1. Introduction
Biological systems often take a gelled state. Understanding the frozen state of gels (i.e., the size and distribution of ice grains) is of practical importance for the implementation of cryopreservation. Water in gels is compartmentalized by the polymer network [
1,
2], and there is a possibility that the polymer network obstructs and retards the diffusional motion of the water molecules in gels [
3], consequently preventing the growth of ice crystals. The study of the freezing behavior and the state of the frozen gels is of basic interest, as they reflect the characteristics of the polymer network (i.e., the flexibility of polymer chains, the size of the compartments, and the extent of the continuity between adjacent compartments which are interrelated via the density of the crosslinks) [
4].
In this connection, the freezing behavior of Sephadex
® (crosslinked dextran) gels has been investigated mainly by DSC (differential scanning calorimetry) [
1,
2] and by various physical techniques [
5]. Stemming from these investigations, different freezing behaviors were indicated depending on the density of the crosslinks. It was found that with the Sephadex
® G25 gel, the water remains partially unfrozen during cooling and the ice crystallizes during the subsequent warming (rewarming), as indicated by DSC. A conceivable scheme of ice crystallization during rewarming is described as follows. Some part of the water in the gel is trapped by the polymer network at the time of freezing initiation when it is cooled, followed by a change in the polymer network that turns it into a glassy state. The glassy water crystallizes during the rewarming, caused by the partial melting of the ice that was previously separated [
5,
6,
7]. The presence of glassy water, however, remains unconfirmed. Even when the water does not turn into a glassy state, small ice crystals are formed in the gel and a powder diffraction pattern has been observed by a two-dimensional X-ray diffraction (XRD) study [
8]. In Sephadex
® gels with crosslinks of both higher and lower densities than that of G25, an anomalous freezing behavior during rewarming was not observed, and the larger ice crystals were considered to have formed, which was also indicated by the two-dimensional XRD study [
8].
As the glass transition temperature (
Tg) of hyper-quenched liquid water is known to be around 136 K (−137 °C) [
9], the
Tg of water in a G25 gel (assumed to be around −50 °C) is too high. The
Tg of the water in the G25 gel was estimated from the temperature where the liquid water disappears in the ESR (Electron Spin Resonance) spectra obtained by using a spin probe method. The water in the G25 gel is partially trapped by the polymer network and turns into a glassy state at the time of freezing initiation, presumably as a consequence of the close interaction with the hydroxy groups located at the surface of the dextran network structures via the hydrogen bonds. The high
Tg value of the water in the gel can thus be explained. In fact, the
Tg of bulk water confined in MCM-41 nanopores was suggested to be 210 K [
10].
The occurrence of ice crystallization during rewarming observed with Sephadex
® G25 gel is characteristic of non-equilibrium freezing after substantial supercooling, and it is not observed after equilibrium freezing [
7]. In this connection, supercooled water is of great interest from a scientific standpoint and has been actively discussed since the 1970s [
11]. Although various thermodynamic properties such as the heat capacity (
Cp), the isothermal compressibility, and so forth have indicated a divergence at around −45 °C, the behavior of said divergence is a matter of concern [
11,
12] and it is still unclear due to the interference by homogeneous nucleation which occurs at around −40 °C. Concerning the amorphous phases of ice, the high-density and low-density amorphous forms of ice and/or polymorphism—where a deeper understanding of the hydrogen bond network in water is required [
13]—have also been intensively discussed. Although the presence of supercooling is a requisite for the observation of ice crystallization during rewarming, the water in the gel freezes at around −22 °C; the degree of supercooling is not very high. Then, the rates of freezing and the resultant change of the polymer network dependent on the degree of supercooling need to be fast for the observation and the occurrence of the glass transition. The analysis of ice crystallization during rewarming observed with a G25 gel might provide valuable information in this connection.
Ice crystals exist in aggregates as ice grains, and small ice grains are formed as a consequence of the formation of small ice crystals. Then, the sizes of the ice grains observed reflect the sizes of ice crystals that are formed. Although it might be true that the size of ice crystals (and therefore the ice grains formed) are different depending on the density of the crosslinks or on the polymer network structure, the information obtained by the diffraction pattern of the two-dimensional XRD is unsatisfactory, as there is no information on the distribution of the ice grains in the gels. The direct observation of ice grains at a high resolution is desirable for the understanding of the frozen state. The observation of the beads by SEM (scanning electron microscope) after freeze-drying is possible. However, there is concern that the technique will lead to changes in the polymer network during the freeze-drying treatment.
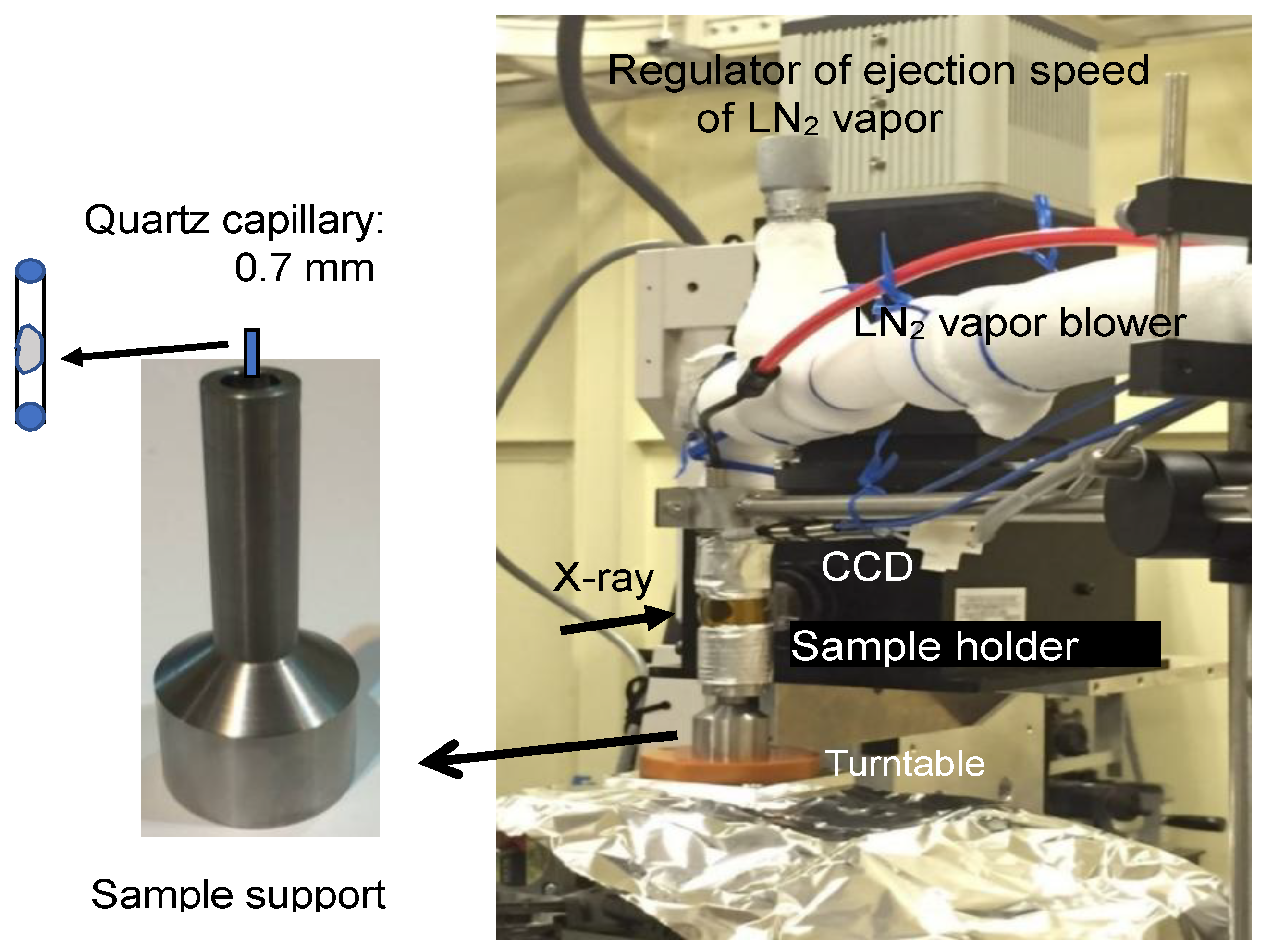
In the present study, the in situ observation of the ice grains formed in Sephadex
® gel beads was conducted by the X-ray CT (computed tomography) imaging technique. Recently, the X-ray CT technique has been improved, making it possible to acquire three-dimensional images of the microstructure of foods or biological systems [
14,
15]. The technique of X-ray CT imaging is based on the detection of the differences in the X-ray absorption rate by the materials that compose the sample. The contrast of the images depends on the wavelength, chromaticity (mono- or poly-), and the brilliance of the X-ray beam applied, as well as the mass density and chemical components of materials. By using synchrotron radiation, a highly brilliant and monochromatic X-ray beam can be provided and a high spatial resolution was made possible. Then, the synchrotron radiation-sourced X-ray CT analysis of the ice grains formed in Sephadex
® beads was conducted at SPring-8, a synchrotron radiation facility in Japan. The detection of ice grains in the micrometer range by using a beam line in the facility was anticipated. The observation of the freeze-dried Sephadex
® beads by SEM was also conducted for comparison.
To understand the frozen state of gels, it is necessary to obtain information about the ordered structure of the polymer network forming the gel as well as the size of the ice grains that are smaller than a micrometer. For that purpose, synchrotron radiation-sourced SAXS (small-angle X-ray scattering) measurements were also carried out at SPring-8. For the analysis of the completeness of ice crystals determining the shape of the ice grains, WAXD (wide-angle X-ray diffraction) measurements were carried out together with SAXS measurements in the XRD study. As the XRD study deals with the structure of dimensions that are smaller than the beads, the term “gel” was used for the XRD experiment instead of “beads.”
The objective of the present study was to analyze the shape and distribution of the ice grains formed in the Sephadex® beads with different crosslink densities, as well as to comprehend the frozen state, especially that of the G25 gel, that leads to ice crystallization during rewarming.
3. Discussion
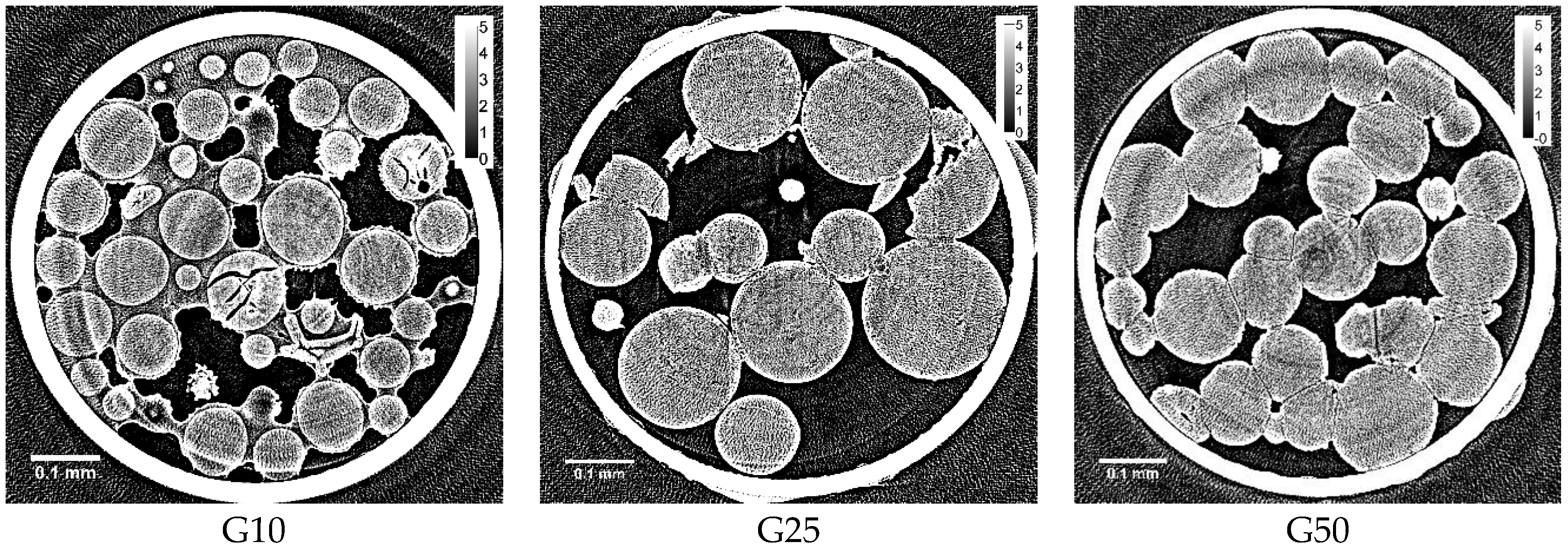
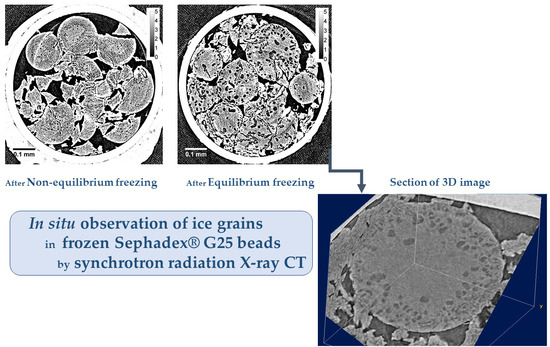
By the present study of X-ray CT measurement using synchrotron radiation, the in situ observation of the ice grains in Sephadex® beads was made possible with a spatial resolution of ~1 μm. As a result, it is necessary to change the interpretation of the size and distribution of the ice grains formed in the beads that was held previously.
By the previous study of the two-dimensional XRD measurement, it was interpreted that ice crystals of larger size were formed in a G10 gel compared to those in G25 and G100 gels, as many clear spots were observed on the three concentric rings due to the diffractions by hexagonal ice in the image [
8]. Moreover, it was considered that the formation of large ice crystals corresponded to the formation of the large ice grains in the internal region of G10 beads. However, clear spots observed by the two-dimensional XRD measurement were probably due to the ice crystallized outside the G10 beads as the unabsorbed water existed, which was indicated by the X-ray CT image obtained after rapid cooling (
Figure 1). Although the water holding capacity of a G10 gel is 1 g water/g dry gel, corresponding to a water content of 50 wt % according to the literature [
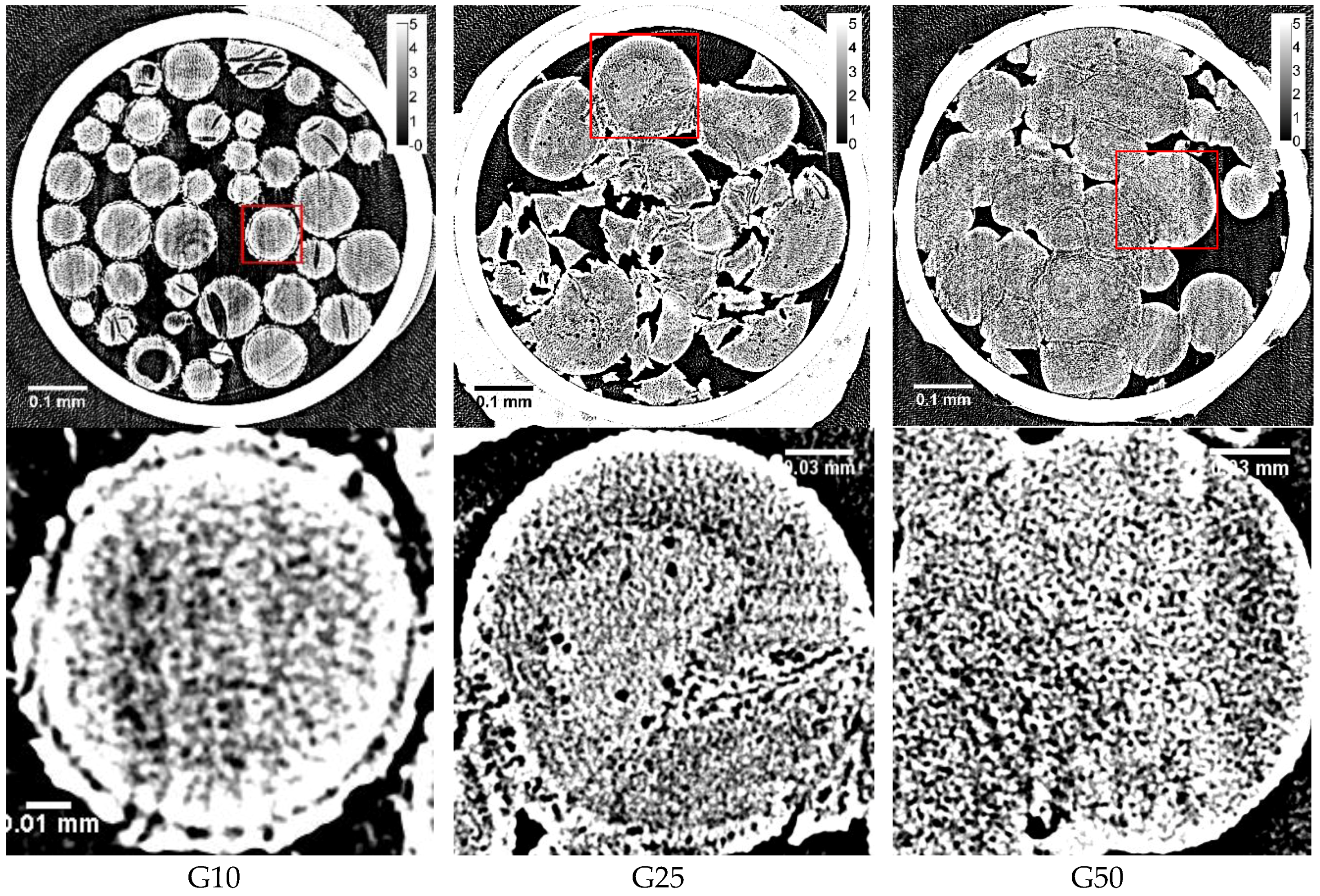
16], it is reasonable to consider that some water remains unabsorbed outside the gel beads because of the inhomogeneous distribution of water in the bead sample. There is another possibility of the observation of clear spots in the two-dimensional XRD image of a G10 gel—the large ice grains may be formed in the region of the beads between the rigid surface-treated layer and the polymer matrix. The existence of this region is suggested by the image in
Figure 2. However, no ice grains larger than ~1 μm were observed in the internal region of G10 beads, even after slow cooling, though most of the water in the beads was frozen. By the DSC measurement, about 70 percent of the water in the Sephadex
® beads was estimated to be frozen during slow cooling [
2]. Looking at the expanded X-ray CT images of the beads (
Figure 2), small structures of ~1 μm seemed to appear in the internal region of the beads. However, they might be artifacts, as the fluctuation of the X-ray absorption rate is considerable, especially at the border between ice and the polymer matrix. Ring artifacts were also observed, as can especially be seen in
Figure 1.
In the internal region of G25 beads, a small number of ice grains of 3‒5 μm in diameter were observed after slow cooling. Although the ice grains were observed in G25 beads, most of the ice grains that formed in the rest of the beads were smaller than ~1 μm, as they were not visible in the image. This is consistent with the results obtained from the two-dimensional XRD study where the powder diffraction pattern was observed [
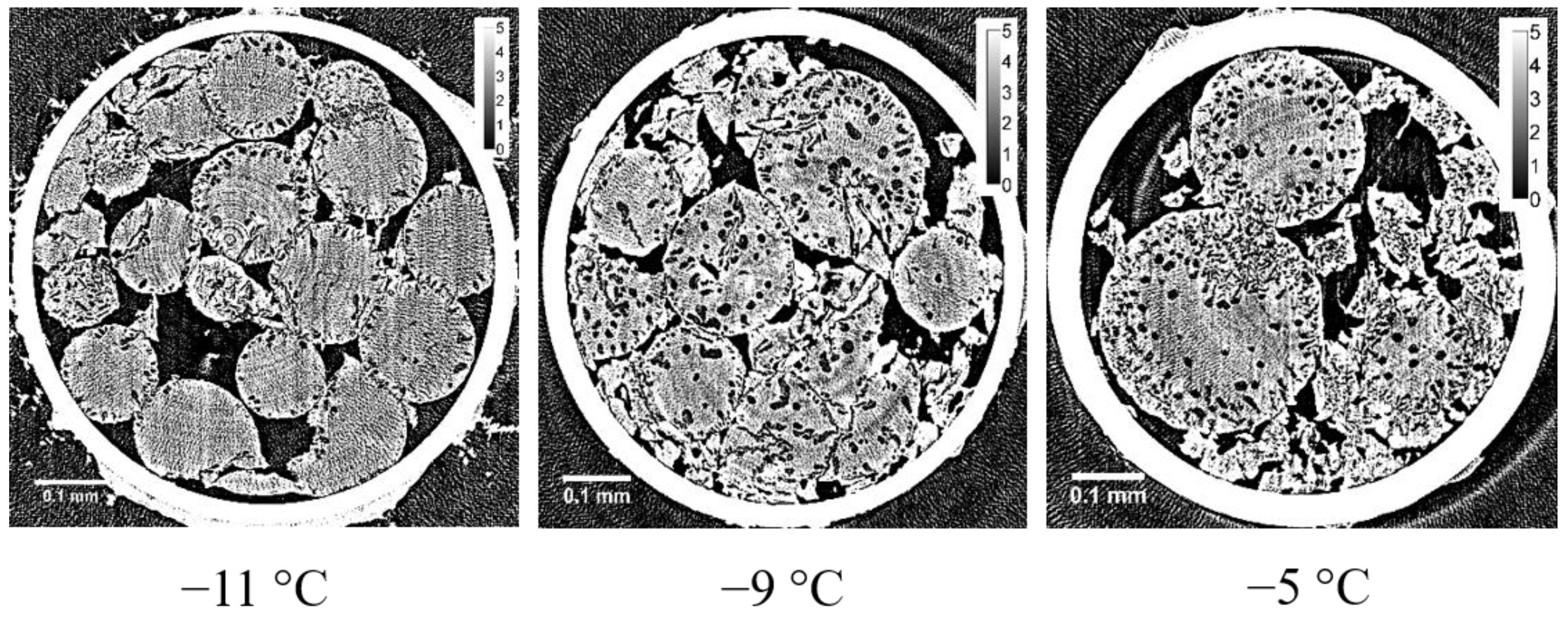
8]. In the X-ray CT image obtained with the refrozen G25 beads re-cooled from −9 °C (the temperature of the completion of ice crystallization during rewarming), many large ice grains larger than 10 μm appeared in the internal region of the beads, which is also consistent with the result of the two-dimensional XRD study where the spotty diffraction pattern indicating the formation of large ice crystals was observed.
There were no surface-treated layers observed in G50 beads. Instead, G50 beads containing 50 wt % water were ready to stick together, probably by means of the tangling bonds of the polymer chains existing on the bead surface. In this connection, G100 beads with lower crosslink densities than G50 beads became too sticky, losing the bead structure when they were added with water [
17], making it difficult to prepare G100 bead samples in quartz capillaries. That is the reason why G50 beads were used in this study instead of G100 beads as the bead sample with low crosslink densities. No ice grains were observed in the CT image of G50 beads. Considering the result obtained by the two-dimensional XRD study using a G100 gel, where pairs of arcs facing each other were seen on the three concentric rings due to the diffractions by hexagonal ice [
8], there is a possibility that long and narrow structures could be observed in the X-ray CT image of G50 beads. However, the width of the structures may have been too narrow (<~1 μm), if they existed, to be recognized by the observation.
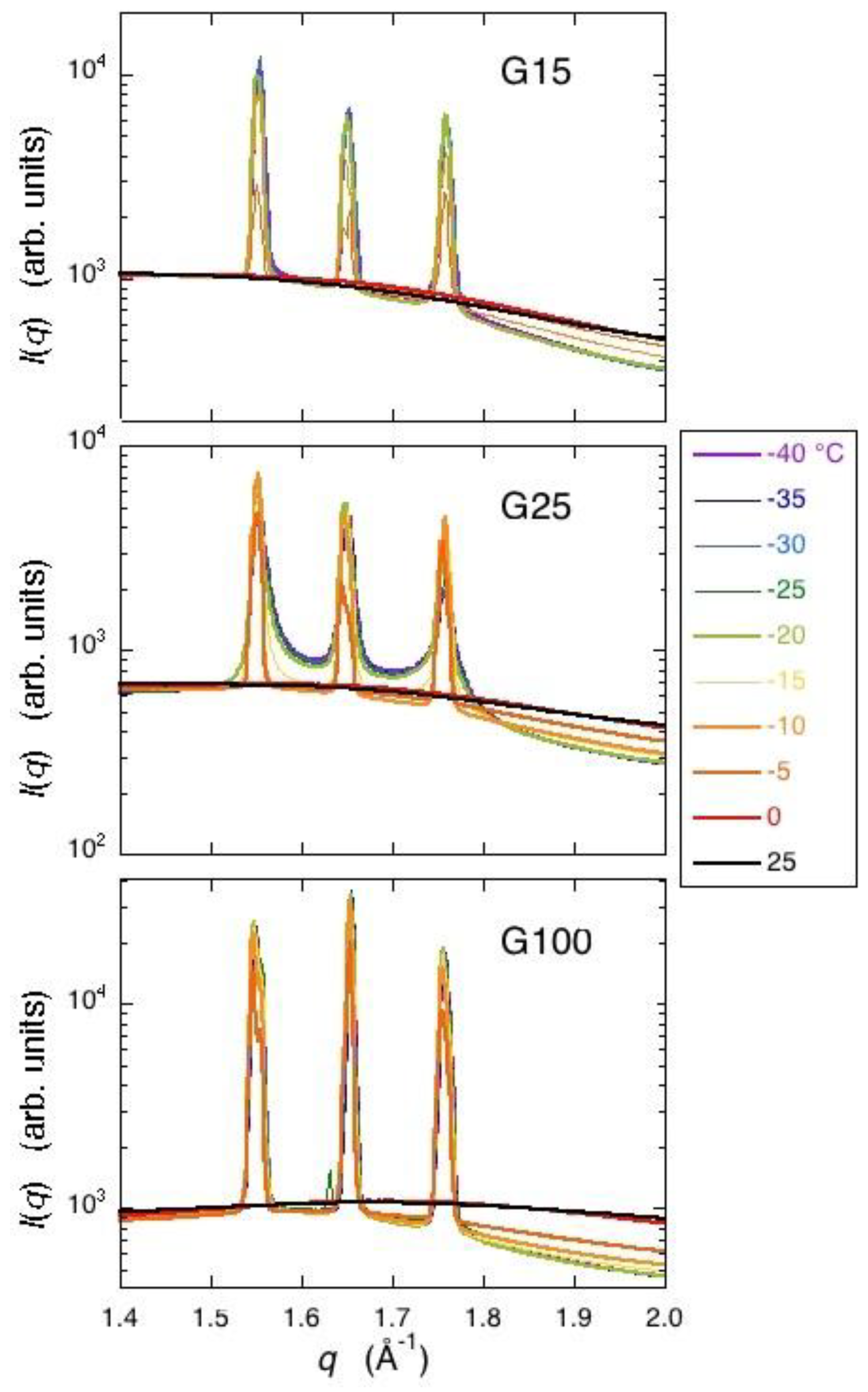
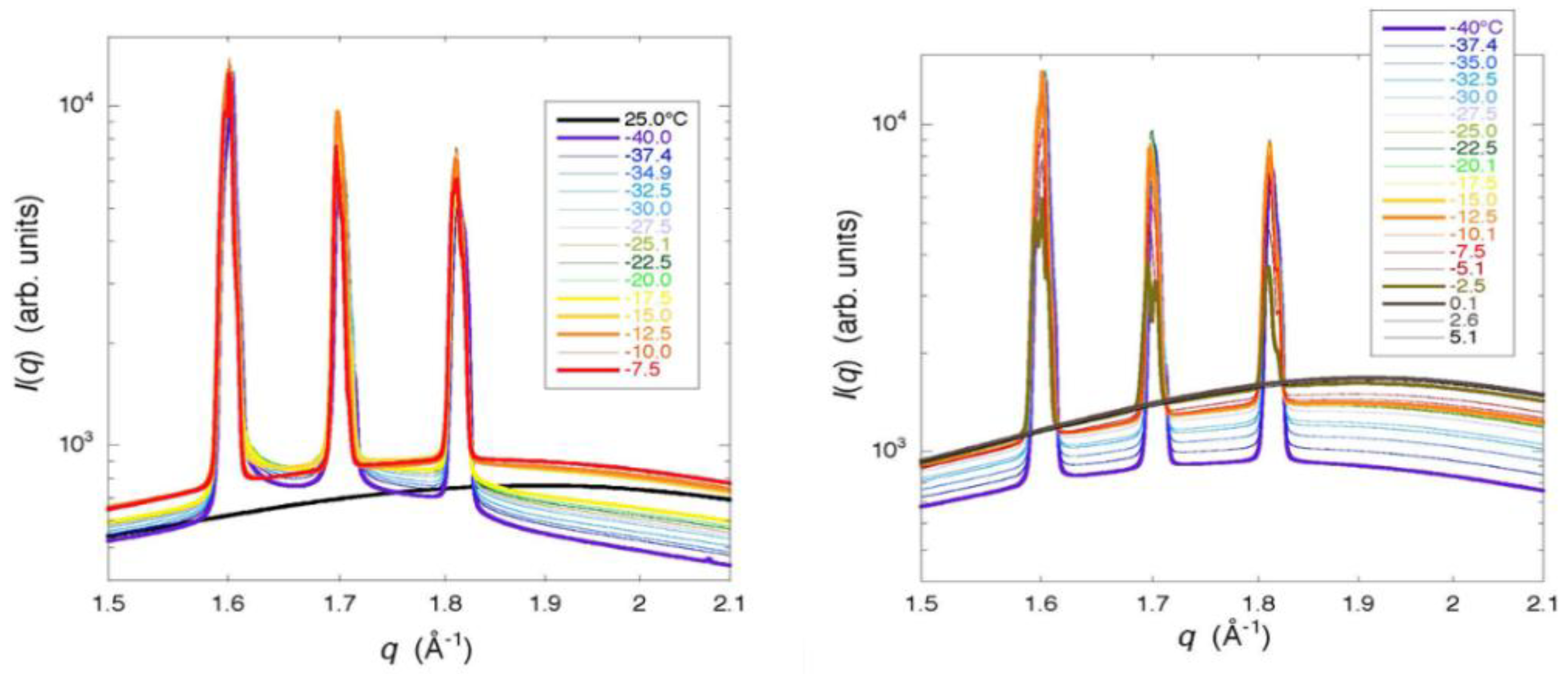
From the results of the WAXD measurement, the formation of small incomplete ice crystals in a G25 gel was indicated, as Bragg peaks showed a remarkable tailing. The result is consistent with that of the two-dimensional XRD study where continuous but dim images due to the diffractions by powder crystals was obtained for hexagonal ice. Moreover, the presence of glassy water in a frozen G25 gel was indicated, though not decisive [
5,
18]. A slight tailing in the Bragg peaks was also observed with a frozen G15 gel, indicating the presence of small ice crystals in the gel with higher crosslink densities than a G25 gel. The finding is consistent with the interpretation of the result obtained with a G10 gel by the X-ray CT measurement. In the case of a G100 gel with lower crosslink densities than a G25 gel, the formation of large and stable ice crystals was indicated by WAXD, which is also consistent with the result obtained by the two-dimensional X-ray measurement, though the presence of long and narrow ice structures was not verified by the X-ray CT measurement as mentioned above. The observation of narrow Bragg peaks with a refrozen G25 gel indicated large and stable ice crystals. Therefore, large ice grains were formed in the beads at the time of ice crystallization during rewarming. This result corresponds with that of the X-ray CT measurement.
It is certain that the frozen state of the Sephadex
® gels depending on the density of the crosslinks was made clearer by the experiments of a synchrotron radiation-sourced X-ray CT and an XRD. However, the frozen state of the gels of submicron order remains unclarified. More precise SAXS measurements are necessary. Concerning ice crystallization during rewarming, the presence of glassy water in a G25 gel is still unconfirmed. Detection of the glass transition in the frozen gel is desirable for this clarification [
10].
4. Conclusions
The frozen state of Sephadex® gels containing 50 wt % water was analyzed by using a synchrotron radiation-sourced X-ray CT and XRD.
By X-ray CT, the ice grains in Sephadex® beads were observed with a spatial resolution of ~1 μm. As a result, ice grains larger than ~1 μm were hardly observed in the beads after slow cooling, independent of the crosslink density. In G25 beads, a small number of ice grains of 3‒5 μm in diameter were observed after slow cooling, but most of the ice grains formed in the beads were smaller than ~1 μm. Ice grains larger than 10 μm in diameter were successfully observed in the refrozen G25 beads re-cooled from the temperature of the completion of ice crystallization during rewarming. These results obtained by the X-ray CT measurement were consistent with those previously obtained by two-dimensional XRD measurement.
The results obtained by XRD—especially by WAXD—corresponded well with those obtained by X-ray CT. The observation of a remarkable tailing in Bragg peaks due to the diffraction by hexagonal ice indicated the formation of small incomplete ice crystals or the presence of glassy water in a G25 gel. With the refrozen G25 gel re-cooled from the temperature of the completion of ice crystallization during rewarming, the tailing in Bragg peaks disappeared, which indicated the formation of large and stable ice crystals, as were observed by X-ray CT.
Finally, the size and distribution of the ice grains formed in Sephadex® beads were found to depend on the crosslink density.