Jetting Dynamics of Burning Gel Fuel Droplets
Abstract
:1. Introduction
2. Results and Discussion
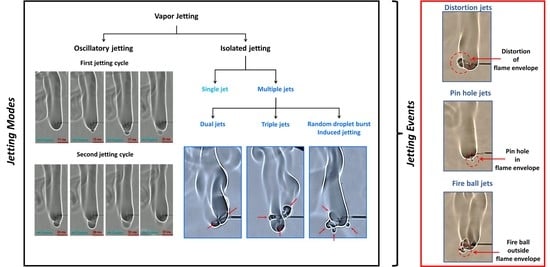
2.1. Jetting Modes
2.2. Jetting Events
2.3. Effect of the Gellant Type on the Jetting Events
3. Conclusions
- The jetting of fuel vapors is responsible for the disruptive combustion behavior of gel fuel droplets and occurs in two potential modes, namely, either oscillatory bursting or isolated bursting, or both, where the latter features single or multiple jets initiating simultaneously.
- Both the jetting modes constitute jetting events which may either distort the flame front or form a fire ball outside the enclosed flame envelope or, alternatively, break the flame front locally and form a pin hole therein. Accordingly, these events are identified as flame distortion, fire ball, and pin hole jets, respectively. The pin hole jets are the highest-velocity jets (~1000–1550 mm/s) and, hence, apply a high local shear to the flame front, thereby causing its localized extinction, which appears as a break in the flame.
- The type of the gellant and the nature of the shell that it forms determine the types of jetting events that will dominate the combustion behavior of a gel fuel droplet.
- The gellants that tend to form thin-weak-flexible shells (HPMC at 3 wt.% in this study) are associated with a low degree of internal pressure build-up and the formation of large rupture sites, which result in low-velocity flame distortion jets (500–870 mm/s). In contrast, the gellants that form thick-strong-rigid shells (MC at 9 wt.% in this study) are associated with a high degree of internal pressure build-up and the formation of tiny, localized rupture sites, which result in high-velocity flame ball (800–1530 mm/s) and pin hole jets (1000–1550 mm/s).
4. Materials and Methods
4.1. Materials
4.2. Experimental Test Facility
4.3. Image Acquisition and Post-Processing: Flame Front Tracking and Reconstruction
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurice, L.Q.; Lander, H.; Edwards, T.; Harrison, W.E., III. Advanced aviation fuels: A look ahead via a historical perspective. Fuel 2001, 80, 747–756. [Google Scholar] [CrossRef]
- Edwards, T. Liquid fuels and propellants for aerospace propulsion: 1903–2003. J. Propuls. Power 2003, 19, 1089–1107. [Google Scholar] [CrossRef]
- Natan, B.; Rahimi, S. The status of gel propellants in year 2000. Int. J. Energetic Mater. Chem. Propuls. 2002, 5, 172–194. [Google Scholar] [CrossRef]
- Hodge, K.; Crofoot, T.; Nelson, S. Gelled propellants for tactical missile applications. In Proceedings of the 35th Joint Propulsion Conference and Exhibit, Los Angeles, CA, USA, 20–24 June 1999; p. 2976. [Google Scholar]
- Ciezki, H.K.; Naumann, K.W.; Weiser, V. Status of gel propulsion in the year 2010 with a special view on the German activities. In Proceedings of the Deutscher Luft-und Raumfahrtkongress 2010, Hamburg, Germany, 31 August–2 September 2010; Volume 2010. [Google Scholar]
- Rahimi, S.; Natan, B. Flow of gel fuels in tapered injectors. J. Propuls. Power 2000, 16, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, S.; Natan, B. Atomization characteristics of gel fuels. In Proceedings of the 34th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Cleveland, OH, USA, 13–15 July 1998; p. 3830. [Google Scholar]
- Rahimi, S.; Natan, B. Numerical Solution of the Flow of Power-Law Gel Propellants in Converging Injectors. Propellants Explos. Pyrotech. 2000, 25, 203–212. [Google Scholar] [CrossRef]
- Chojnacki, K.; Feikema, D. Atomization studies of gelled bipropellant simulants using planar laser induced fluorescence. In Proceedings of the 31st Joint Propulsion Conference and Exhibit, San Diego, CA, USA, 10–12 July 1995; p. 2423. [Google Scholar]
- Green, J.; Rapp, D.; Roncace, J. Flow visualization of a rocket injector spray using gelled propellant simulants. In Proceedings of the 27th Joint Propulsion Conference, Sacramento, CA, USA, 24–26 June 1991; p. 2198. [Google Scholar]
- Urbon, B.C. Atomization and Combustion of a Gelled, Metallized Slurry Fuel. Master’s Thesis, Naval Postgraduate School, Monterey, CA, USA, 1992. [Google Scholar]
- Solomon, Y.; Natan, B. Experimental investigation of the combustion of organic-gellant-based gel fuel droplets. Combust. Sci. Technol. 2006, 178, 1185–1199. [Google Scholar] [CrossRef]
- Solomon, Y.; Natan, B.; Cohen, Y. Combustion of gel fuels based on organic gellants. Combust. Flame 2009, 156, 261–268. [Google Scholar] [CrossRef]
- Mishra, D.P.; Patyal, A. Effects of initial droplet diameter and pressure on burning of ATF gel propellant droplets. Fuel 2012, 95, 226–233. [Google Scholar] [CrossRef]
- Mishra, D.P.; Patyal, A.; Padhwal, M. Effects of gellant concentration on the burning and flame structure of organic gel propellant droplets. Fuel 2011, 90, 1805–1810. [Google Scholar] [CrossRef]
- Law, C.K. Internal boiling and superheating in vaporizing multicomponent droplets. AIChE J. 1978, 24, 626–632. [Google Scholar] [CrossRef]
- Wang, C.H.; Law, C.K. Microexplosion of fuel droplets under high pressure. Combust. Flame 1985, 59, 53–62. [Google Scholar] [CrossRef]
- Wang, C.H.; Liu, X.Q.; Law, C.K. Combustion and microexplosion of freely falling multicomponent droplets. Combust. Flame 1984, 56, 175–197. [Google Scholar] [CrossRef]
- Miglani, A.; Nandagopalan, P.; John, J.; Baek, S.W. Oscillatory bursting of gel fuel droplets in a reacting environment. Sci. Rep. 2017, 7, 3088. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Nie, W.; He, H. Unsteady combustion model of nonmetalized organic gel fuel droplet. Energy Fuels 2012, 26, 6627–6639. [Google Scholar] [CrossRef]
- Miglani, A.; Basu, S. Coupled mechanisms of precipitation and atomization in burning nanofluid fuel droplets. Sci. Rep. 2015, 5, 15008. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Miglani, A. Combustion and heat transfer characteristics of nanofluid fuel droplets: A short review. Int. J. Heat Mass Transf. 2016, 96, 482–503. [Google Scholar] [CrossRef]
- Miglani, A.; Basu, S.; Kumar, R. Insight into instabilities in burning droplets. Phys. Fluids 2014, 26, 032101. [Google Scholar] [CrossRef] [Green Version]
- Miglani, A.; Basu, S.; Kumar, R. Suppression of instabilities in burning droplets using preferential acoustic perturbations. Combust. Flame 2014, 161, 3181–3190. [Google Scholar] [CrossRef]
- Miglani, A.; Basu, S. Effect of particle concentration on shape deformation and secondary atomization characteristics of a burning nanotitania dispersion droplet. J. Heat Transf. 2015, 137, 102001. [Google Scholar] [CrossRef]
- Shinjo, J.; Xia, J.; Ganippa, L.C.; Megaritis, A. Physics of puffing and microexplosion of emulsion fuel droplets. Phys. Fluids 2014, 26, 103302. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Karmakar, S.; Som, S.K. Puffing and micro-explosion behavior in combustion of butanol/Jet A-1 and acetone-butanol-ethanol (ABE)/Jet A-1 fuel droplets. Combust. Sci. Technol. 2017, 189, 1796–1812. [Google Scholar] [CrossRef] [Green Version]
- Avulapati, M.M.; Ganippa, L.C.; Xia, J.; Megaritis, A. Puffing and micro-explosion of diesel–biodiesel–ethanol blends. Fuel 2016, 166, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Solomon, Y.; DeFini, S.J.; Pourpoint, T.L.; Anderson, W.E. Gelled monomethyl hydrazine hypergolic droplet investigation. J. Propuls. Power 2013, 29, 79–86. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; He, Z.; Wu, J. Experimental study on the combustion and microexplosion of freely falling gelled unsymmetrical dimethylhydrazine (UDMH) fuel droplets. Energies 2012, 5, 3126–3136. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R.; Anderson, W. Droplet burning of JP-8/silica gels. In Proceedings of the 48th AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2010; p. 421. [Google Scholar]
- Kunin, A.; Natan, B.; Greenberg, J.B. Theoretical model of the transient combustion of organic-gellant-based gel fuel droplets. J. Propuls. Power 2010, 26, 765–771. [Google Scholar] [CrossRef]
- Nachmoni, G.A.D.; Natan, B. Combustion characteristics of gel fuels. Combust. Sci. Technol. 2000, 156, 139–157. [Google Scholar] [CrossRef]
- Cho, K.Y.; Pourpoint, T.L.; Son, S.F.; Lucht, R.P. Microexplosion investigation of monomethylhydrazine gelled droplet with OH planar laser-induced fluorescence. J. Propuls. Power 2013, 29, 1303–1310. [Google Scholar] [CrossRef]
- John, J.; Nandagopalan, P.; Baek, S.W.; Miglani, A. Rheology of solid-like ethanol fuel for hybrid rockets: Effect of type and concentration of gellants. Fuel 2017, 209, 96–108. [Google Scholar] [CrossRef]
- Settles, G.S. Schlieren and Shadowgraph Techniques: Visualizing Phenomena in Transparent Media; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Agarwal, V.; Chitkariya, P.; Miglani, A.; Nandagopalan, P.; John, J.; Kankar, P.K. Deep learning-based image processing for analyzing combustion behavior of gel fuel droplets. In Smart Electrical and Mechanical Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 65–85. [Google Scholar]
- Moses, K.; Miglani, A.; Kankar, P.K. Deep CNN-based damage classification of milled rice grains using a high-magnification image dataset. Comput. Electron. Agric. 2022, 195, 106811. [Google Scholar]
- Patil, S.; Miglani, A.; Kankar, P.K.; Roy, D. Deep learning-based methods for detecting surface defects in steel plates. In Smart Electrical and Mechanical Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 87–107. [Google Scholar]


















| Flame Disruption Event | HPMC-3% (mm/s) | MC-9% (mm/s) |
|---|---|---|
| Flame distortion | 800 ± 5% | 873 ± 7% |
| Fireball outside the flame | 1095 ± 10% | 1030 ± 8% |
| Pin ejections | 1313 ± 9% | 1155 ± 6% |
| Gellant | Weight % | Ethanol (Wt. %) | De-Ionized Water (Wt. %) | Yield Stress (Pa) |
|---|---|---|---|---|
| HPMC | 3 | 82 | 15 | 23.23 2.62 |
| MC | 9 | 77 | 14 | 398 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, J.; Miglani, A.; John, J.; Nandagopalan, P.; Shaikh, J.; Kankar, P.K. Jetting Dynamics of Burning Gel Fuel Droplets. Gels 2022, 8, 781. https://doi.org/10.3390/gels8120781
Sharma J, Miglani A, John J, Nandagopalan P, Shaikh J, Kankar PK. Jetting Dynamics of Burning Gel Fuel Droplets. Gels. 2022; 8(12):781. https://doi.org/10.3390/gels8120781
Chicago/Turabian StyleSharma, Janmejai, Ankur Miglani, Jerin John, Purushothaman Nandagopalan, Javed Shaikh, and Pavan Kumar Kankar. 2022. "Jetting Dynamics of Burning Gel Fuel Droplets" Gels 8, no. 12: 781. https://doi.org/10.3390/gels8120781