Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs
Abstract
:1. Introduction
2. Results and Discussion
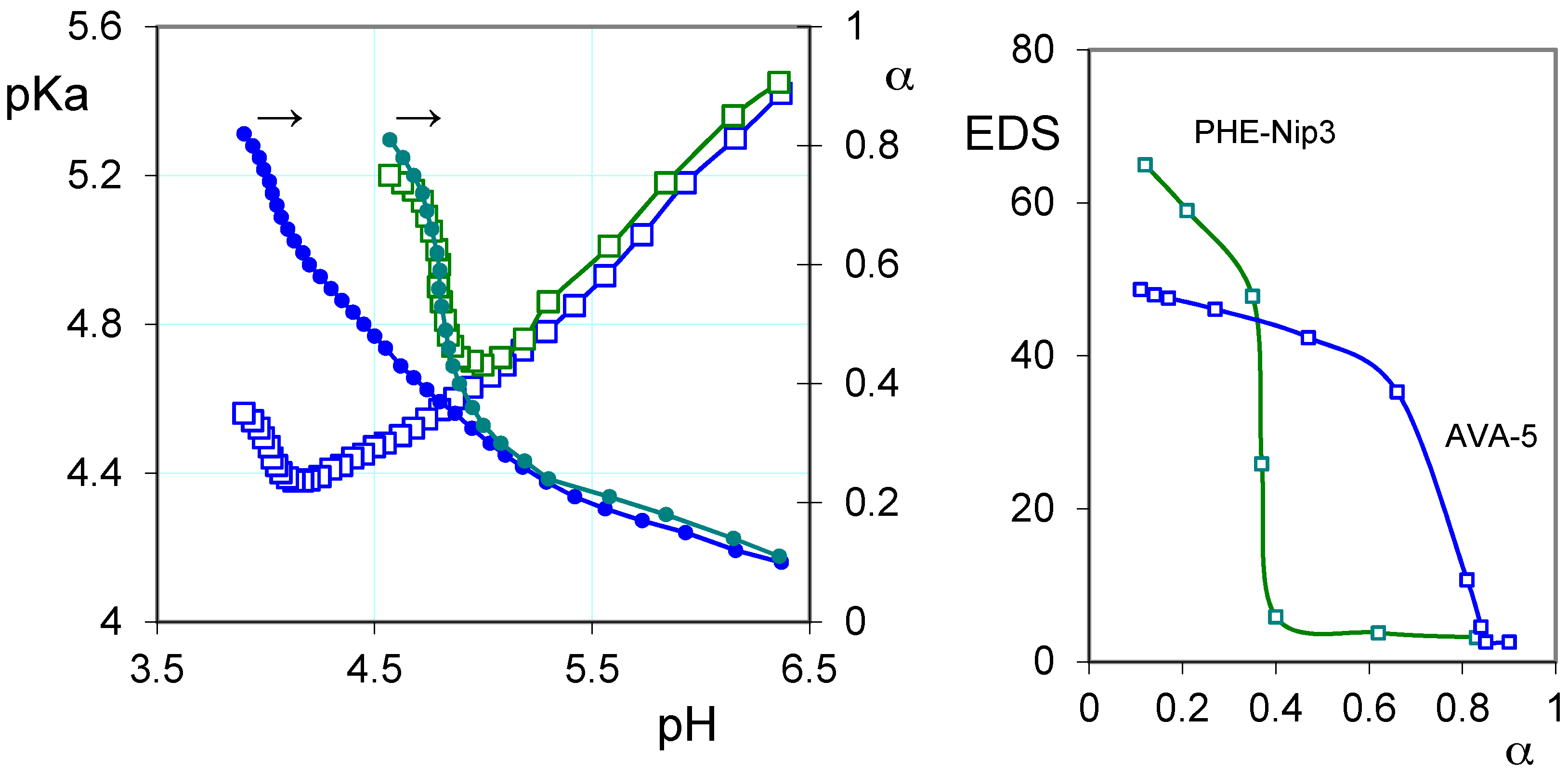
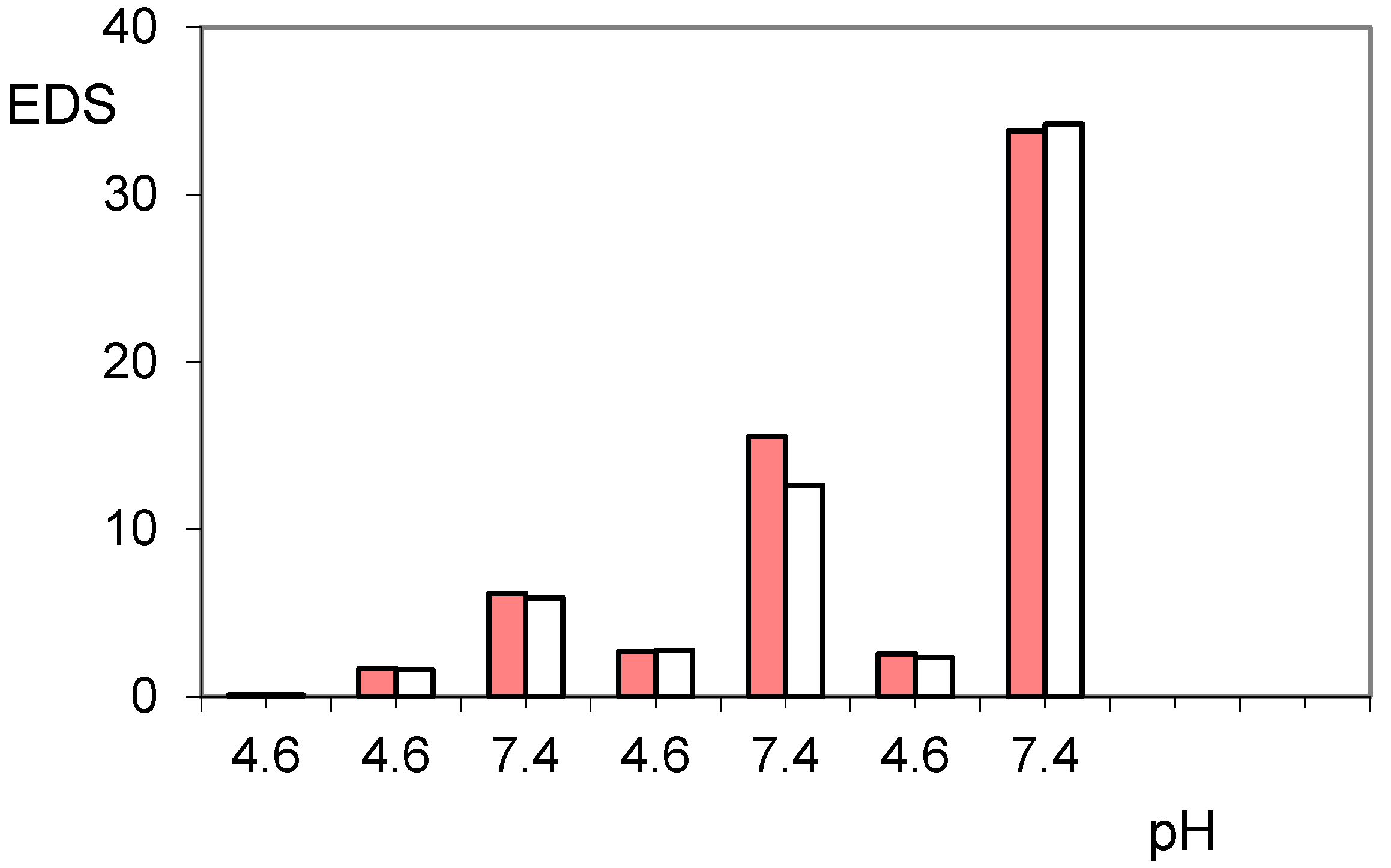
2.1. Protonation Study
2.2. Loading of Paroxetine and Duloxetine
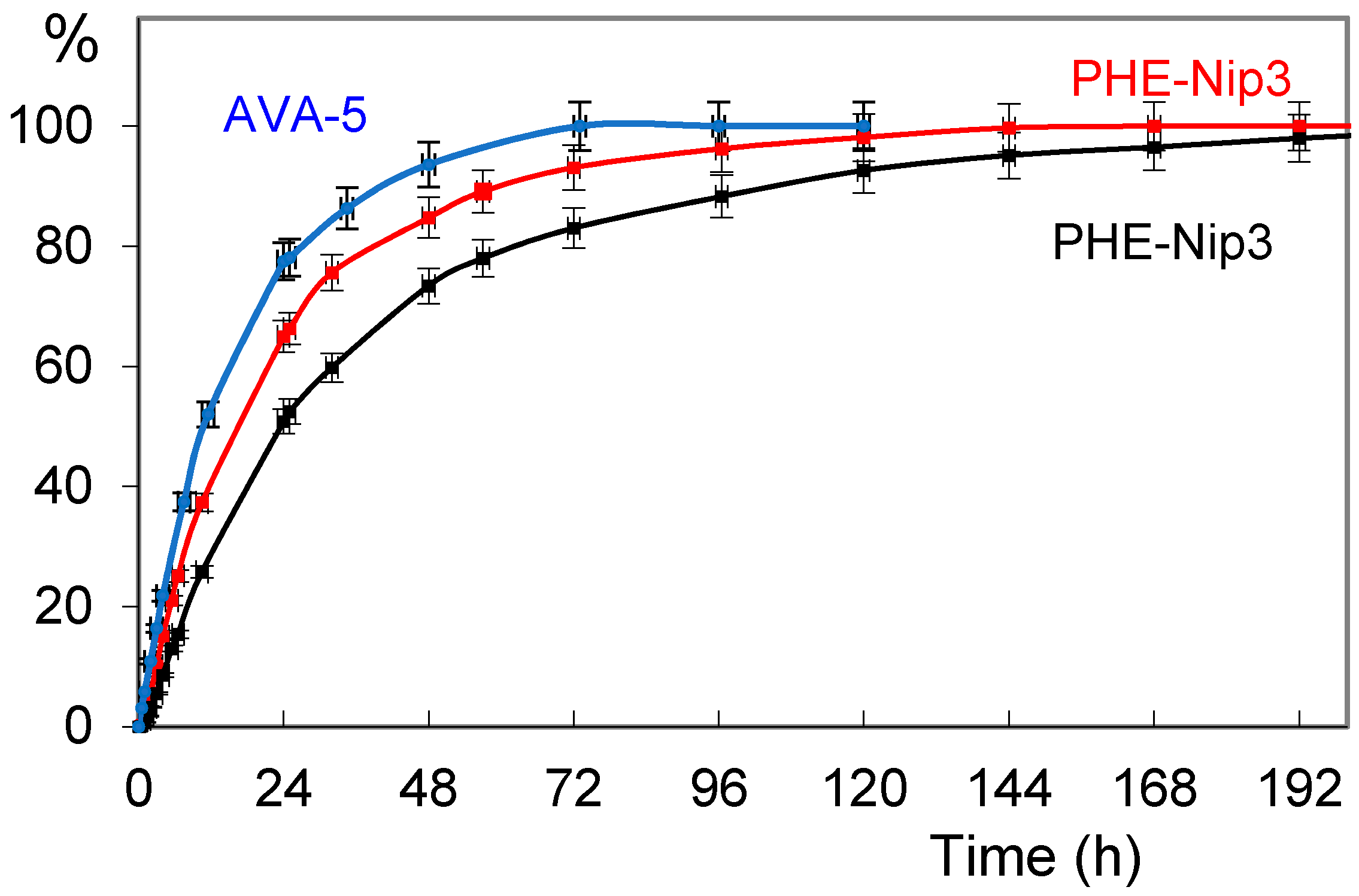
2.3. Release of Paroxetine and Duloxetine from the Hydrogels PHE-Nip3 and AVA-5
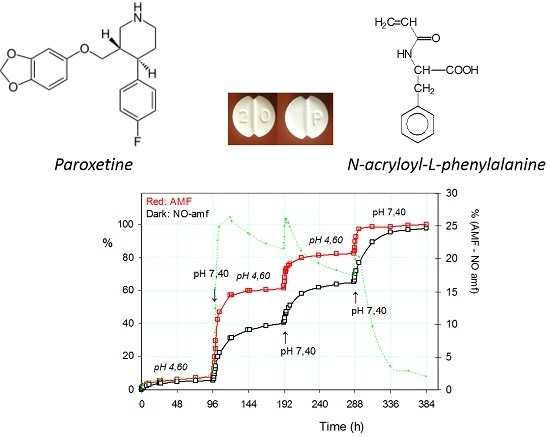
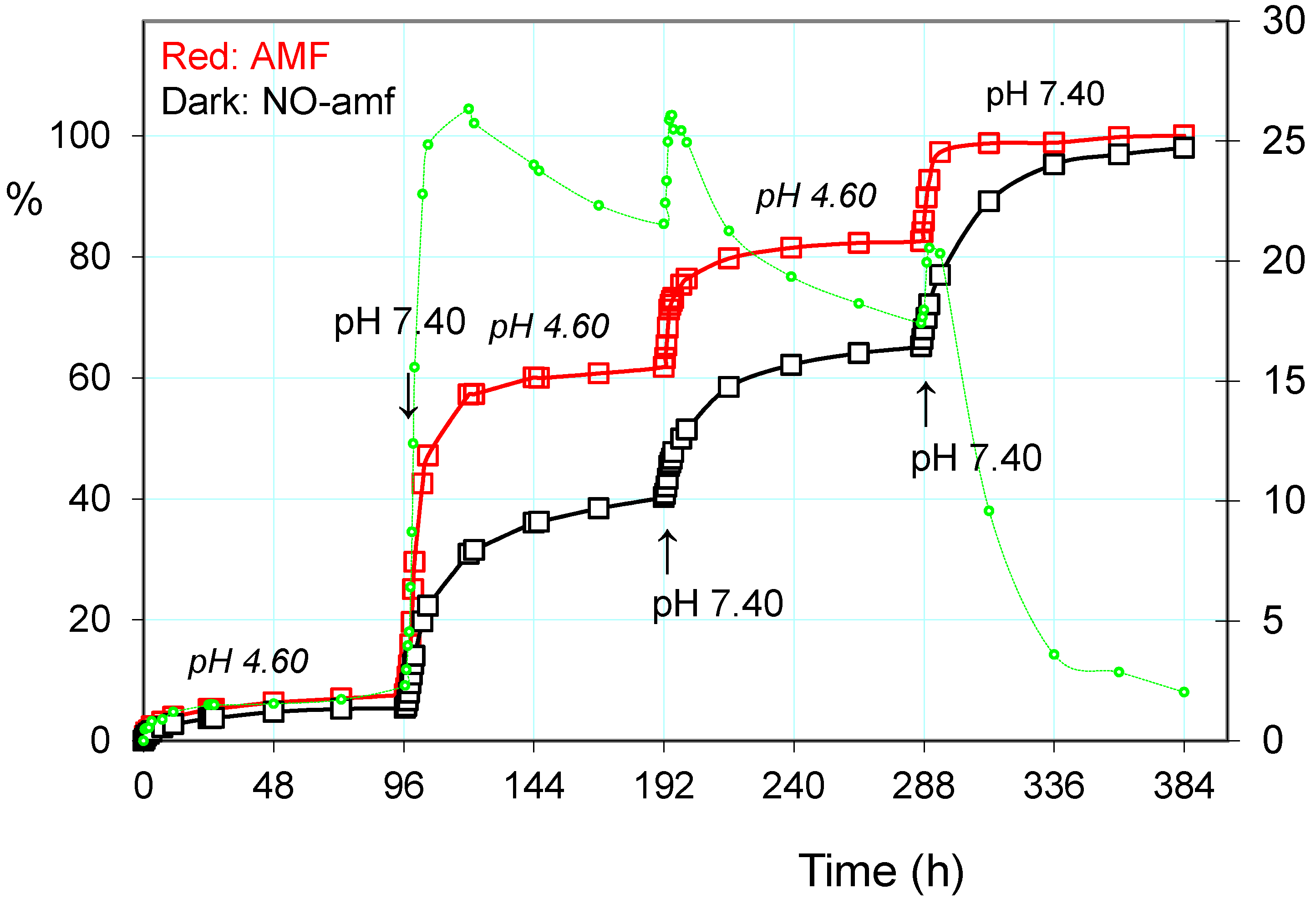
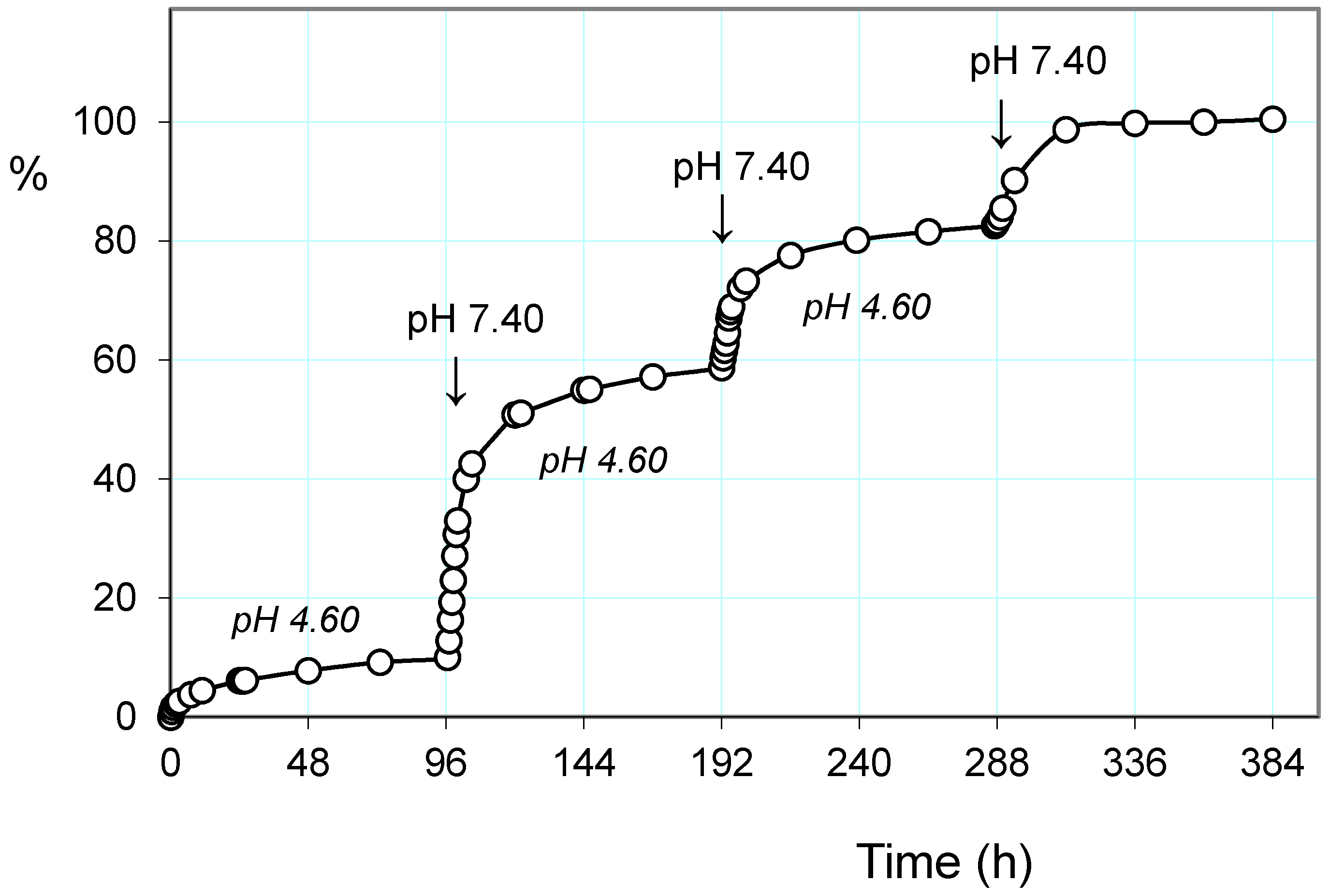
2.3.1. Release of the Drug: Effect of pH and Alternating Magnetic Field (AMF)
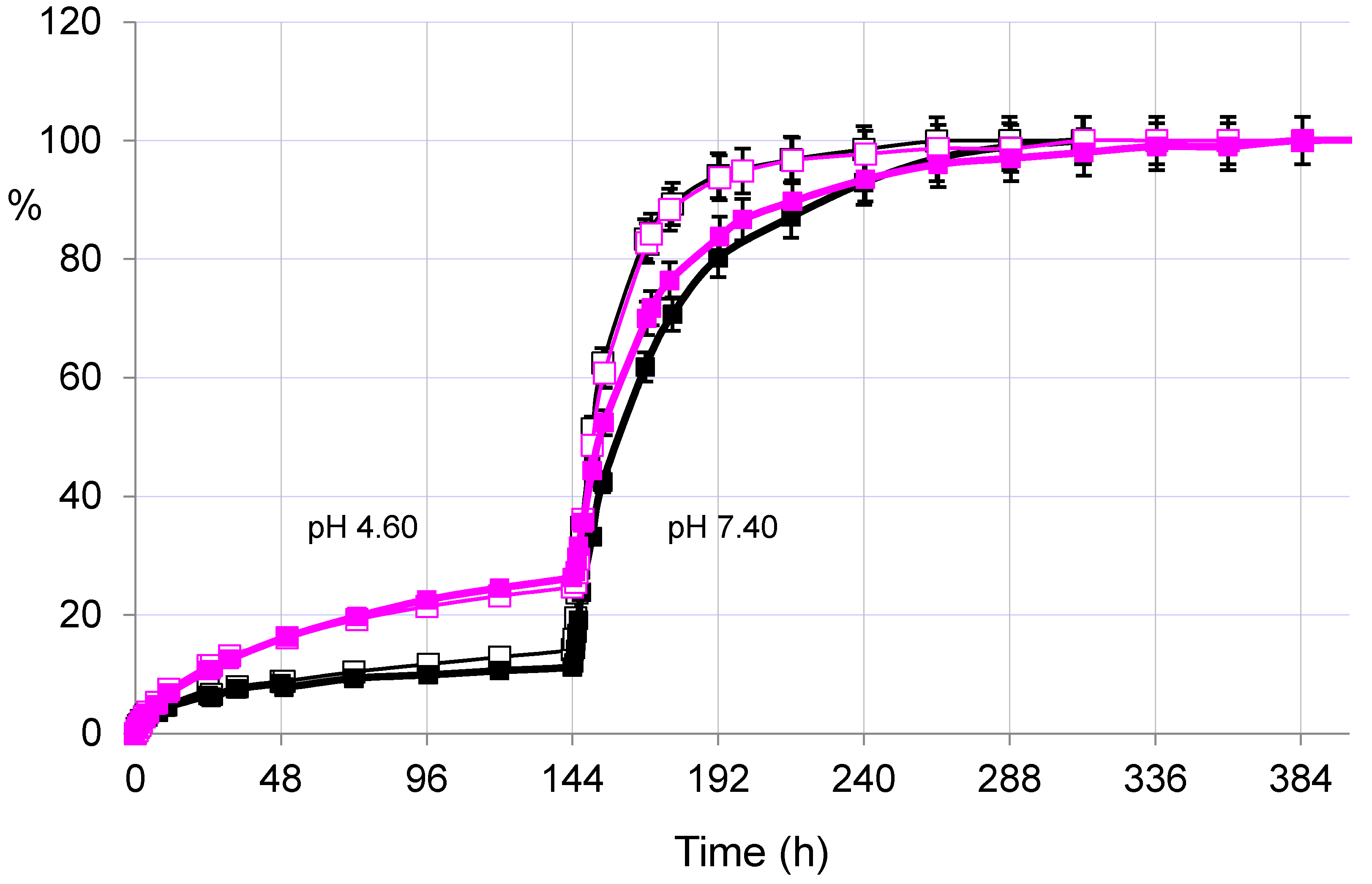
2.3.2. Pulsatile Drug Release
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Loading of the Drug
4.3. Release of the Drug
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMF | Alternating magnetic field |
| EDS | Equilibrium degree of swelling |
References
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Mather, M.L.; Tomlins, P.E. Hydrogels in regenerative medicine: Towards understanding structure-function relationships. Regen. Med. 2010, 5, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I. Multiple stimuli-responsive hydrogels based on α-aminoacid residues for drug delivery. In Smart Materials for Drug Delivery; Alvarez-Lorenzo, C., Concheiro, A., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 199–227. [Google Scholar]
- Casolaro, M.; Casolaro, I. Stimuli-responsive hydrogels bearing α-aminoacid residues: A potential platform for future therapies. J. Biomed. Eng. Med. Dev. 2016. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future. Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar]
- Casolaro, M.; Casolaro, I. Multiple stimuli-responsive hydrogels for metal-based drug therapy. Polymers 2012, 4, 964–985. [Google Scholar] [CrossRef]
- Schild, H.G.; Tirrel, D.A. Microcalorimetric detection of lower critical solution temperature in aqueous polymer solutions. J. Phys. Chem. 1990, 94, 4352–4356. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Chem. 1968, A2, 1441–1455. [Google Scholar] [CrossRef]
- Casolaro, M.; Cini, R.; del Bello, B.; Ferrali, M.; Maellaro, E. A cisplatin/hydrogel complex in cancer therapy. Biomacromolecules 2009, 10, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; del Bello, B.; Maellaro, E. Hydrogel containing l-valine residue as a platform for cisplatin chemotherapy. Collids Surf. B Biointerfaces 2011, 88, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Serinelli, F.; Consumi, M.; Magnani, A.; Casolaro, M.; Cini, R. Release studies from smart hydrogels as carriers for piroxicam and copper(II)-oxicam complexes as anti-inflammatory and anti-cancer drugs. X-ray structures of new copper(II)-piroxicam and isoxicam complex molecules. J. Inorg. Biochem. 2008, 102, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Casolaro, M.; Magnani, A.; Sega, A.; Chiasserini, L.; Messori, L.; Gabbiani, C.; Valiahdi, S.M.; Jakupec, M.; Keppler, B.K.; et al. New platinum-oxicam complexes as anti-cancer drugs. Synthesis, characterization, release studies from smart hydrogels, evaluation of reactivity with selected proteins and cytotoxic activity in vitro. J. Inorg. Biochem. 2010, 104, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Cini, R.; Defazio, S.; Tamasi, G.; Casolaro, M.; Messori, L.; Casini, A.; Morpurgo, M.; Hursthouse, M. fac-{Ru(CO)3}2+-core complexes and design of metal-based drugs. Synthesis, structure, and reactivity of Ru-thiazole derivative with serum proteins and absorption-release studies with acryloyl and silica hydrogels as carriers in physiological media. Inorg. Chem. 2007, 46, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Bernini, C.; Corbini, G.; Owens, N.F.; Messori, L.; Scaletti, F.; Massai, L.; Giudice, P.L.; Cini, R. Synthesis, spectroscopic and DFT structural characterization of two novel ruthenium(III) oxicam complexes. In vivo evaluation of anti- inflammatory and gastric damaging activities. J. Inorg. Biochem. 2014, 134, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I.; Bottari, S.; del Bello, B.; Maellaro, E.; Demadis, K.D. Long-term doxorubicin release from multiple stimuli-responsive hydrogels based on α-amino-acid residues. Eur. J. Pharm. Biopharm. 2014, 88, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I.; Lamponi, S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. Eur. J. Pharm. Biopharm. 2012, 80, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I. Controlled release of antidepressant drugs by multiple stimuli-sensitive hydrogels based on α-aminoacid residues. J. Drug Deliv. Sci. Technol. 2015, 30, 82–89. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The clinical pharmacology and use of paroxetine, a new selective serotonin reuptake inhibitor. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1994, 14, 127–138. [Google Scholar]
- Knadler, M.P.; Lobo, E.; Chappell, J.; Bergstrom, R. Duloxetine: Clinical pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2011, 50, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Sanchez, C.; Reines, E.H.; Montgomery, S.A. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int. Clin. Psychopharmacol. 2014, 29, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.R.; Hamilton, T.C.; Norton, J.; Petersen, E.N.; Poyser, R.H.; Thormählen, D. Relative lack of cardiotoxicity of paroxetine in animal models. Acta Psychiatr. Scand. 1989, 350, 27–30. [Google Scholar] [CrossRef]
- Wright, C.L.; Mist, S.D.; Ross, R.L.; Jones, K.D. Duloxetine for the treatment of fibromyalgia. Expert Rev. Clin. Immunol. 2010, 6, 745–756. [Google Scholar] [CrossRef] [PubMed]
- DrugBank: Small Molecule Drugs. Available online: www.drugbank.ca/drugs (accessed on 15 July 2016).
- Casolaro, M. Thermodynamics of multiple stimuli-responsive polyelectrolytes with complexing ability towards the copper(II) ion. React. Polym. 1994, 23, 71–83. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, UK, 1995. [Google Scholar]
- Satarkar, N.S.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Elvira, C.; Gallardo, A.; San Roman, J.; Cifuentes, A. Covalent Polymer-Drug Conjugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Vilara, G.; Tulla-Puchea, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Dobrynina, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Casolaro, M. Vinyl polymers containing l-valine and l-leucine residues: Thermodynamic behaviour of homopolymers and copolymers with N-isopropylacrylamide. Macromolecules 1995, 28, 2351–2358. [Google Scholar] [CrossRef]
- Casolaro, M.; Paccagnini, E.; Mendichi, R. Stimuli-responsive polymers based on l-phenylalanine residues: Protonation thermodynamics of free polymers and cross-linked hydrogels. Macromolecules 2005, 38, 2460–2468. [Google Scholar] [CrossRef]
- Peppas, N.A.; Korsmeyer, R.W. Dynamically swelling hydrogels in controlled release applications. In Hydrogels in Medicine and Pharmacy; Peppas, N.A., Ed.; CRC Press: Boca Raton, FL, USA, 1987; p. 109. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Barbucci, R.; Giani, G.; Fedi, S.; Bottari, S.; Casolaro, M. Biohydrogels with magnetic nanoparticles as crosslinker: Characteristics and potential use for controlled antitumor drug-delivery. Acta Biomater. 2012, 8, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Prouty, M.D.; Guo, Z.; Golub, V.O.; Cumar, C.S.S.R.; Lvov, Y.M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Liu, T.Y.; Huang, H.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir 2008, 24, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Wang, C.; Xu, R.; Chen, Z.; Gu, N. Magnetically sensitive alginate-templated polyelectrolyte multilayer microcapsules for controlled release of doxorubicin. J. Phys. Chem. C 2010, 114, 7673–7679. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.-H.; Liu, K.-H.; Liu, D.-M.; Chen, S.-Y. Study on controlled drug permeation of magnetic-sensitive ferrogels: Effect of Fe3O4 and PVA. J. Control. Release 2008, 126, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Bottari, S.; Cappelli, A.; Mendichi, R.; Ito, Y. Vinyl polymers based on l-histidine residues. Part 1. The thermodynamics of poly(ampholyte)s in the free and in the cross-linked gel form. Biomacromolecules 2004, 5, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.R.; Hashmi, S.; Naik, J.B. UV spectrophotometric method development and validation for determination of paroxetine hydrochloride in pharmaceutical dosage form. Int. J. Pharm. Pharm. Sci. 2010, 2, 43–45. [Google Scholar]
- Anderson, D.; Reed, S.; Lintemoot, J.; Kegler, S.; DeQuintana, S.; Sandberg, M.; Muto, J. A first look at duloxetine (Cimbalta®) in a post-mortem laboratory. J. Anal. Toxicol. 2006, 30, 576–580. [Google Scholar] [CrossRef] [PubMed]
| Chemical Structure | pKa | Chemical Structure | pKa |
|---|---|---|---|
| Paroxetine | 9.77 a | Duloxetine | 9.7 a |
 |  | ||
| Trazodone | 6.89 b | Citalopram | 9.42 b |
 |  | ||
| N-acryloyl-l-phenylalanine | 3.34 c | N-acryloyl-l-valine | 3.47 c |
 |  |
| Hydrogel | Drug | AMF a | k | n | R2 b |
|---|---|---|---|---|---|
| PHE-Nip3 | Parox | N | 0.0246 | 0.96 | 0.993 |
| Y | 0.0247 | 1.26 | 0.995 | ||
| Dulox | N | 0.0572 | 0.84 | 0.990 | |
| Y | 0.0594 | 0.93 | 0.990 | ||
| AVA-5 | Parox | - | 0.0593 | 0.91 | 0.998 |
| Dulox | - | 0.0591 | 1.04 | 0.996 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casolaro, M.; Casolaro, I. Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs. Gels 2016, 2, 24. https://doi.org/10.3390/gels2040024
Casolaro M, Casolaro I. Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs. Gels. 2016; 2(4):24. https://doi.org/10.3390/gels2040024
Chicago/Turabian StyleCasolaro, Mario, and Ilaria Casolaro. 2016. "Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs" Gels 2, no. 4: 24. https://doi.org/10.3390/gels2040024