. [Google Scholar]
- Gortner, R.A.; Hoffman, W.F. The gelation of dibenzoyl-L-cystine. J. Am. Chem. Soc. 1921, 43, 2199. [Google Scholar]
- Menger, F.M.; Caran, K.L. Anatomy of a gel. Amino acid derivatives that rigidify water at submillimolar concentrations. J. Am. Chem. Soc. 2000, 47, 11679–11691. [Google Scholar] [CrossRef]
- Odriozoa, I.; Loinaz, I.; Pomposo, J.A.; Grande, H.J. Gold–glutathione supramolecular hydrogels. J. Mater. Chem. 2007, 17, 4843–4845. [Google Scholar] [CrossRef]
- Casuso, P.; Carrasco, P.; Loinaz, I.; Grande, H.J.; Odriozola, I. Converting drugs into gelators: Supramolecular hydrogels from N-acetyl-l-cysteine and coinage-metal salts. Org. Biomol. Chem. 2010, 8, 5455–5458. [Google Scholar] [CrossRef]
- Casuso, P.; Carrasco, P.; Loinaz, I.; Cabanero, G.; Grande, H.-J.; Odriozola, I. Argentophilic hydrogels: Elucidating the structure of neutral versus acidic system. Soft Matter 2011, 7, 3627–3633. [Google Scholar] [CrossRef]
- Casuso, P.; Vicente, A.P.-S.; Iribar, H.; Gutierrez-Rivera, A.; Izeta, A.; Loinaz, I.; Cabanero, G.; Grande, H.-J.; Odriozola, I.; Dupin, D. Aurophilically cross-linked “dynamic” hydrogels mimicking healthy synovial fluid properties. Chem. Comm. 2014, 50, 15199–15201. [Google Scholar] [CrossRef]
- Khizhnyak, S.D.; Komarov, P.V.; Ovchinnikov, M.M.; Zherenkova, L.V.; Pakhomov, P.M. Mechanism of gelation in low-concentration aqueous solutions of silver nitrate with l-cysteine and its derivatives. Soft Matter 2017, 13, 5168–5184. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Averkin, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. l-Cysteine/AgNO2 low molecular weight gelators: Self-assembly and suppression of MCF-7 breast cancer cells. Soft Matter 2020, 16, 9669–9673. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Averkin, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. l-Cysteine as a reducing/capping/gel-forming agent for the preparation of silver nanoparticle composites with anticancer properties. Soft Matter 2022, 18, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Vishnevetskii, D.V.; Averkin, D.V.; Efimov, A.A.; Lizunova, A.A.; Ivanova, A.I.; Pakhomov, P.M.; Ruehl, E. Ag/α-Ag2MoO4/h-MoO3 nanoparticle based microspheres: Synthesis and photosensitive properties. Soft Matter 2021, 17, 10416–10420. [Google Scholar] [CrossRef] [PubMed]
- Vishnevetskii, D.V.; Averkin, D.V.; Efimov, A.A.; Lizunova, A.A.; Shamova, O.V.; Vladimirova, E.V.; Sukhareva, M.S.; Mekhtiev, A.R. L-cysteine and N-acetyl-L-cysteine mediated synthesis of nanosilver-based sols and hydrogels with antibacterial and antibiofilm properties. J. Mater. Chem. B 2023, 11, 5794–5804. [Google Scholar] [CrossRef]
- Potapenkova, T.V.; Vishnevetskii, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. Effect of dispersed phase concentration on gelation and formation of silver nanoparticles in aqueous solutions of L-cysteine and silver nitrite. Russ. Chem. Bull. 2022, 71, 2123–2129. [Google Scholar] [CrossRef]
- Andrianova, Y.V.; Vishnevetskii, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. Gelation processes in an aqueous solution of L-cysteine/AgNO3 under the influence of metal salts with various valencies. Russ. Chem. Bull. 2023, 72, 2171–2179. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Semenova, E.M.; Averkin, D.V.; Mekhtiev, A.R. Behavior and bioactive properties of aqueous L-cysteine–AgNO3 solution at different pH. Mend. Commun. 2023, 33, 431–432. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Abrashitova, K.A.; Kuklin, A.I.; Orekhov, A.S.; Vasiliev, A.L.; Iliopoulos, I.; Philippova, O.E. Viscoelastic synergy and microstructure formation in aqueous mixtures of nonionic hydrophilic polymer and charged wormlike surfactant micelles. Macromolecules 2017, 50, 339–348. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Makarov, A.V.; Kuklin, A.I.; Iliopoulos, I.; Philippova, O.E. Role of Charge of Micellar Worms in Modulating Structure and Rheological Properties of their Mixtures with Nonionic Polymer. Macromolecules 2018, 51, 213–221. [Google Scholar] [CrossRef]
- Löwenberg, C.; Julich-Gruner, K.K.; Neffe, A.T.; Behl, M.; Lendlein, A. Salt-induced shape-memmory effect in gelatin-based hydrogels. Biomacromolecules 2020, 6, 2024–2031. [Google Scholar] [CrossRef]
- Alekseev, V.G.; Semenov, A.N.; Pakhomov, P.M. Complexation of Ag+ ions with L-cysteine. Russ. J. Inorg. Chem 2012, 57, 1041–1044. [Google Scholar] [CrossRef]
- He, Z.; Xiong, L. Drug Controlled Release and Biological Behavior of Poly(D,L-Lactide-Co-Glycolide) Microspheres. J. Macromol. Sci. Part B 2011, 50, 1154–1161. [Google Scholar] [CrossRef]
- Ji, W.; Afsar, N.U.; Wu, B.; Sheng, F.; Shehzad, M.A.; Ge, L.; Xu, T. In-situ crosslinked SPPO/PVA composite membranes for alkali recovery via diffusion dialysis. J. Membr. Sci. 2019, 590, 117267. [Google Scholar] [CrossRef]
- Ajith, C.; Deshpande, A.P.; Varughese, S. Proton conductivity in crosslinked hydrophilic ionic polymer system: Competitive hydration, crosslink heterogeneity, and ineffective domains. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1087–1101. [Google Scholar] [CrossRef]
- Kudoh, Y.; Kojima, T.; Abe, M.; Oota, M.; Yamamoto, T. Proton conducting membranes consisting of poly(vinyl alcohol) and poly(styrene sulfonic acid): Crosslinking of poly(vinyl alcohol) with and without succinic acid. Solid State Ion. 2013, 253, 189–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly(vinyl alcohol) with glyoxal. J. Polym. Res. 2009, 17, 725–730. [Google Scholar] [CrossRef]
- Xu, S.; Shen, L.; Li, C.; Wang, Y. Properties and pervaporation performance of poly(vinyl alcohol) membranes crosslinked with various dianhydrides. J. Appl. Polym. Sci. 2018, 135, 46159. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Poly-vinyl alcohol (PVA) crosslinked by trisodium trimetaphosphate (STMP) and sodium hexametaphosphate (SHMP): Effect of molecular weight, pH and phosphorylating agent on length of spacing arms, crosslinking density and water interaction. J. Mol. Struct. 2020, 1202, 127264. [Google Scholar] [CrossRef]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New crosslinked cast films based on poly(vinyl alcohol): Preparation and physico-chemical properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, J.-Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(vinyl alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers·PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 55. Retrospective view on the more than 40 years of studies performed in the A.N. Nesmeyanov Institute of Organoelement Compounds with respect of the cryostructuring processes in polymeric systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Mahnama, H.; Dadbin, S.; Frounchi, M.; Rajabi, S. Preparation of biodegradable gelatin/PVA porous scaffolds for skin regeneration. Artif. Cells Nanomed. Biotechnol. 2016, 45, 928–935. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, W.; Liu, C.; Tian, M.; Liu, P. A novel poly (vinyl alcohol)/poly (ethylene glycol) scaffold for tissue engineering with a unique bimodal open-celled structure fabricated using supercritical fluid foaming. Sci. Rep. 2019, 9, 9534. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.D.; Sposito, C.V.J.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Luther, E.M.; Koehler, Y.; Diendorf, J.; Epple, M.; Dringen, R. Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 2011, 22, 375101. [Google Scholar] [CrossRef]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; BraydichStolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Kakcinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed, Mater. Res. A. 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Lin, S.; Ji, Z.; Thomas, C.R.; Li, L.; Mecklenburg, M.; Meng, H.; Wang, X.; Zhang, H.; Xia, T.; et al. Surface Defects on Plate-Shaped Silver Nanoparticles Contribute to Its Hazard Potential in a Fish Gill Cell Line and Zebrafish Embryos. ACS Nano 2012, 6, 3745–3759. [Google Scholar] [CrossRef] [PubMed]
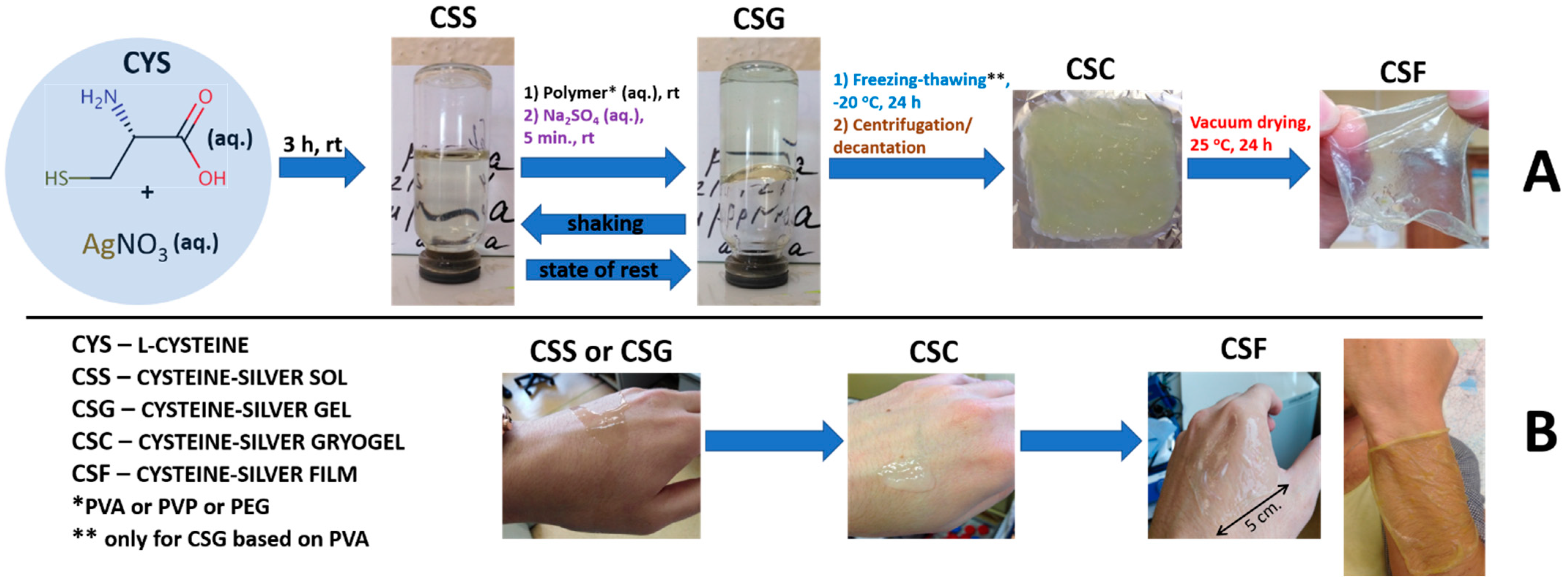
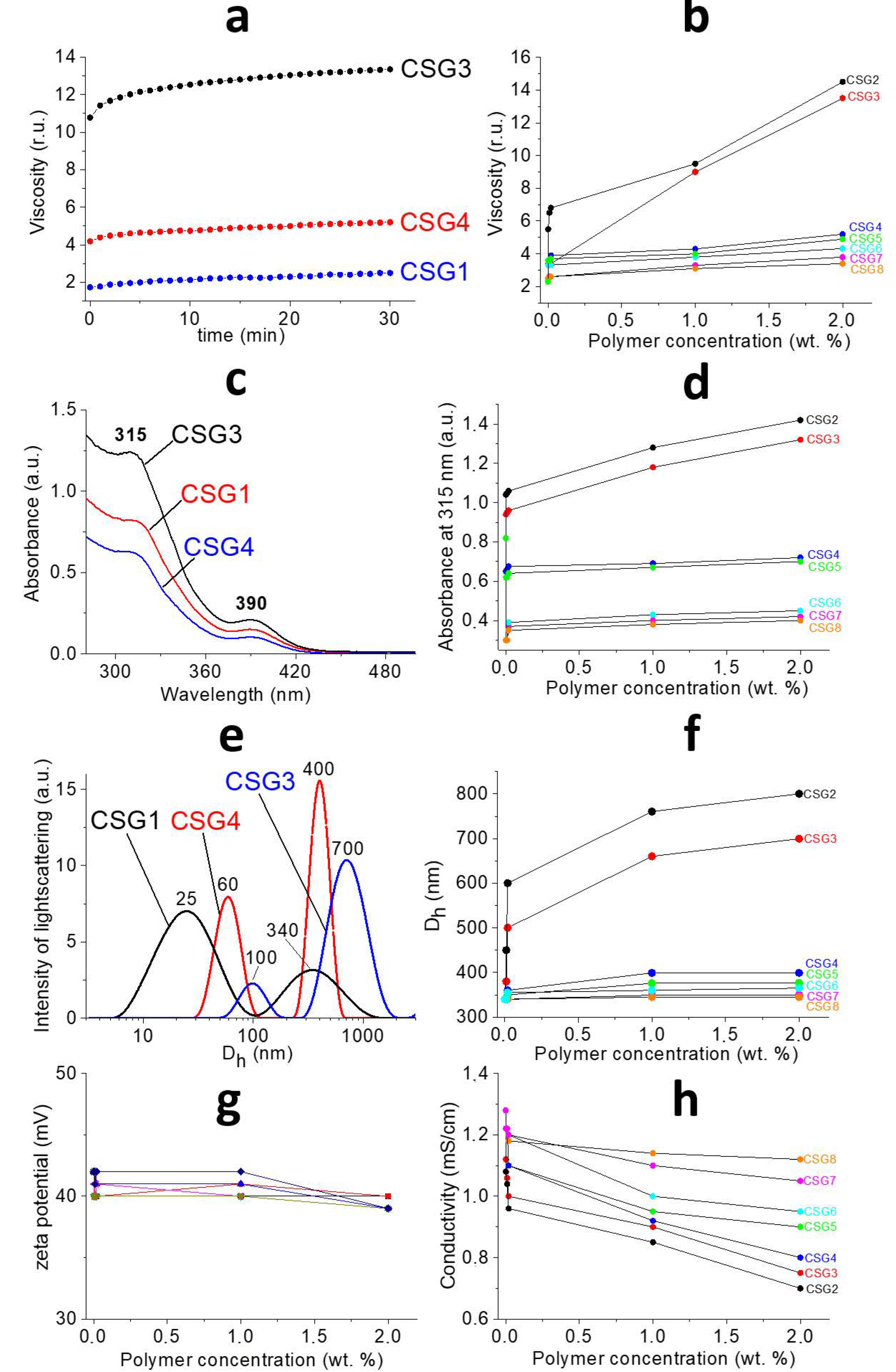
| Entry | Polymer (Molecular Weight kDa) |
|---|---|
| CSG1 CSG2 | No polymer PVA (100 kDa) |
| CSG3 | PVA (50 kDa) |
| CSG4 | PVP (40 kDa) |
| CSG5 | PVP (20 kDa) |
| CSG6 | PEG (40 kDa) |
| CSG7 | PEG (6 kDa) |
| CSG8 | PEG (0.4 kDa) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnevetskii, D.V.; Mekhtiev, A.R.; Averkin, D.V.; Polyakova, E.E. Cysteine–Silver–Polymer Systems for the Preparation of Hydrogels and Films with Potential Applications in Regenerative Medicine. Gels 2023, 9, 924. https://doi.org/10.3390/gels9120924
Vishnevetskii DV, Mekhtiev AR, Averkin DV, Polyakova EE. Cysteine–Silver–Polymer Systems for the Preparation of Hydrogels and Films with Potential Applications in Regenerative Medicine. Gels. 2023; 9(12):924. https://doi.org/10.3390/gels9120924
Chicago/Turabian StyleVishnevetskii, Dmitry V., Arif R. Mekhtiev, Dmitry V. Averkin, and Elizaveta E. Polyakova. 2023. "Cysteine–Silver–Polymer Systems for the Preparation of Hydrogels and Films with Potential Applications in Regenerative Medicine" Gels 9, no. 12: 924. https://doi.org/10.3390/gels9120924





