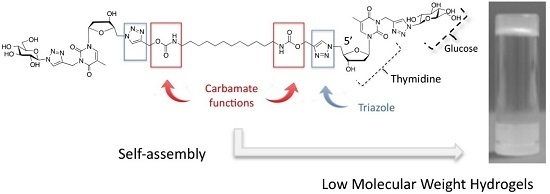
Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators
Abstract
:1. Introduction
2. Results and Discussion
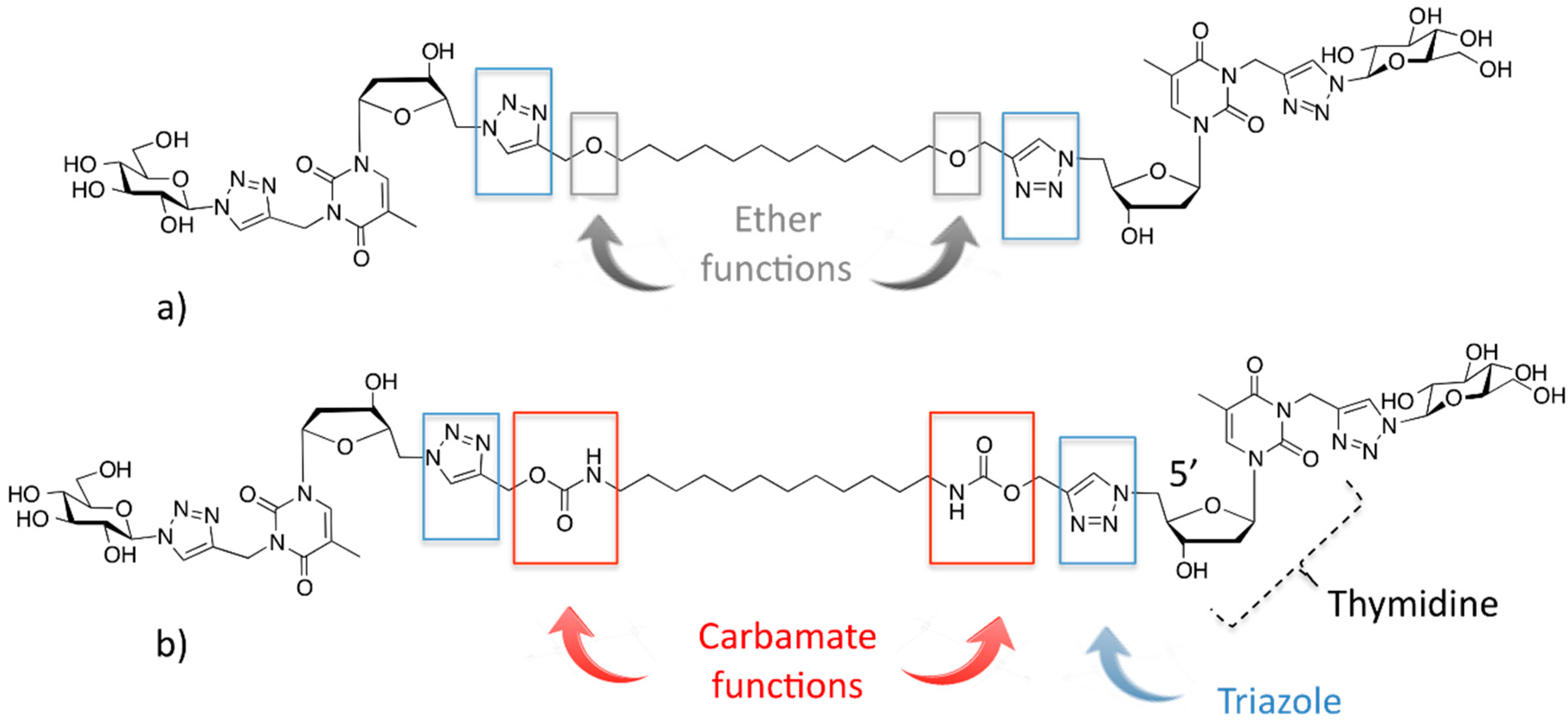
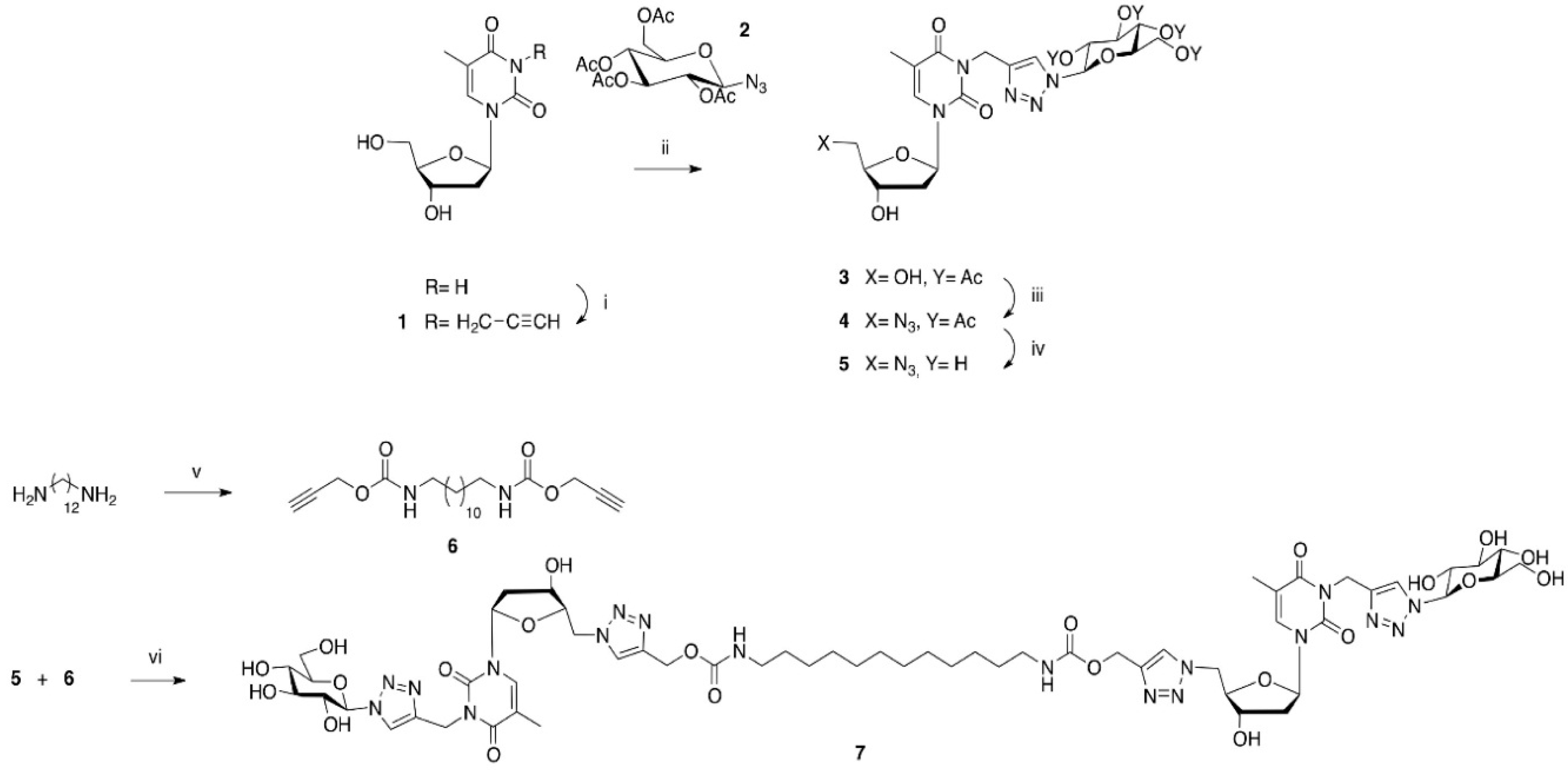
2.1. Synthesis of the Carbamate-Based Bolaamphiphiles
2.2. Physicochemical Studies
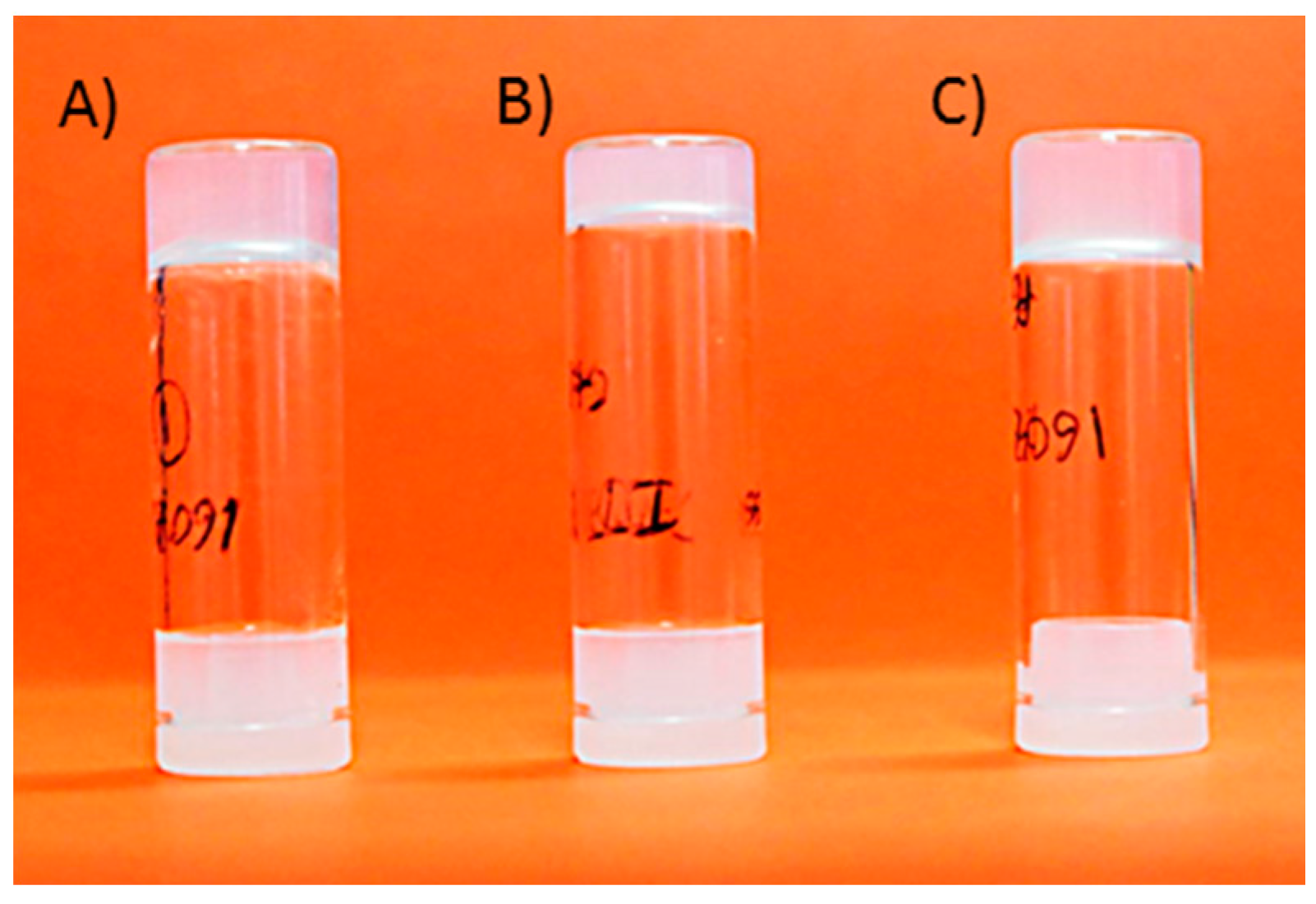
2.2.1. Gel Formation Assays
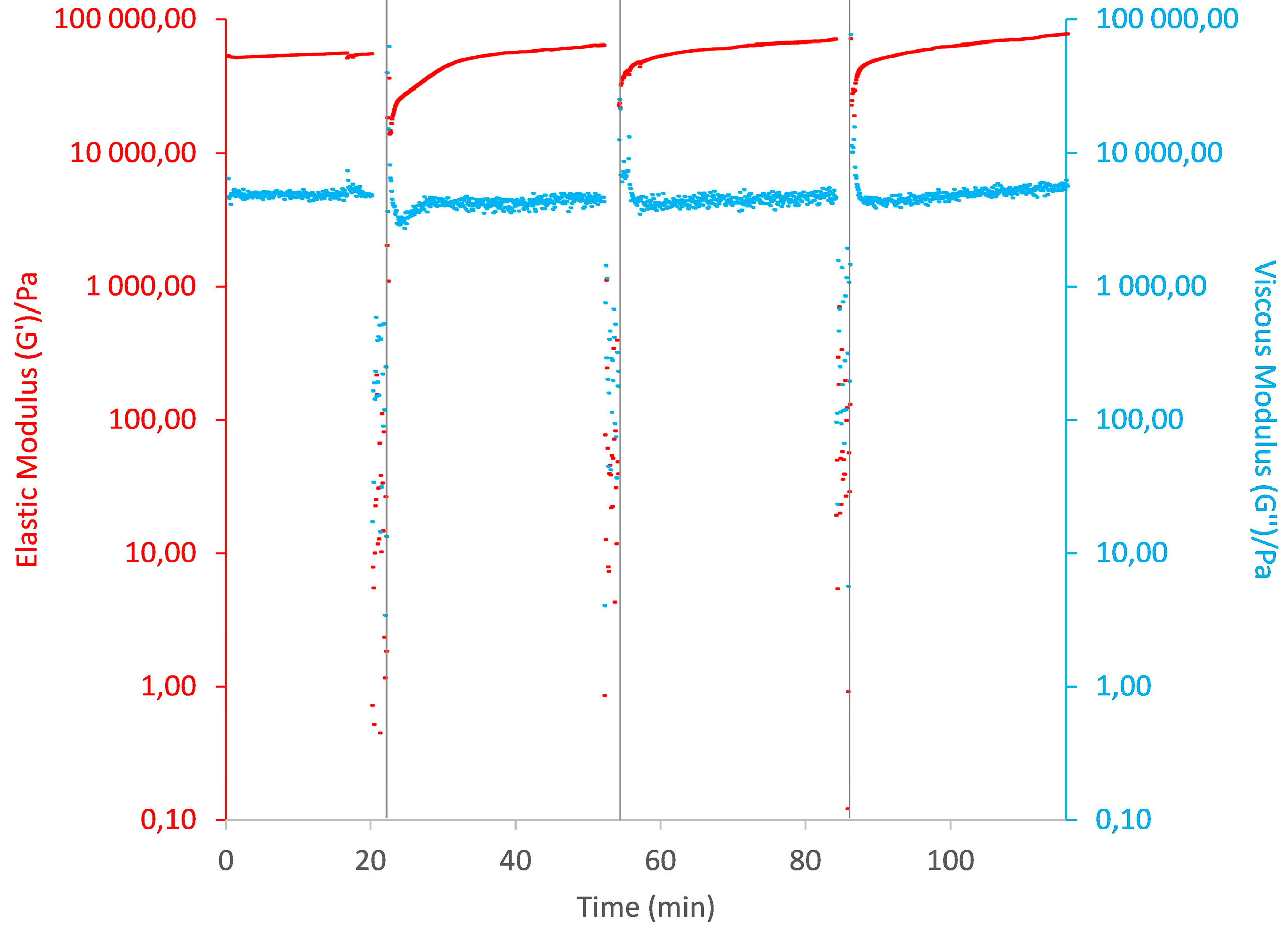
2.2.2. Rheological Studies
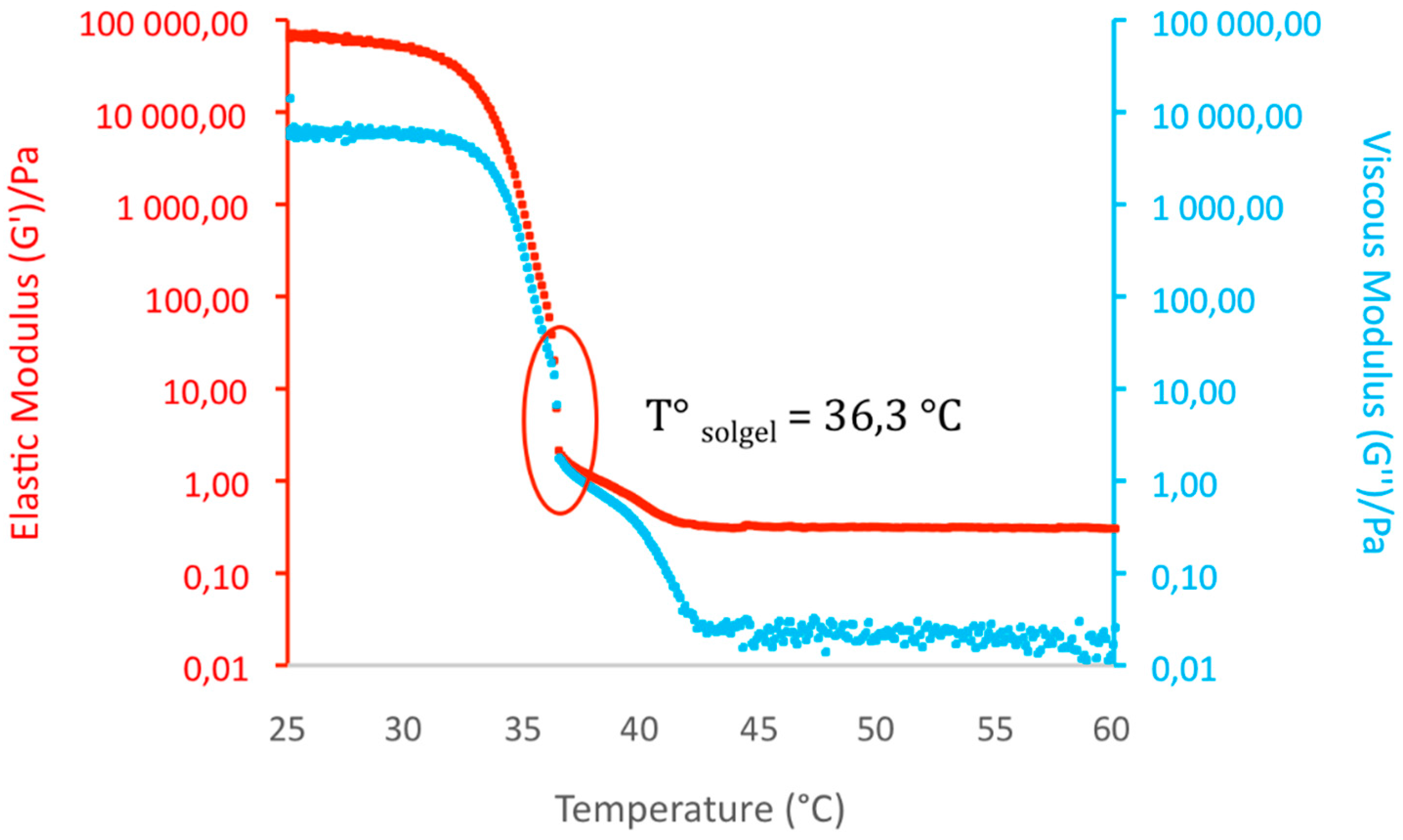
2.2.3. Gel–Sol Transition Temperature (Tgel)
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Synthesis
N-3-Propargylthymidine (1)
1-Deoxy-1-azido-2,3,4,6-tetra-O-acetyl-β-d-glucopyranose (2)
N-3-[1-((β-d-Glucopyranosidetetraacetate)-1H-1,2,3-triazol-4-yl)methyl]thymidine (3)
5′-Deoxy-N-3-[1-((β-d-glucopyranosidetetraacetate)-1H-1,2,3-triazol-4-yl)methyl] azidothymidine (4)
5′-Deoxy-N-3-[1-((β-d-glucopyranoside)-1H-1,2,3-triazol-4-yl)methyl]azido thymidine (5)
1,12-Dodecanediyl-bis-N-propargylcarbamate (6)
1,12-Diaminododecanediyl-N,N′-bis-[5′-(4-methyloxycarbonyl-1H-1,2,3-triazole-1-yl)-N-3-((1-(β-d-glucopyranoside)-1H-1,2,3-triazole-4-yl)methyl)-5′-deoxy thymidine] (7) (GNBA-carbamate)
4.3. Rheology
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steed, J.W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, P.P.; Hariharan, M.; Ramaiah, D. A Supramolecular ON–OFF–ON Fluorescence Assay for Selective Recognition of GTP. J. Am. Chem. Soc. 2006, 128, 11334–11335. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; le Meins, J.-F.; Latxague, L.; et al. A theromosensitive hydrogel for tissue engineering. eCM 2012, 23, 147–160. [Google Scholar]
- Tiller, J.C. Increasing the local concentration of drugs by hydrogel formation. Angew. Chem. Int. Ed. 2003, 42, 3072–3075. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Barthélémy, P. Glycosyl-nucleoside lipids as low-molecular-weight gelators. Langmuir 2009, 25, 8447–8450. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Bernard, J.; Staedel, C.; Barthélémy, P. Glycosyl-nucleoside-lipid based supramolecular assembly as a nanostructured material with nucleic acid delivery capabilities. Chem. Commun. 2009, 5127–5129. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Brun, C.; Arnion, H.; Staedel, C.; Barthélémy, P. Glycosyl-nucleoside fluorinated amphiphiles as components of nanostructured hydrogels. Tetrahedron Lett. 2010, 51, 1012–1015. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Brard, M.; Richter, W.; Benvegnu, T.; Plusquellec, D. Synthesis and supramolecular assemblies of bipolar archaeal glycolipid analogues containing a cis-1,3-disubstituted cyclopentane ring. J. Am. Chem. Soc. 2004, 126, 10003–11012. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Blume, A. Self-assembly of bipolar amphiphiles. Curr. Opin. Colloid Interface Sci. 2007, 12, 138–147. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, T.; Huang, J. Recent advances in the mixed systems of bolaamphiphiles and oppositely charged conventional surfactants. J. Colloid Interface Sci. 2009, 337, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Blume, A. Single-Chain Bolaphospholipids: Temperature-Dependent Self-assembly and Mixing Behavior with Phospholipids. Adv. Plan. Lipid Bilayers 2012, 16, 93–128. [Google Scholar]
- Blume, A.; Drescher, S.; Graf, G.; Kçhler, K.; Meister, A. Self-assembly of different single-chain bolaphospholipids and their miscibility with phospholipids or classical amphiphiles. Adv. Colloid Interface Sci. 2014, 208, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. Engl. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143. [Google Scholar] [CrossRef] [PubMed]
- Horton, D. Methods in Carbohydrate Chemistry; Lemieux, U., Whistler, R.L., Wolfrom, M.L., BeMiller, J.N., Eds.; Academic Press Inc.: New York, NY, USA, 1963; Volume II, pp. 433–437. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latxague, L.; Gaubert, A.; Maleville, D.; Baillet, J.; Ramin, M.A.; Barthélémy, P. Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators. Gels 2016, 2, 25. https://doi.org/10.3390/gels2040025
Latxague L, Gaubert A, Maleville D, Baillet J, Ramin MA, Barthélémy P. Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators. Gels. 2016; 2(4):25. https://doi.org/10.3390/gels2040025
Chicago/Turabian StyleLatxague, Laurent, Alexandra Gaubert, David Maleville, Julie Baillet, Michael A. Ramin, and Philippe Barthélémy. 2016. "Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators" Gels 2, no. 4: 25. https://doi.org/10.3390/gels2040025
APA StyleLatxague, L., Gaubert, A., Maleville, D., Baillet, J., Ramin, M. A., & Barthélémy, P. (2016). Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators. Gels, 2(4), 25. https://doi.org/10.3390/gels2040025