Irreversible Swelling Behavior and Reversible Hysteresis in Chemically Crosslinked Poly(vinyl alcohol) Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Behavior in Aqueous/Organic Mixtures
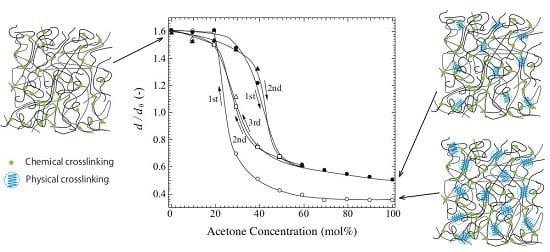
2.2. Swelling Ratio in DMSO/Acetone Mixed Solvent
2.3. Change in Microcrystallites in the DMSO/Acetone Mixed Solvent
2.4. Origin of Reversible Change with a Large Hysteresis
3. Conclusions
4. Experimental
4.1. Sample Preparation
4.2. Measurements of Swelling Ratio and FT-IR
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tanaka, T. Gels. Sci. Am. 1981, 244, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Ushio, K.; Kumar, P.; Ikeuchi, K.; Hyon, S.H.; Nakamura, T.; Fujita, H. Development of artificial cartilage. Proc. Inst. Mech. Eng. H J. Eng. Med. 2000, 214, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chang, Y.S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Nambu, M. Japanese Patent Kokai. Japan Patent 59/56446, 1984. [Google Scholar]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol). Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Otsuka, E.; Suzuki, A. A simple method to obtain a swollen PVA gel crosslinked by hydrogen bonds. J. Appl. Polym. Sci. 2009, 114, 10–16. [Google Scholar] [CrossRef]
- Otsuka, E.; Suzuki, A. Swelling properties of physically cross-linked PVA gels prepared by a cast-drying method. In Gels: Structures, Properties, and Functions; Springer: Berlin/Heidelberg, Germany, 2009; pp. 121–126. [Google Scholar]
- Otsuka, E.; Sugiyama, M.; Suzuki, A. Network microstructure of PVA cast gels observed by SAXS measurements. J. Phys. Conf. Ser. 2010, 247, 012043. [Google Scholar] [CrossRef]
- Wang, B.; Mukataka, S.; Kodama, M.; Kokufuta, E. Viscometric and light scattering studies on microgel formation by γ-ray irradiation to aqueous oxygen-free solutions of poly(vinyl alcohol). Langmuir 1997, 13, 6108–6114. [Google Scholar] [CrossRef]
- Yoshii, F.; Makuuchi, K.; Darwis, D.; Iriawan, T.; Razzak, M.T.; Rosiak, J.M. Heat resistance poly(vinyl alcohol) hydrogel. Radiat. Phys. Chem. 1995, 46, 169–174. [Google Scholar] [CrossRef]
- Praptowidodo, V.S. Influence of swelling on water transport through PVA-based membrane. J. Mol. Struct. 2005, 739, 207–212. [Google Scholar] [CrossRef]
- Dai, W.S.; Barbari, T.A. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly(vinyl alcohol). J. Membr. Sci. 1999, 156, 67–79. [Google Scholar] [CrossRef]
- Kudo, S.; Otsuka, E.; Suzuki, A. Swelling behavior of chemically crosslinked PVA gels in mixed solvents. J. Polym. Sci. B 2010, 48, 1978–1986. [Google Scholar] [CrossRef]
- Otsuka, E.; Kudo, S.; Sugiyama, M.; Suzuki, A. Effects of microcrystallites on swelling behavior in chemically crosslinked poly(vinyl alcohol) gels. J. Polym. Sci. B 2011, 49, 96–102. [Google Scholar] [CrossRef]
- Otsuka, E.; Sugiyama, M.; Suzuki, A. Formation and destruction of physical crosslinks by mild treatments in chemically crosslinked poly(vinyl alcohol) gels. Polym. Bull. 2011, 67, 1215–1226. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a non-ionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Shinyashiki, N.; Shimomura, M.; Ushiyama, T.; Miyagawa, T.; Yagihara, S. Dynamics of Water in Partially Crystallized Polymer/Water Mixtures Studied by Dielectric Spectroscopy. J. Phys. Chem. B 2007, 111, 10079–10087. [Google Scholar] [CrossRef] [PubMed]
- Nkhwa, S.; Lauriaga, K.F.; Kemal, E.; Deb, S. Poly(vinyl alcohol): Physical Approaches to Designing Biomaterials for Biomedical Applications. Conf. Pap. Sci. 2014, 2014, 403472. [Google Scholar] [CrossRef]
- Miya, M.; Iwamoto, R.; Mima, S. FT-IR study of intermolecular interactions in polymer blends. J. Polym. Sci. B 1984, 22, 1149–1151. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Peppas, N.A. Dissolution mechanism of semicrystalline poly(vinyl alcohol) in water. J. Polym. Sci. B 1996, 34, 1339–1346. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.J.; Jang, J. Effect of silica nanofillers on isothermal crystallization of poly(vinyl alcohol): In situ ATR-FTIR study. Polym. Test. 2008, 27, 360–367. [Google Scholar] [CrossRef]
- Hirashima, Y.; Suzuki, A. Roles of Hydrogen Bonding on the Volume Phase Transition of Ionized Poly(N-isopropylacrylamide) Gels. J. Phys. Soc. Jpn. 2004, 73, 404–411. [Google Scholar] [CrossRef]
- Sato, H.; Hirashima, Y.; Suzuki, A. Reswelling transition of poly(sodium acrylate) gels due to destruction of hydrogen bonds observed by ATR FTIR spectroscopy. J. Appl. Polym. Sci. 2007, 105, 3809–3816. [Google Scholar] [CrossRef]
- Annaka, M.; Tanaka, T. Multiple phases of polymer gels. Nature 1992, 355, 430–432. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamemaru, K.; Usui, S.; Hirashima, Y.; Suzuki, A. Irreversible Swelling Behavior and Reversible Hysteresis in Chemically Crosslinked Poly(vinyl alcohol) Gels. Gels 2018, 4, 45. https://doi.org/10.3390/gels4020045
Kamemaru K, Usui S, Hirashima Y, Suzuki A. Irreversible Swelling Behavior and Reversible Hysteresis in Chemically Crosslinked Poly(vinyl alcohol) Gels. Gels. 2018; 4(2):45. https://doi.org/10.3390/gels4020045
Chicago/Turabian StyleKamemaru, Keiichiro, Shintaro Usui, Yumiko Hirashima, and Atsushi Suzuki. 2018. "Irreversible Swelling Behavior and Reversible Hysteresis in Chemically Crosslinked Poly(vinyl alcohol) Gels" Gels 4, no. 2: 45. https://doi.org/10.3390/gels4020045