1. Introduction
Aerogels are delicate materials prepared from wet gels by very careful removal of the solvent in a special drying process, which can preserve the original gel structure without collapse. The main reason for collapse is the presence of capillary forces, originating from the surface tension of the liquid filling the gel structure, as well as the adhesion of the liquid to the capillary walls. Under simple atmospheric drying, capillary forces may destroy the original gel structure, thereby leading to significant shrinking and cracking. To avoid structural damages, measures include eliminating the surface tension (supercritical drying); blocking the mobility of the gel and solvent (freeze-drying); or changing the polarities of the capillary walls versus the solvent (ambient drying, or spring-back drying) [
1]. Since several modifications of the original processes have been developed so far, their classification may be less straightforward in certain cases.
Freeze-drying is an excellent technique for the removal of water (or some other solvents) from sensitive biological tissues in the frozen state, and is successfully applied to the aerogel production as well [
2,
3]. Freeze-drying has previously resulted in mostly powder-like materials when used for inorganic aerogels; however, most recently, an improved technique has been published, which can produce monolithic pieces [
4].
Ambient (or spring-back) drying is an attractive method of aerogel production since it does not use any complicated instruments and can be scaled up to industrial use [
5,
6,
7,
8]. According to the latest developments, small transparent silica monoliths can also be prepared [
9]. There is also, a new spray-drying technique to produce powdery aerogels [
10].
Supercritical or critical point drying (SCD) is the most frequently applied technique for aerogel production. It is capable of producing not only powdery but large and optically clear monolithic samples, which are used, i.e., in the Cherenkov-counters [
11,
12,
13,
14,
15,
16]. A significant advantage of the SCD processes is that the solvent used in the gelation process may be selected from a very wide range, and the technique is not limited to certain types of gel materials and is rather quite universal. Two basic strategies are the high temperature (HT) and the low temperature (LT) drying [
17].
In the HT process, the solvent itself is heated above its critical point after a pre-pressurization step. Since the critical point of the most common organic solvents is above 200 °C, and the critical pressure is in the range of 40–80 bar, supercritical conditions can be reached by heating wet samples covered with the solvent in a sealed autoclave, followed by slow depressurization [
18,
19,
20,
21,
22]. The process and the instrumentation are simple; it requires no pumps, and may produce aerogels with hydrophobic surface modification directly. Unfortunately, organic solvents represent a fire hazard in case of accidental and uncontrolled release, and the high temperature may damage heat-sensitive materials. A modification of the HT process is when the temperature is kept a bit under the critical temperature of the solvent [
23,
24].
Supercritical carbon dioxide is used for low temperature drying due to its low critical temperature (31 °C), environmentally friendly nature and non-flammability. In the process, wet gel samples are periodically or continuously flushed with supercritical CO
2 until all solvents are removed. Regardless of the periodicity, both processes require heat exchangers, and a liquid CO
2 pump, which is a valued part of the instrument. The continuous LT process may require larger volume of CO
2, but thorough optimization and modification to an industrial scale can significantly reduce the specific drying costs [
25,
26]. Under supercritical conditions, some functionalization of the aerogel skeleton can also be achieved [
27]. Due to the high scientific and industrial importance of the aerogels, significant efforts were taken to study the effects of drying conditions, diffusion, chemical composition, and drying temperature profiles on the quality of aerogels [
28,
29,
30,
31,
32,
33,
34,
35].
Our aim was to develop an environment-friendly, solvent-saving, simple and low running-cost system for the preparation of aerogels from gel casting to supercritical drying. An additional requirement was that the construction should be student-friendly, as it was intended for all levels of research, including undergraduate studies. In this paper, we concentrate on the design and work experiences of our gel casting, aging, solvent exchange and medium temperature (MT) pumpless supercritical CO2 drying system.
2. Results and Discussion
As has been shown in the introduction, liquid organic solvents may be transformed into their supercritical state by simple heating of the drying vessel after certain pre-pressurization with an inert gas. Our concept was to apply that simple approach to liquid carbon dioxide, which by itself provides the necessary pre-pressure that can transform it into the supercritical state by simple heating, without additional steps. The final pressure of the drying chamber is basically determined by the relative volume of the liquid CO2 introduced in the chamber, as well as the final temperature. The volume of carbon dioxide intake can be controlled by the initial temperature of the dryer (provided the CO2 cylinder is kept at constant room temperature). The required drying pressure is then determined by the final heating temperature of the chamber. Our new process uses the pressure of the carbon dioxide cylinder to provide liquid flows, and is combined with a double temperature control protocol to achieve a wide range of available drying pressures and temperatures. As is shown in the forthcoming sections, this simple method may substitute complicated and costly supercritical pumping systems and provide crack-free monolithic aerogels made of a variety of materials.
The basic technology steps in our aerogel-making protocol are gel casting, aging, solvent exchange, and supercritical drying. Aerogel synthesis in general starts with controlled chemical hydrolysis of precursors, followed by polycondensation of the hydrolysis products, yielding sol particles first, which grow in time and finally form a self-standing gel. Here we give a detailed description of all the steps (not just the drying process) and show some practical hints in order to provide guidelines or a manual for laboratories preparing to get involved in aerogel research, though some of them might be useful for advanced laboratories as well.
2.1. Gel Casting, Molds
The first issue is deciding which mold can be used for gel casting. The mold should be chemically resistant, easy to use and, in our case, a cost-effective one. Polymers, like poly(vinyl chloride), polystyrene and polypropylene are prone to stick to the gel too strongly, resulting in damages of the monoliths when removed from the mold. Using commercial silicone spray to cover the surface may help in some cases, but silicone oil residue may build in the surface of the gel, which may be unwanted. Poly(tetrafluoro ethylene) (PTFE) proved to be the most advantageous construction material. Unfortunately, machined PTFE molds with proper enclosures were not available, thus we combined materials to solve the problem.
2.1.1. Cylindrical Molds
For cylindrical-shaped samples, machined PVC tubes with removable internal PTFE liner is used, which keeps the costs low and provides undamaged gel monoliths (
Figure 1a). Pushing-out of the gel from the mold directly to the drying frame is performed very carefully, with the help of a PTFE plunger. It has a coaxial bore and two surface channels to allow passing air into the mold and prevent sticking of the gel to the plunger. The base plate of the mold can be made from the same materials as the mold, but we prefer thin glass plates. Inside the tube, the base surface is covered with a round PTFE liner. The base plate is stuck to the PVC tube by melted paraffin, applied by a brush; paraffin solidifies upon cooling and keeps the parts together with sufficient force to allow moving of molds filled with the reaction mixture or gel. It provides a chemically resistant and easy-to-break seal, which can be removed (break off) with little rotating force, leaving the lower gel surface undamaged (
Figure 1c). Due to limited strength of the connection, we do not use that technique for a volume larger than 60 mL. After casting, the molds are sealed tightly with a double layer of a flexible laboratory film (Parafilm) or with aluminum foil, to prevent evaporation. The surface of the gel is covered with a few milliliters of solvent layer (i.e., methanol), when it is aged in the mold.
2.1.2. Rectangular Molds
Rectangular-shaped gels are cast in a custom-made U-shaped silicone template covered on two sides with glass plates (
Figure 1b). The internal glass surface is covered with thin PTFE foil stuck to the surface by a high-vacuum silicone grease. The parts are held together by spring clamps (
Figure 1d). After casting, the mold is sealed with a rectangular silicone plug to prevent evaporation. The surface of the gel is covered with a few milliliters of solvent layer (i.e., methanol), when it is aged in the mold. Removal of the gel is performed by laying down the set on the table without clamps, rotating and sliding aside the top cover glass plate, followed by stretching out of the U-template. Finally, the gel is transferred to the drying frame by sliding in (this step requires some practice, and the help of a thin PTFE sheet placed on the frame temporarily).
2.2. Frames, Sample Holders, Containers
Drying frames or shaped sample holders are made of punched aluminum plates of 0.75 mm thickness. They can be made into any shape fairly easily, and their preparation requires very little practice. Cylindrical frames can be assembled with aluminum rivets, but we have found open split structure with a bottom inbound edge covered with filter paper discs quite satisfactory (
Figure 2a).
Rectangular frames are designed to be foldable, as the transfer of the monoliths requires more freedom of movements (
Figure 2b). The wet gels are transferred, treated and stored in the frames throughout the entire process. Since evaporation of the solvent (that is drying-out of the gel) must be avoided, all aging, chemical treatment and solvent exchange steps are performed in well-closed containers. Instead of using laboratory cylinders with ground joints, we found commercial fruit jars to be reliable and inexpensive containers. The lid fits with a thread hermetically, and the internal surface of the lid is covered with a thin plastic layer. The sizes of the frames are designed to fit the jars, meaning that four cylindrical, two thick or four thin rectangular frames can be placed in a jar at a time (
Figure 2c,d). Approximately 350–500 mL of aging mixture or exchanging solvent is filled in the jars, and the samples must be fully covered with liquid all the time.
2.3. Aging, Functionalization
Aging may require a longer time to stabilize the gel structure and let the bonds form completely, and sometimes it may need the use of addition reagents [
28,
36,
37,
38,
39,
40,
41]. Since it is a very specific process, we will not elaborate on the chemicals used, but refer the reader to the corresponding literature. Aging can be performed either in the original mold (sealed hermetically), or in separate vessels after wet gels are placed in the frames.
Chemical modification, such as hydrophobization, can be performed on the wet gels. For making a hydrophobic silica surface, we use hexamethyl disilazane as a reagent in dry methanol. Trimethyl chlorosilane must be avoided when the gels are in aluminum frames. Hydrochloric acid is liberated in the process, and it corrodes aluminum fairly rapidly. In such cases, a manually punched polypropylene cylinder is used.
2.4. Solvent Exchange
Solvent exchange is a critical part of the process, because water content must be removed from the wet gels very carefully. Our supercritical drying process uses acetone as the final solvent, but other organic solvents can be handled as well. Residual water may damage the gel structure upon drying, resulting in opalescent, shrunk and cracked aerogels instead of transparent and crack-free monoliths.
In general, the solvent exchange is performed over several steps. Although using a copious amount of fresh and anhydrous exchanging solvents is the easiest way to perform the task, unfortunately, that would result in a copious volume of waste solvent, which should be avoided. We have developed a solvent-saving procedure to reduce solvent consumption. In general, the gelation reaction is performed in a mixture of an organic solvent (typically methanol, ethanol, isopropanol) and water, plus catalyst. After aging and modifications, we soak the gels in a mixture of the same solvent plus acetone, increasing the acetone content in 25% steps to 100%, then soak the gels in pure acetone; each step lasts at least one day or more. After that we transfer the gels in the solvent regeneration extractor, and distill fresh acetone over them for at least three days (
Figure 3a).
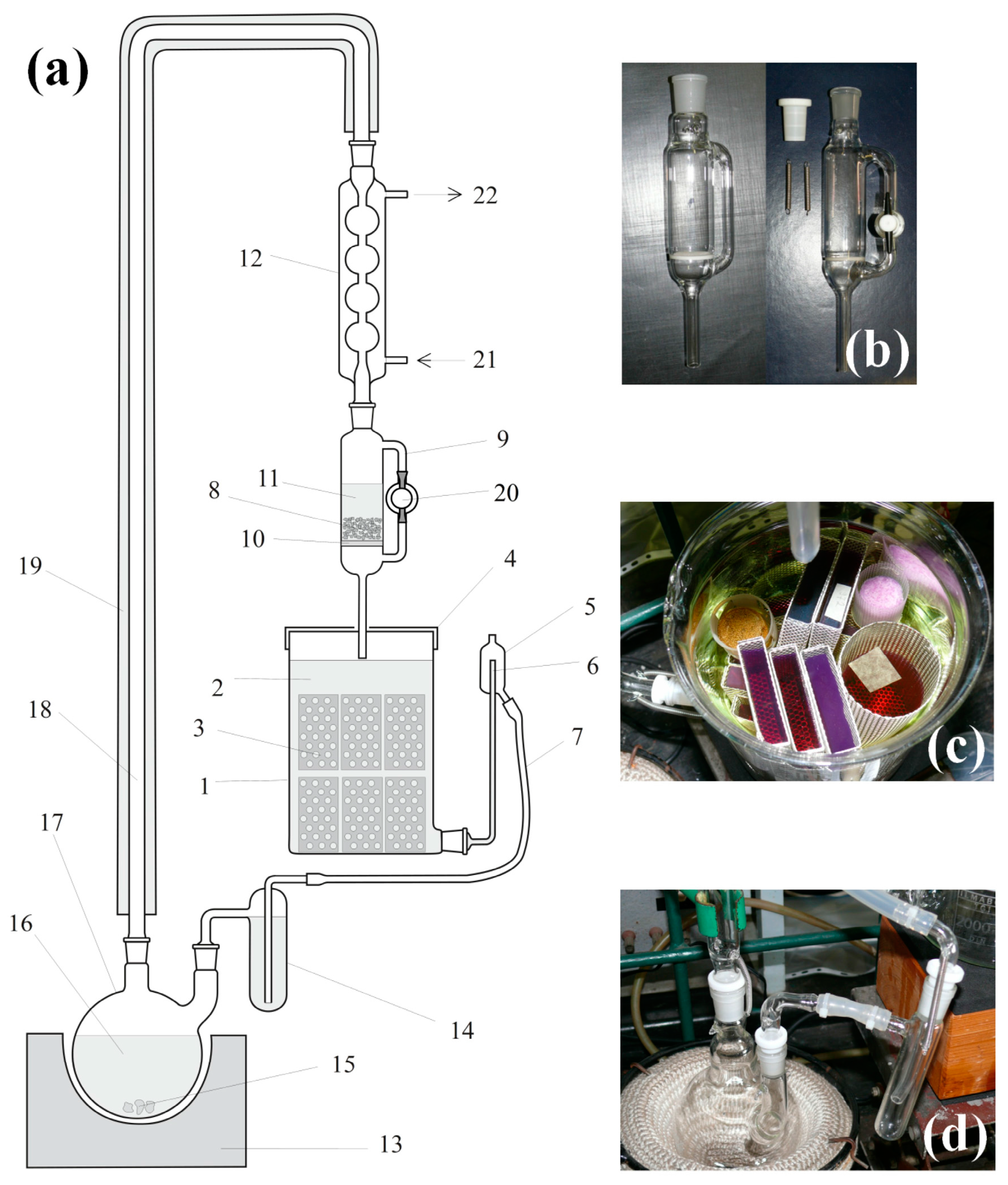
2.5. Continuous Solvent Regeneration and Extraction Unit
Structural drawing of the continuous-mode solvent regeneration and extraction unit is in
Figure 3. The main units of the extractor are the distillation flask (
Figure 3d), the drying unit (
Figure 3b), and the extraction chamber (
Figure 3c). Detailed description of the extraction process can be found in
Appendix A.
The extraction chamber can be used for adsorption of different materials, like indicators, in the gels. In
Figure 3c, all violet- and red-colored gels were stained by treating them with crystal violet, and then the excess was extracted with acetone. When the solvent exchange/extraction is complete, gels are stored in closed fruit jars under dry acetone.
Special attention should be paid to the proper sample handling in the extractor chamber in order to avoid possible cross-contaminations. As a general rule, gel samples containing functionalizing agents, surface modification reagents, or highly soluble materials like fluorescent dyes should be handled separately. Only gels of identical types should be extracted in the same solution.
2.6. Pumpless Supercritical CO2 Dryer
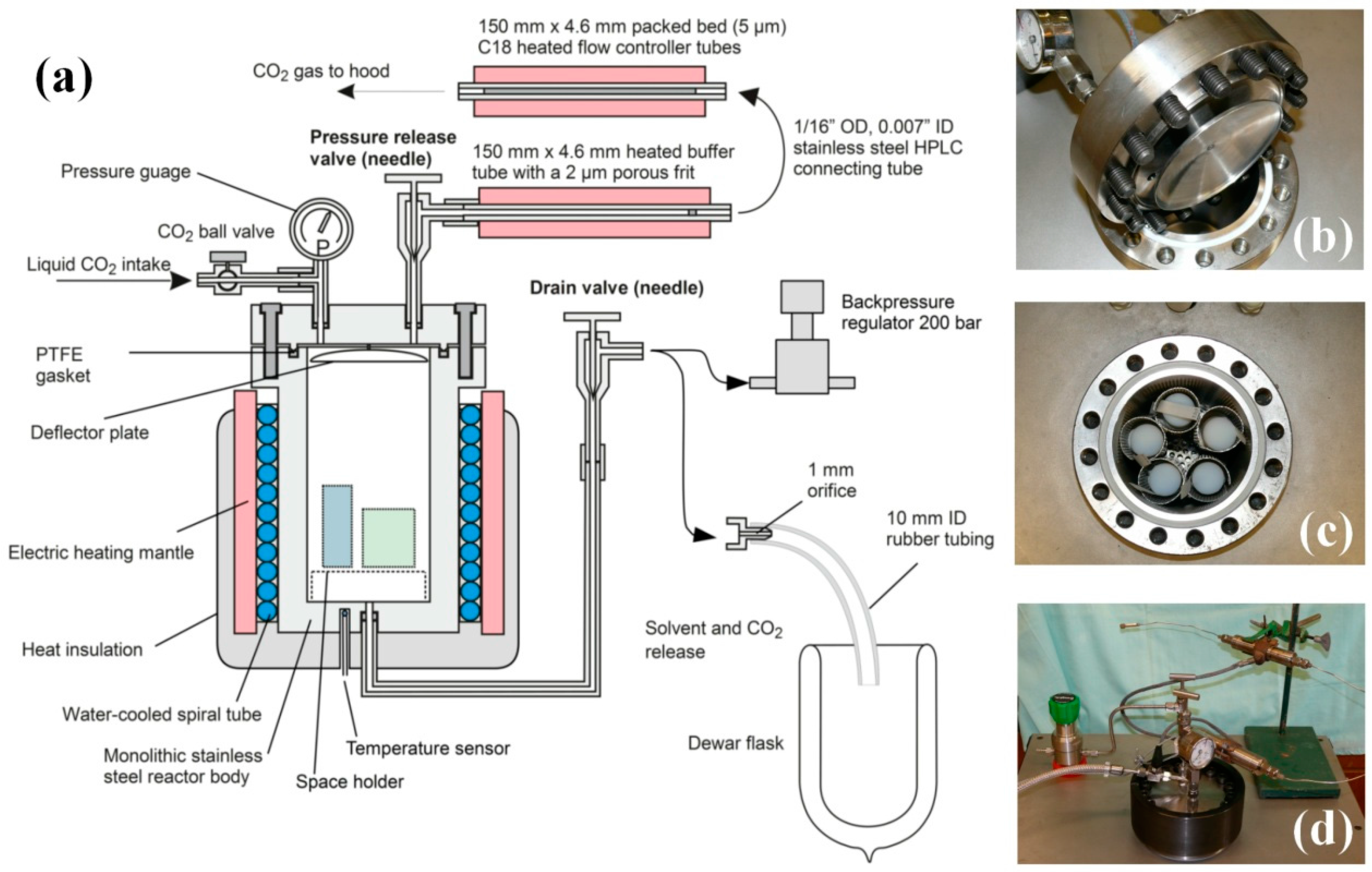
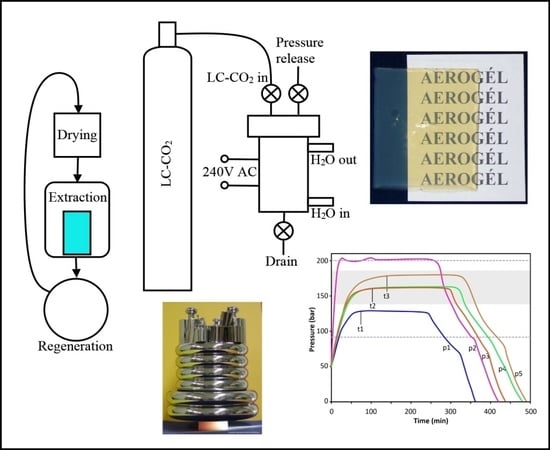
2.6.1. Dryer Construction
We have designed a supercritical dryer, which does not require the use of a liquid CO
2 pump, and the system is operated by the original pressure of the CO
2 cylinder, plus the pressure increase of carbon dioxide occurring in the liquid to supercritical transition. The cross-sectional drawing of the system is in
Figure 4a. Panels b–d in
Figure 4 shows critical parts of the dryer: (b) the lid with male-type joint, pressure gauge and deflector plate; (c) the reactor body with a female-type joint with PTFE gasket, as well as freshly dried cylindrical aerogel monoliths, and (d) the assembled dryer unit with safety lock ring (dark), heated flow-controller tubes and back pressure regulator. More technical details can be found in
Appendix B.
2.6.2. Liquid CO2 Extraction and Supercritical Drying
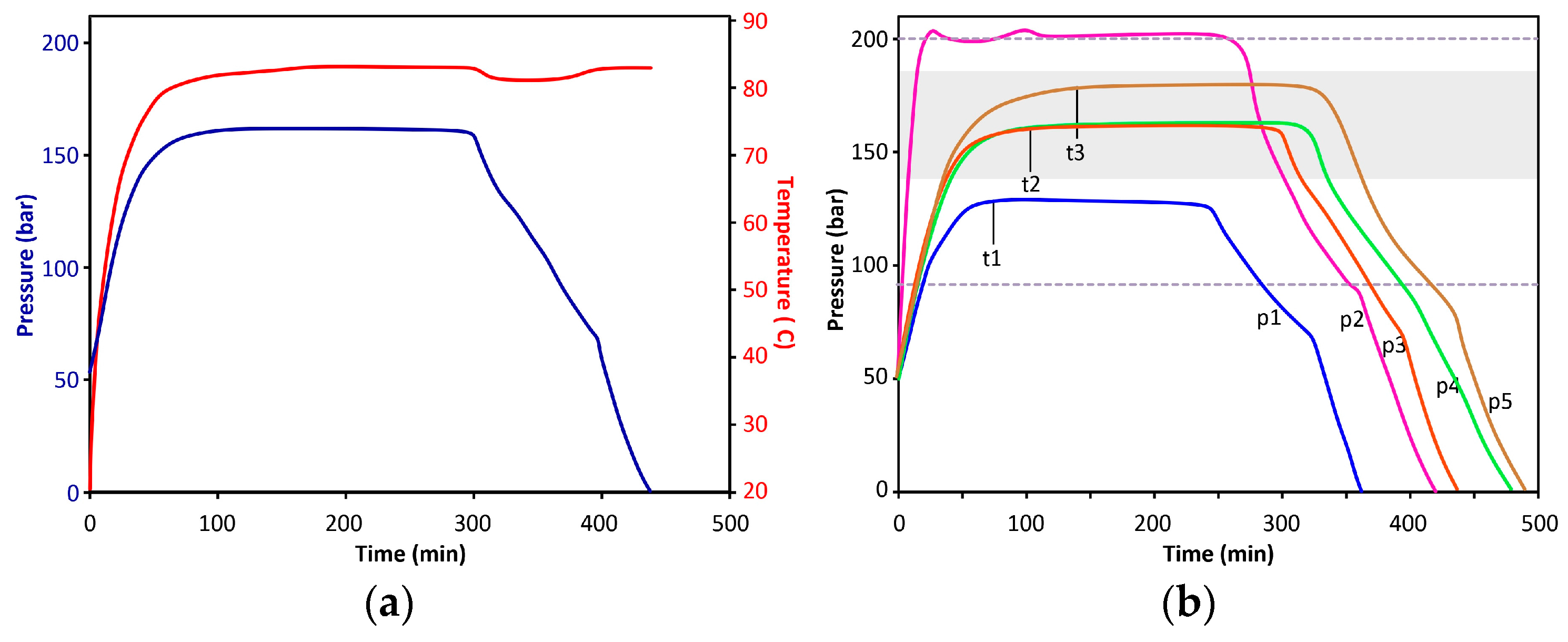
Supercritical drying of wet gels is combined with a liquid CO2 extraction of acetone. Solubility of acetone in liquid carbon dioxide is quite limited, thus in the first step, acetone is replaced with liquid CO2 and drained. In the second step, acetone residue is washed out with liquid CO2. In the third step, the reactor is sealed, heated to approx. 80 °C, equilibrated for 3 h, then depressurized at a rate of approx. 1–2 bar/min.
The technical steps of the extraction and drying process are summarized in
Figure S1 and described in
Appendix C. A typical internal temperature and pressure diagram can be seen in
Figure 5a. Other characteristic pressure diagrams, including examples of undercharged and overcharged reactors, are shown in
Figure 5b. The time necessary to reach pressure stabilization (t1–t3) depends on the starting volume of LC-CO
2 in the reactor, as well as, to a lesser extent, the number of aerogel monoliths.
A linear depressurization curve of the chamber would be ideal to provide the lowest level of mechanical stress to the aerogel monoliths. However, it can be hardly achieved by simple manual control. The depressurization process may be calculated/modelled by using the van der Waals equation as shown in
Appendix D.
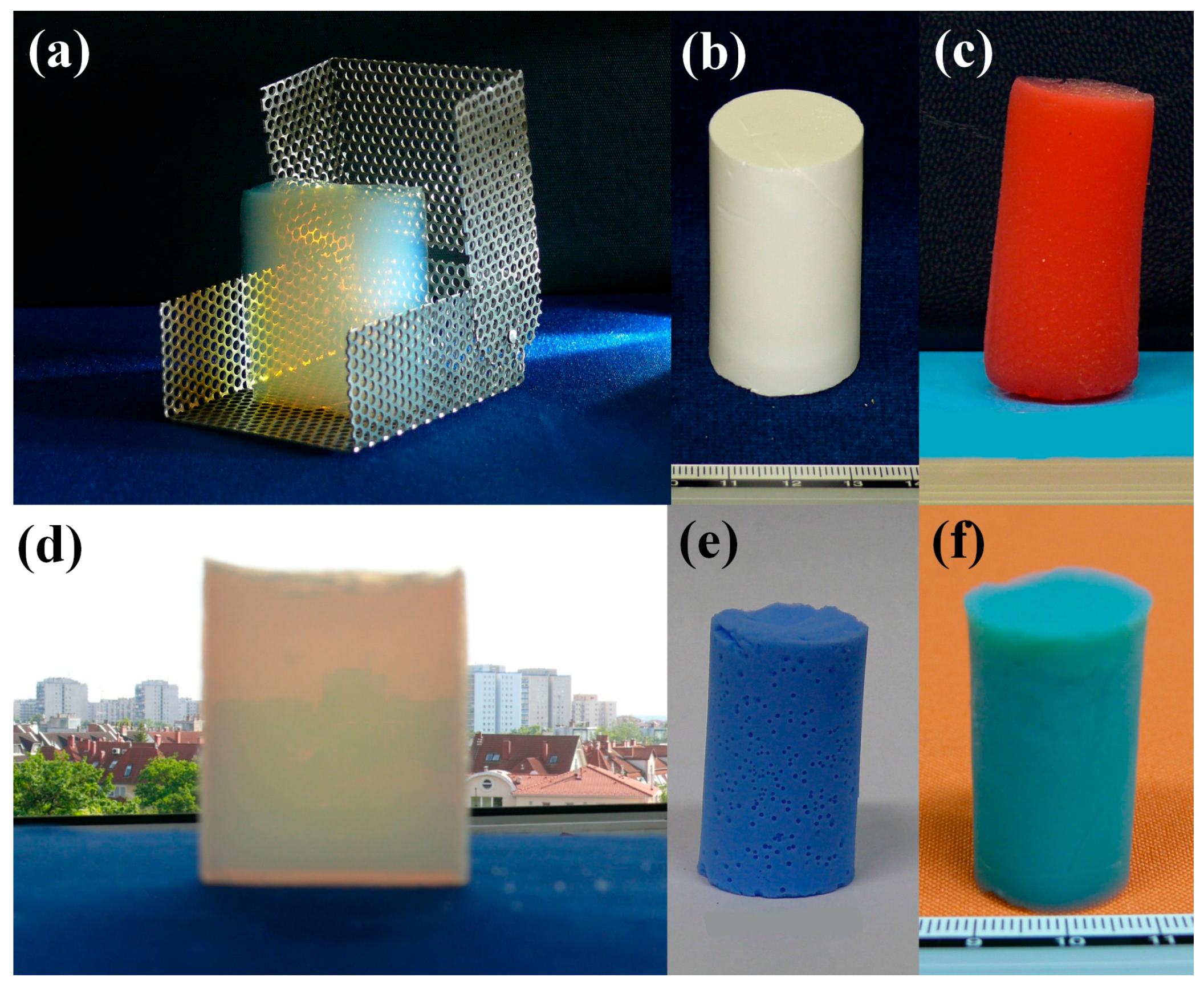
So far, a large number of aerogel samples have been synthesized by the method described above. Not only silica aerogels, but their covalently functionalized derivatives, organic and inorganic hybrids and composites, iron oxohydroxide, alumina and tin(IV) oxide gels were also prepared. A few examples of monolithic aerogels can be seen in
Figure 6.
3. Conclusions
The sample preparation technique, from gel casting to solvent exchange, presented here represents a clear and simple way to produce wet gel samples for supercritical drying. Most of the components can be found in an average chemical laboratory, or can be manufactured by a glass-blowing artist, or even made manually.
3.1. Continuous Extraction and Solvent Exchange
The continuous extractor provides significant solvent volume reduction in the process, thus reducing material costs and toxic waste management fees. The work principle is clear; it combines a continuous solvent recirculation, regeneration and drying unit with continuous extraction of the wet gels. Furthermore, it gives the possibility of chemical modification of the wet gels in the extraction chamber, followed by direct and complete washing out of the reactants with freshly distilled solvent. The unit can be used for the most commonly used solvents, and the construction allows the instrument to be scaled up (or down), if necessary. A smaller 200 mL unit is also in use for the extraction and solvent exchange of individual samples, which cannot be kept in the large unit, due to leaking of either chemical functionalizing agents or fluorescent dyes.
3.2. Comparison of High, Low and Medium Temperature Drying Processes
The principle we use in the MT supercritical dryer is a hybrid of two basic drying strategies, namely the HT and LT approaches, combined with a liquid CO2 extraction/solvent exchange step. We use supercritical carbon dioxide drying like in the LT techniques. However, SC-CO2 is generated in situ from liquid CO2, as in the HT process, where the solvent is heated up above its critical temperature. The medium temperature process (MT) we use (80 °C) applies higher temperature than the LT processes (40 °C), but is much lower than that used in the HT processes (above 200 °C). The basic difference from LT techniques is that our MT process does not require the use of a costly cooled CO2 pump. The system is operated by the pressure of the CO2 cylinder, and the supercritical medium is generated from liquid CO2 directly in the dryer. As a prerequisite for successful operation, all samples should be free of any other solvents, which could increase the critical point, or would form a low-solubility liquid phase within the gels. Removal of solvents is achieved by a semi-continuous liquid CO2 extraction before the supercritical transition, and it is performed also in the dryer unit.
The net full processing time, including LC-CO2 extraction and supercritical drying, is approximately 16–18 h. The length of supercritical drying only is 6–8 h. Liquid CO2 consumption is 4–5 kg/batch. (One batch may contain ten cylinders of 28 mm OD × 50–60 mm L dimensions, or four prisms of 2.2 × 5 × 6 cm3, or two prisms of 2.2 × 5 × 6 cm3 plus four prisms of 1.1 × 5 × 6 cm3 dimensions, or twelve prisms of 1.1 × 5 × 6 cm3 dimensions).
3.3. Characteristics and Long Time User’s Experiences with the MT Supercritical Dryer
The pumpless MT supercritical dryer has been in operation in our laboratory for nine years, and around 200 dryings have been performed thus far.
The most significant advantage of the construction is that it requires no CO2 pump and heat exchangers, significantly reducing the investment cost. All flow operations are powered by the pressure of the carbon dioxide cylinder. It has a very simple, seamless and sturdy construction. Only a one-phase 240 V AC (2 kW) power line and communal cold running water supply are required. The rolling frame allows high mobility and ease of transportation. Only the main PTFE gasket has to be replaced regularly (after approximately 10–15 dryings); other gaskets do not need replacement for years. Due to ease of handling, there is no need for high level technical training. The dryer has been successfully operated by undergraduate and graduate students for the last eight years.
We have also found a few disadvantages of the system. The most important drawback is that it is controlled manually and requires continuous (but not permanent) personal supervision, generally two work shifts per drying. Although heating is automatic, decompression is manually controlled, thus smooth operation depends on the operator’s skills. Efficacy of filling the reactor with liquid CO2 (and thus the final pressure) depends on the temperatures of cooling water and the temperature of the CO2 bottle, although the process works fine in a wide range of conditions. Needle valves are prone to over tightening and require regular maintenance.
4. Materials and Methods
4.1. Materials
All materials were of reagent grade except as noted and used without any purification. Ammonia solution (25%), technical grade methanol and acetone was purchased from Molar Chemicals Kft. (Halásztelek, Hungary). Argon gas cylinder (4.6) and carbon dioxide cylinder equipped with dip tube (Carbogen) were provided by Linde Gáz Magyarország Zrt (Budapest, Hungary). Silane reagents and anhydrous magnesium sulfate were purchased from Sigma-Aldrich (Budapest, Hungary). Steel pressure tubings, pressure gauge, connecting elements, needle valves, and back pressure regulator were purchased from Swagelok and Linde Gáz Magyarország Zrt. (Budapest, Hungary).
4.2. Instruments
Parts of the continuous glass extractors were custom made by András Donka in the glass-blower workshop of the Department of Inorganic and Analytical Chemistry, University of Debrecen, Debrecen, Hungary.
The supercritical dryer was manufactured and assembled in the workshop of the Nuclear Research Institute of Hungarian Academy of Science (Atomki, Debrecen, Hungary). Rolling frame, support unit, heating and cooling system were custom made by Goodwill Trade Kft. (Hajdúböszörmény, Hungary).
4.3. Synthesis of Silica Aerogels by Acid-Base Catalyzed Process (Samples in Figure 6a,f)
The synthesis is based on a general acid-base catalyzed process widely known in the literature. However, we use boiling temperature in the acid hydrolysis step to reduce the necessary time. Reaction conditions and reagent concentrations are set to reach the gelation point within 8–15 min. Two solutions (A and B) were prepared. Solution A contained tetraethyl orthosilicate (TEOS) (10.00 mL, 44.78 mmol) and aqueous hydrochloric acid solution (1000 µL, 0.20 M) in ethanol (44.0 mL). Solution B contained aqueous ammonia solution (1500 µL, 25% m/m) and ethanol (16.0 mL). Solution A was heated to boiling and refluxed for 5.0 min, then cooled down to room temperature in a cold water bath. The solution was transferred into a 100 mL Erlenmeyer flask, magnetically stirred, and solution B was added under vigorous stirring. The reaction mixture was poured in a rectangular mold and let to gel and age in the mold for one day. The surface was covered with a layer of ethanol. After aging, the gel was transferred into a drying frame and soaked first in ethanol, then in increasing acetone level ethanol until 100% acetone was reached. After three days in acetone, the sample was placed in the continuous extractor and dried and extracted with acetone for three days. The sample was put in the supercritical dryer and dried according to the protocol described in
Appendix C. Pristine aerogel received after SCD was heated in a furnace at 500 °C for 8 h to reach final strength; the 1.8 cm thick aerogel monolith can hold 10 kg weight easily, without cracking (see
Figure S2).