Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials
Abstract
:1. Introduction
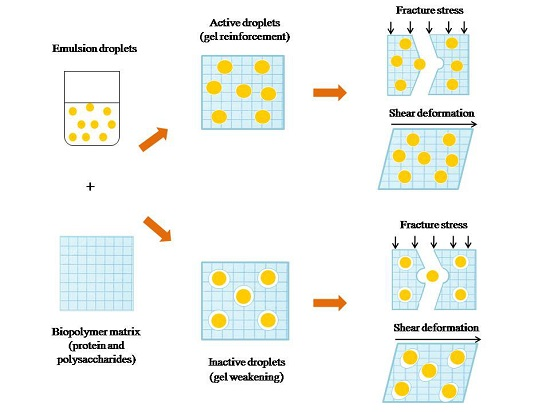
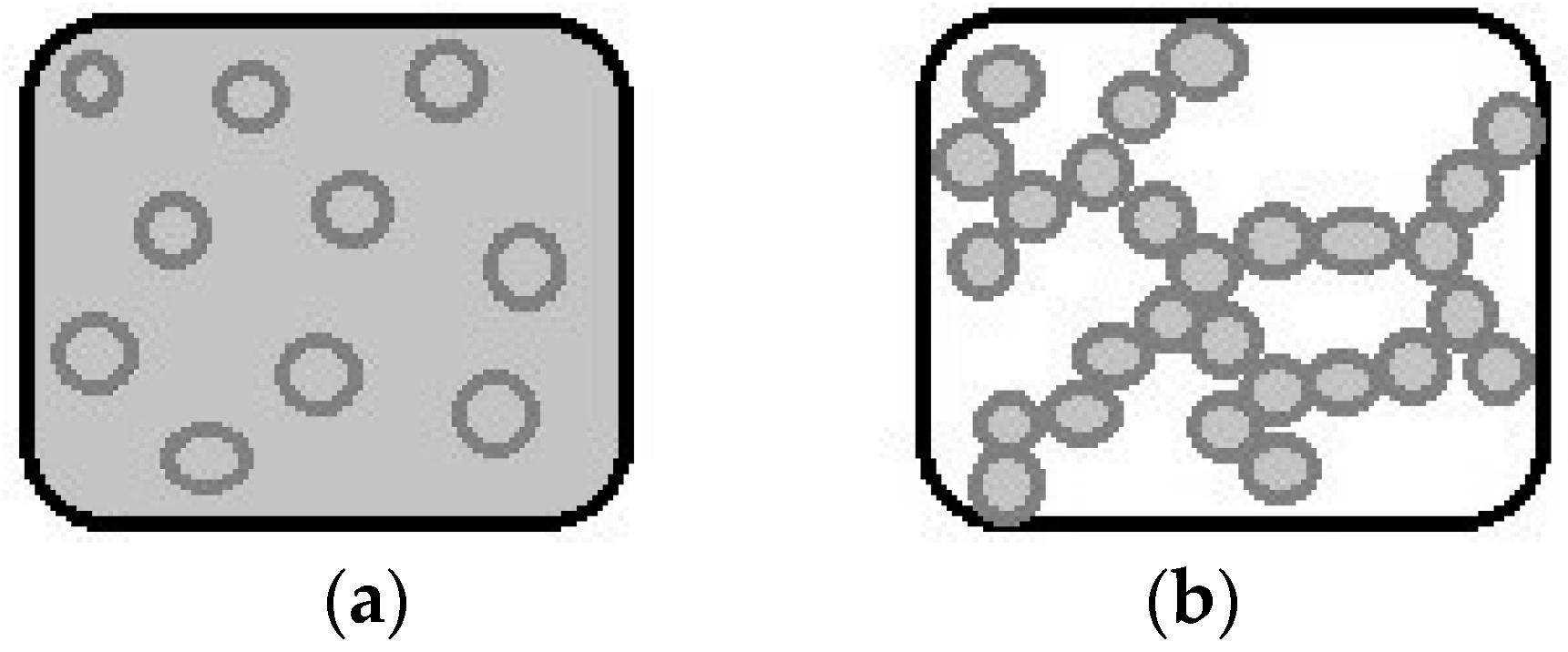
2. Definition and Characteristics of Emulsion-Filled Gels
Parameters that Impact the Rheological Behavior of Emulsion-Filled Gels
3. Rheological Techniques for the Characterization of Emulsion-Filled Gels
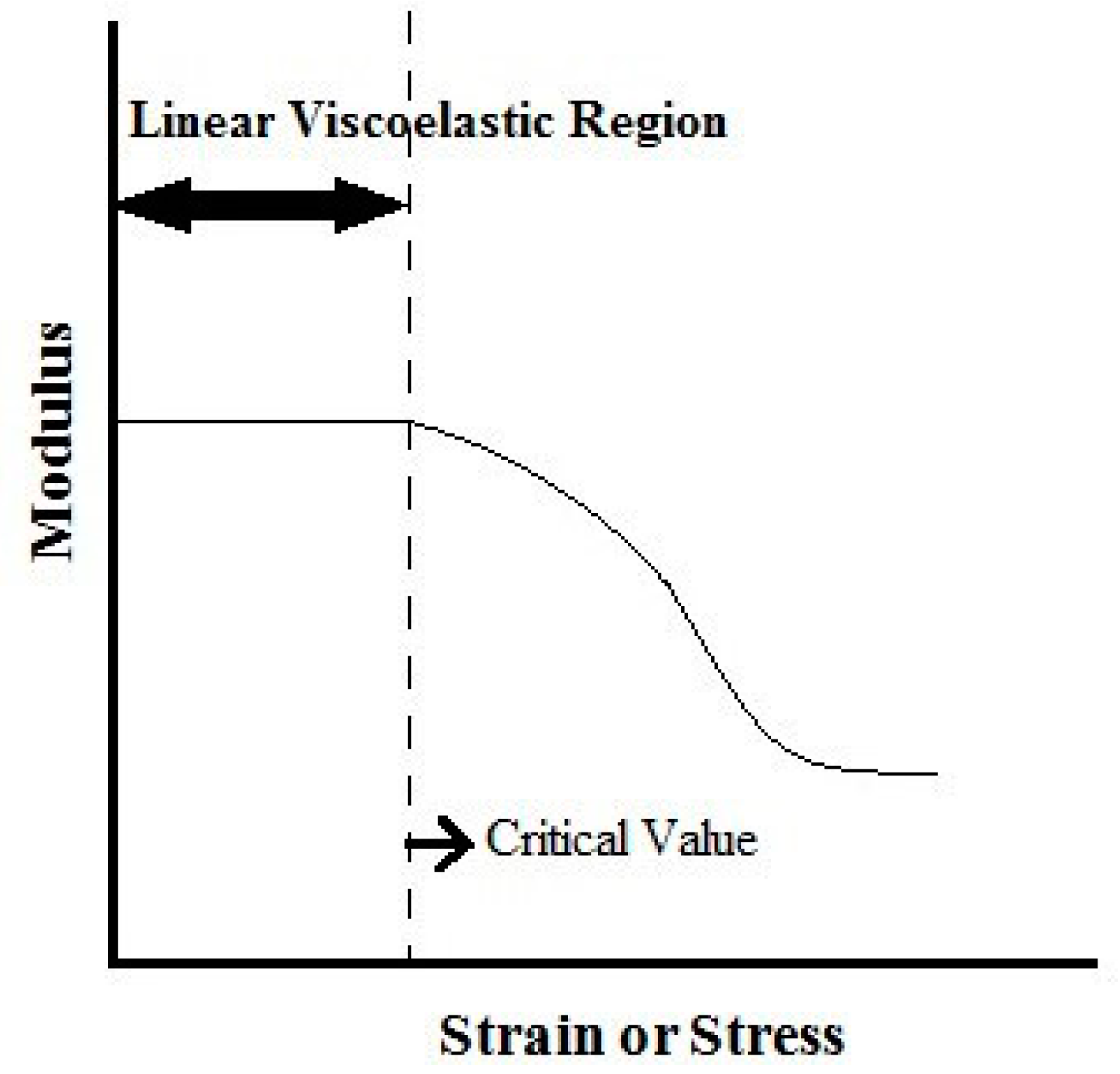
3.1. Dynamic Oscillatory Tests
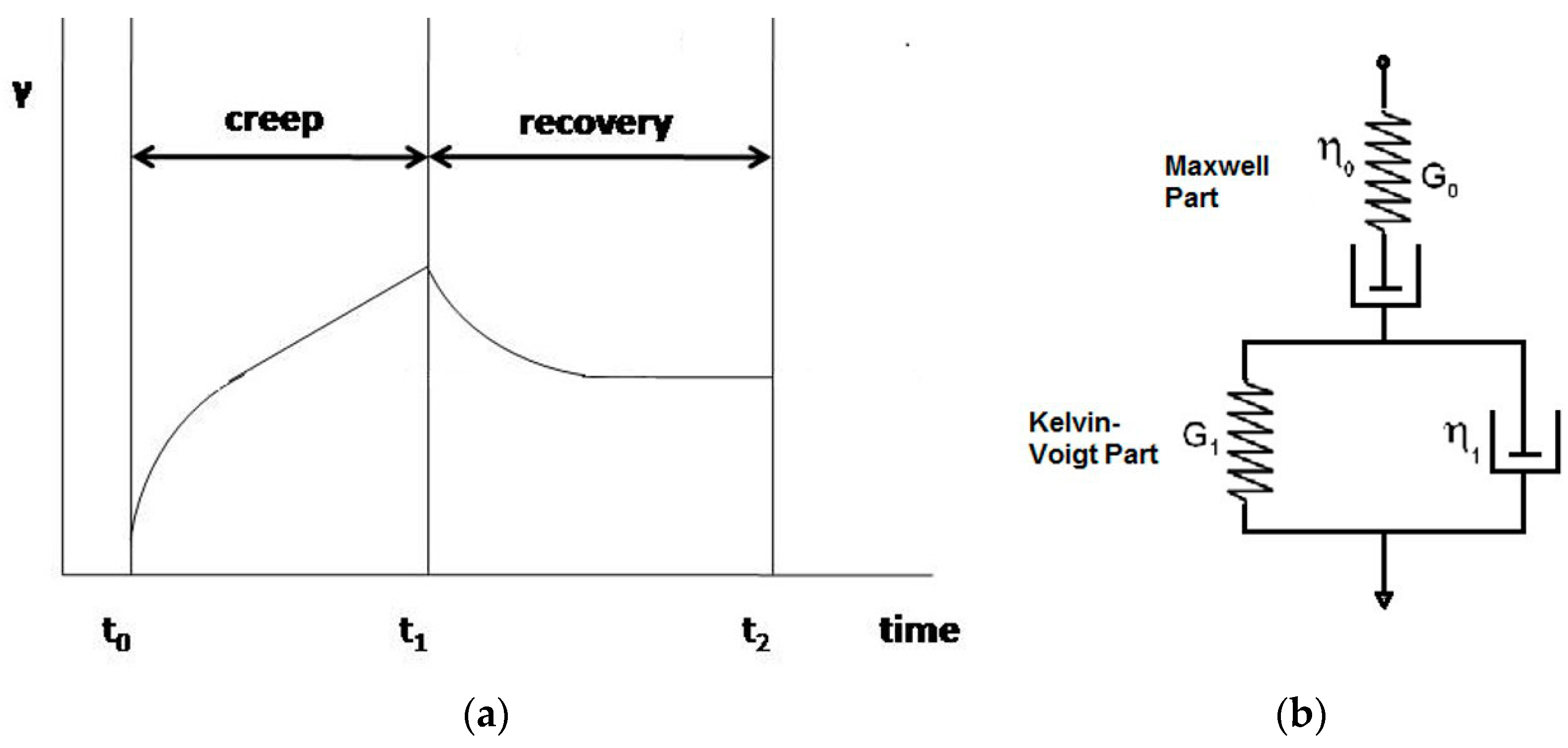
3.2. Creep-Recovery Tests
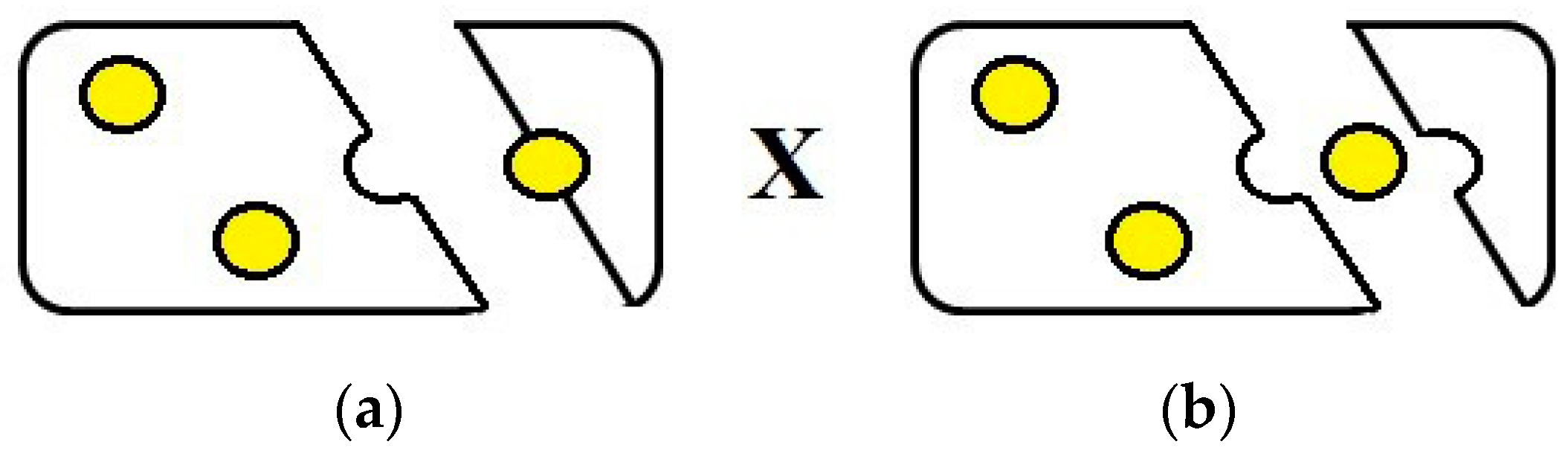
3.3. Large Deformation Tests and the Correlation of Emulsion-Filled Gel Textural Properties and Sensory Perception
4. Conclusions and Future Perspectives
- (i)
- different biopolymers as gelling agents;
- (ii)
- oil type and content and its physicochemical properties;
- (iii)
- interaction between emulsion droplets and biopolymeric matrix;
- (iv)
- production processes.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Norton, I.; Moore, S.; Fryer, P. Understanding food structuring and breakdown: Engineering approaches to obesity. Obes. Rev. 2007, 8, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Rosner, B.A.; Hennekens, C.H.; Willett, W.C. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997, 337, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.; Daniels, S.R.; Eckel, R.H.; Engler, M.; Howard, B.V.; Krauss, R.M.; Lichtenstein, A.H.; Sacks, F.; St Jeor, S.; Stampfer, M.; et al. Summary of the scientific conference on dietary fatty acids and cardiovascular health: Conference summary from the nutrition committee of the American Heart Association. Circulation 2001, 103, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Statrulat, I.; Britten, M.; Salmieri, S.; Fustier, P.; St-Gelais, D.; Champagne, C.P.; Lacroix, M. Enrichment of cheese with bioactive lipophilic compounds. J. Funct. Foods 2014, 6, 8–59. [Google Scholar] [CrossRef]
- Oliver, L.; Scholten, E.; van Aken, G.A. Effect of fat hardness on large deformation rheology of emulsion-filled gels. Food Hydrocoll. 2015, 43, 299–310. [Google Scholar] [CrossRef]
- Van Aken, G.A.; Vingerhoeds, M.H.; de Wijk, R.A. Textural perception of liquid emulsions: Role of oil content, oil viscosity and emulsion viscosity. Food Hydrocoll. 2011, 25, 789–796. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Houze’, G.; Cases, E.; Colas, B.; Cayot, P. Viscoelastic properties of acid milk gel as affected by fat nature at low level. Int. Dairy J. 2015, 15, 1006–1016. [Google Scholar] [CrossRef]
- Lorenzo, G.; Zaritzky, N.; Califano, A. Rheological analysis of emulsion-filled gels based on high acyl gellan gum. Food Hydrocoll. 2013, 30, 672–680. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Giarnetti, M.; Summo, C.; Pasqualone, A.; Minervini, F.; Caponio, F. Production and characterization of emulsion-filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015, 45, 30–40. [Google Scholar] [CrossRef]
- Van Aken, G.A.; Oliver, L.; Scholten, E. Rheological effect of particle clustering in gelled dispersions. Food Hydrocoll. 2015, 48, 102–109. [Google Scholar] [CrossRef]
- Barrantes, E.; Tamine, A.Y.; Sword, A.M.; Muir, D.D.; Kaláb, M. The manufacture of set-type natural yoghurt containing different oils—2: Rheological properties and microstructure. Int. Dairy J. 1996, 6, 827–837. [Google Scholar] [CrossRef]
- Lucey, J.A.; Munro, P.A.; Singh, H. Effects of heat treatment and whey protein addition on the rheological properties and structure of acid skim milk gels. Int. Dairy J. 1999, 9, 275–279. [Google Scholar] [CrossRef]
- Pereira, R.; Matia-Merino, L.; Jones, V.; Singh, H. Influence of fat on the perceived texture of set acid milk gels: A sensory perspective. Food Hydrocoll. 2006, 20, 305–313. [Google Scholar] [CrossRef]
- Van Vliet, T. Rheological properties of filled gels. Influence of filler matrix interaction. Colloid Polym. Sci. 1988, 266, 518–524. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of surface character of filler particles on rheology of heat-set whey protein emulsion gels. Colloids Surf. B Biointerfaces 1999, 12, 373–381. [Google Scholar] [CrossRef]
- Ring, S.; Stainsby, G. Filler reinforcement gels. Prog. Food Nutr. Sci. 1982, 6, 323–329. [Google Scholar]
- Sala, G.; van de Velde, F.; Cohen Stuart, M.; van Aken, G. Oil droplet release from emulsion-filled gels in relation to sensory perception. Food Hydrocoll. 2007, 21, 977–985. [Google Scholar] [CrossRef]
- Oliver, L.; Berndsen, L.; van Aken, G.; Scholten, E. Influence of droplet clustering on the rheological properties of emulsion-filled gels. Food Hydrocoll. 2015, 50, 74–83. [Google Scholar] [CrossRef]
- Sala, G.; van Vliet, T.; Stuart, M.A.C.; van de Velde, F.; van Aken, G.A. Deformation and fracture of emulsion-filled gels: Effect of gelling agent concentration and oil droplet size. Food Hydrocoll. 2009, 23, 1853–1863. [Google Scholar] [CrossRef]
- Sala, G.; van Vliet, T.; Stuart, M.A.C.; van Aken, G.A.; van de Velde, F. Deformation and fracture of emulsion-filled gels: Effect of oil content and deformation speed. Food Hydrocoll. 2009, 23, 1381–1393. [Google Scholar] [CrossRef]
- Kim, K.-H.; Renkema, J.M.S.; van Vliet, T. Rheological properties of soybean protein isolate gels containing emulsion droplets. Food Hydrocoll. 2001, 15, 295–302. [Google Scholar] [CrossRef]
- Chojnicka, A.; Sala, G.; Kruif, C.G.; van de Velde, F. The interactions between oil droplets and gel matrix affect the lubrication properties of sheared emulsion-filled gels. Food Hydrocoll. 2009, 23, 1038–1046. [Google Scholar] [CrossRef]
- Sato, A.C.K.; Moraes, K.E.F.P.; Cunha, R.L. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll. 2014, 34, 184–192. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; van Delft, M.; van de Velde, F.; van Boekel, M.A.J.S.; Stieger, M. Dynamic texture perception and oral processing of semi-solid food gels: Part 1: A comparison between QDA, progressive profiling and TDS. Food Hydrocoll. 2015, 43, 207–217. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- Dickinson, E. Structure formation in casein-based gels, foams, and emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 3–11. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Li, D.; Wang, L.; Bi, C.; Adhikari, B. Effect of gums on the rheological characteristics and microstructure ofacid-induced SPI-gum mixed gels. Carbohydr. Polym. 2014, 108, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hernàndez-Balada, E.; Taylor, M.M.; Phillips, J.G.; Marmer, W.N.; Brown, E.M. Properties of biopolymers produced by transglutaminase treatment of whey protein isolate and gelatin. Bioresour. Technol. 2009, 100, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, T.; Feng, S.; Xu, Q.; Zheng, T.; Zhou, M.; Chu, X.; Huang, X.; Lu, X.; Pan, S.; et al. Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrason. Sonochem. 2016, 29, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Yamamoto, Y. Rheology of Milk Protein Gels and Protein-Stabilized Emulsion Gels Crosslinked with Transglutaminase. J. Agric. Food Chem. 1996, 44, 1371–1377. [Google Scholar] [CrossRef]
- Line, V.L.S.; Remondetto, G.E.; Subirade, M. Cold gelation of b-lactoglobulin oil-in-water emulsions. Food Hydrocoll. 2005, 19, 269–278. [Google Scholar] [CrossRef]
- Kokini, J.; van Aken, G. Discussion session on food emulsions and foams. Food Hydrocoll. 2006, 20, 438–445. [Google Scholar] [CrossRef]
- Van der Poel, C. On the rheology of concentrated dispersions. Rheol. Acta 1958, 1, 198–205. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Viscoelastic properties of protein-stabilized emulsions: Effect of protein-surfactant interactions. J. Agric. Food Chem. 1998, 46, 91–97. [Google Scholar] [CrossRef] [PubMed]
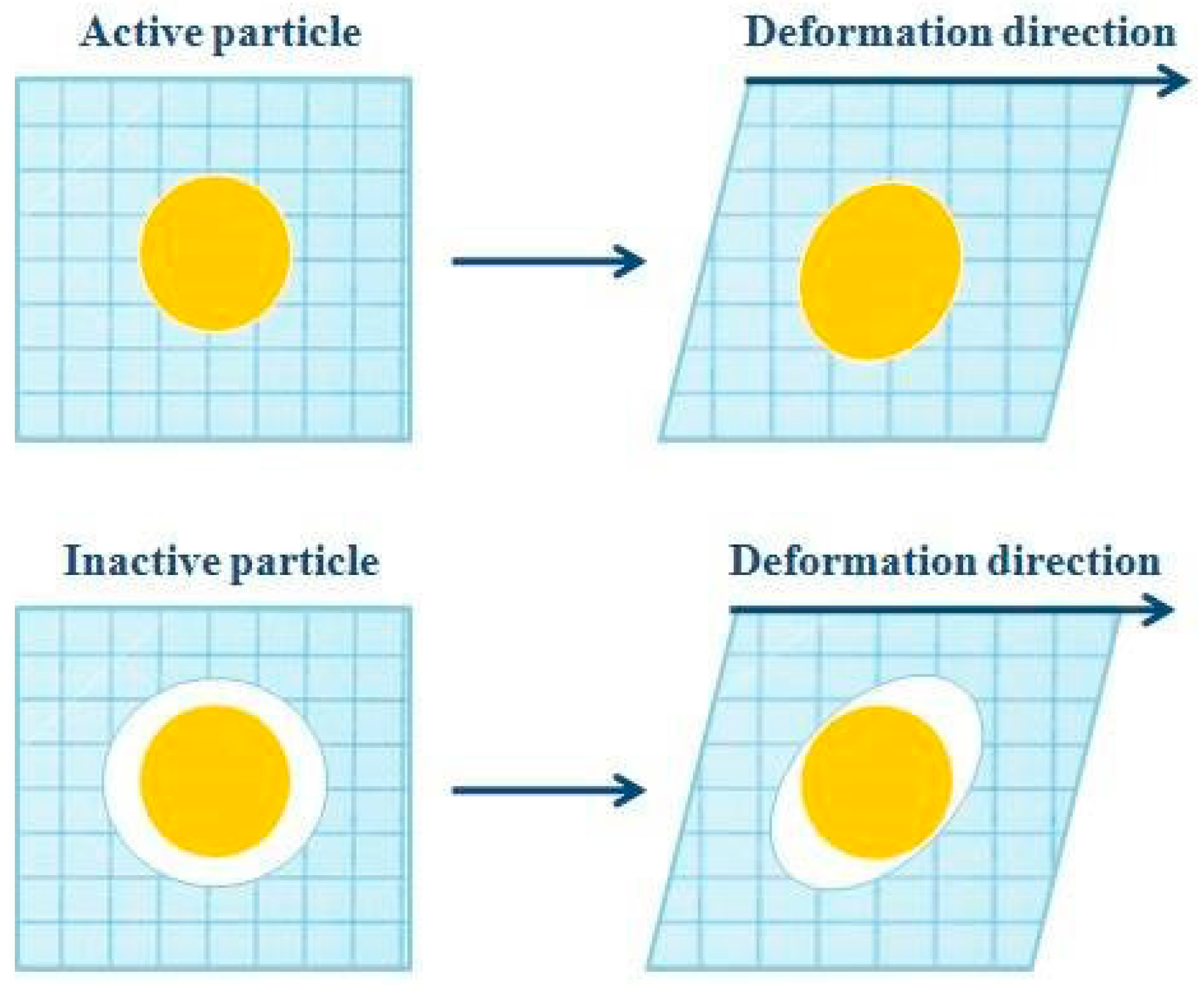
- Dickinson, E.; Chen, J. Heat-set whey protein emulsion gels: Role of active and inactive filler particles. J. Dispers. Sci. Technol. 1999, 20, 197–213. [Google Scholar] [CrossRef]
- Smith, J.C. Simplification of van der Poel/s Formula for the Shear Modulus of a Particulate Composite. J. Res. Natl. Bur. Stand. A Phys. Chem. 1975, 79A, 419–423. [Google Scholar] [CrossRef]
- Pal, R. Complex shear modulus of concentrated suspensions of solid spherical particles. J. Colloid Interface Sci. 2002, 245, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.B.; Nielsen, L.E. Dynamic mechanical properties of particulate filled composites. J. Appl. Polym. Sci. 1970, 14, 1449–1471. [Google Scholar] [CrossRef]
- Palierne, J.F. Linear rheology of viscoelastic emulsions with interfacial tension. Rheol. Acta 1990, 29, 204–214. [Google Scholar] [CrossRef]
- Pal, R. New models for effective Young's modulus of particulate composites. Compos. B 2005, 36, 513–523. [Google Scholar] [CrossRef]
- Pal, R. A new linear viscoelastic model for emulsions and suspensions. Polym. Eng. Sci. 2008, 48, 1250–1253. [Google Scholar] [CrossRef]
- Oliver, L.; Wieck, L.; Scholten, E. Influence of matrix inhomogeneity on the rheological properties of emulsion-filled gels. Food Hydrocoll. 2016, 52, 116–125. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Bowland, E.L.; Hardin, C.C. Factors that determine the fracture properties and microstructure of globular protein gels. Food Hydrocoll. 1995, 9, 237–249. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. The breakdown properties of heat-set whey protein emulsion gels in the human mouth. Food Hydrocoll. 2013, 33, 215–224. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Miao, S. Study on the Rheological Properties and Volatile Release of Cold-Set Emulsion-Filled Protein Gels. J. Agric. Food Chem. 2014, 62, 11420–11428. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Monahan, F.J.; Kinsella, J.E. Effect of emulsion droplets on the rheology of whey protein isolate gels. J. Texture Stud. 1993, 24, 411–422. [Google Scholar] [CrossRef]
- Dickinson, E.; Hong, S.T. Influence of water-soluble nonionic emulsifier on the rheology of heat-set protein-stabilized emulsion gels. J. Agric. Food Chem. 1995, 43, 2560–2566. [Google Scholar] [CrossRef]
- Ye, A.; Taylor, S. Characterization of cold-set gels produced from heated emulsions stabilized by whey protein. Int. Dairy J. 2009, 19, 721–727. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Effects of different oils on the properties of soy protein isolate emulsions and gels. Food Res. Int. 2009, 42, 925–932. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Rheology and microstructure of myofibrillar protein–plant lipid composite gels: Effect of emulsion droplet size and membrane type. J. Food Eng. 2011, 106, 318–324. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Microstructure and elastic modulus of phase-separated gelatin starch hydrogels containing dispersed oil droplets. Food Hydrocoll. 2013, 30, 333–342. [Google Scholar] [CrossRef]
- Yang, M.; Liu, F.; Tang, C.H. Properties and microstructure of transglutaminase-set soy protein-stabilized emulsion gels. Food Res. Int. 2013, 52, 409–418. [Google Scholar] [CrossRef]
- Liu, K.; Stieger, M.; van der Linden, E.; van de Velde, F. Fat droplet characteristics affect rheological, tribological and sensory properties of food gels. Food Hydrocoll. 2015, 44, 244–259. [Google Scholar] [CrossRef]
- Murakatete, N.; Zhang, C.; Hategekimana, J.; Karangwa, E.; Yufei, H. Soybean oil volume fraction effects on the rheology characteristics and gelation behavior of glucono-d-lactone and calcium sulfate-induced tofu gels. J. Texture Stud. 2015. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Tadros, T.F. Correlation of viscoelastic properties of stable and flocculated suspensions with their interparticle interactions. Adv. Colloid Interface Sci. 1996, 68, 97–200. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of monoglycerides and diglycerol-esters on viscoelasticity of heat-set whey protein emulsion gels. Int. J. Food Sci. Technol. 1999, 34, 493–501. [Google Scholar] [CrossRef]
- Steffe, J.F.R. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: Lansing, MI, USA, 1996; pp. 318–321. [Google Scholar]
- Yilmaz, M.T.; Karaman, S.; Dogan, M.; Yetim, H.; Kayacer, A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis (TPA) parameters. J. Food Eng. 2012, 108, 327–336. [Google Scholar] [CrossRef]
- Dolz, M.; Hernández, M.J.; Delegido, J. Creep and recovery experimental investigation of low oil content food emulsions. Food Hydrocoll. 2008, 22, 421–427. [Google Scholar] [CrossRef]
- Lorenzo, G.; Checmarev, G.; Zaritzky, N.; Califano, A. Linear viscoelastic assessment of cold gel-like emulsions stabilized with bovine gelatin. LWT Food Sci. Technol. 2011, 44, 457–464. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; Strijbosch, V.M.G.; van den Broek, A.W.M.; van de Velde, F.; Stieger, M. Uncoupling the impact of fracture properties and compositionon sensory perception of emulsion-filled gels. J. Texture Stud. 2015. [Google Scholar] [CrossRef]
- Lillford, P.J. Mechanism of fracture in foods. J. Texture Stud. 2001, 32, 397–417. [Google Scholar] [CrossRef]
- Heath, R.M.; Prinz, J.F. Oral processing of foods and the sensorial evaluation of texture. In Food Texture: Measurement and Perception; Rosenthal, A.J., Ed.; Aspen Publishers: New York, NY, USA, 1999. [Google Scholar]
- Malone, M.E.; Appelqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Van Vliet, T. On the relation between texture perception and fundamental mechanical parameters for liquid and time dependent solids. Food Qual. Preference 2002, 13, 227–236. [Google Scholar] [CrossRef]
- Van Vliet, T.; Walstra, P. Large deformation and fracture behaviour of gels. Faraday Discuss. 1995, 101, 359–370. [Google Scholar] [CrossRef]
- Van Vliet, T.; van Aken, G.A.; de Jongh, H.H.J.; Hamer, R.J. Colloidal aspects of texture perception. Adv. Colloid Interface Sci. 2009, 150, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, T.; Luyten, H.; Walstra, P. Fracture and yielding of gels. In Food Polymers, Gels and Colloids; Dickinson, E., Ed.; Royal Society of Chemistry: Cambridge, UK, 1991; pp. 392–403. [Google Scholar]
- Van Vliet, T. Mechanical properties of concentrated food gels. In Food Macromolecules and Colloids; Dickinson, E., Lorient, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 1995; pp. 447e–455e. [Google Scholar]
- Yang, X.I.N.; Rogers, N.R.; Berry, T.K.; Foegeding, E.A. Modeling the rheological properties of cheddar cheese with different fat contents at various temperatures. J. Texture Stud. 2011, 42, 331–348. [Google Scholar] [CrossRef]
- Van Vliet, T.; Luyten, H.; Walstra, P. Time dependent fracture behaviour of food. In Food Colloids and Polymer, Stability and Mechanical Properties; Dickinson, E., Walstra, P., Eds.; Royal Society of Chemistry: Cambridge, UK, 1993; Volume 113, pp. 175–190. [Google Scholar]
- Kim, K.-H.; Gohtani, S.; Yamano, Y. Effects of oil droplets in physical and sensory properties of o/w emulsion agar gels. J. Texture Stud. 1996, 27, 655–670. [Google Scholar] [CrossRef]
- Gwartney, E.A.; Larick, D.K.; Foegeding, E.A. Sensory texture and mechanical properties of stranded and particulate whey protein emulsion gels. J. Food Sci. 2004, 69, 333–339. [Google Scholar] [CrossRef]
- Sala, G.; van Aken, G.; Cohen-Stuart, M.A.; van de Veld, F. Effect of droplet-matrix interaction on large deformation properties of emulsion-filled gels. J. Texture Stud. 2007, 38, 511–535. [Google Scholar] [CrossRef]
- Sala, G.; de Wijk, R.A.; van de Velde, F.; van Aken, G.A. Matrix properties affect the sensory perception of emulsion-filled gels. Food Hydrocoll. 2008, 22, 353–363. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; van de Velde, F.; van Boekel, M.A.J.S.; Stieger, M. Dynamic texture perception and oral processing of semi-solid food gels: Part 2: Impact of breakdown behaviour on bolus properties and dynamic texture perception. Food Hydrocoll. 2015, 49, 61–72. [Google Scholar] [CrossRef]
- De Lavergne, D.M.; Tournier, C.; Dominique, B.; Salles, C.; van de Velde, F.; Stieger, M. Dynamic texture perception, oral processing behaviour and bolus properties of emulsion-filled gels with and without contrasting mechanical properties. Food Hydrocoll. 2016, 52, 648–660. [Google Scholar] [CrossRef]
- Mosca, A.C.; Rocha, J.A.; Sala, G.; van de Velde, F.; Stieger, M. Inhomogeneous distribution of fat enhances the perception of fat-relatedsensory attributes in gelled foods. Food Hydrocoll. 2012, 27, 448–455. [Google Scholar] [CrossRef]
- Brandt, M.A.; Skinner, E.Z.; Coleman, J.A. Texture profile method. J. Food Sci. 1963, 28, 404–409. [Google Scholar] [CrossRef]
- Kramer, A.; Szczesniak, A.S. Texture Measurements of Foods: Psychophysical Fundamentals: Sensory, Mechanical, and Chemical Procedures, and Their Interrelationships; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1973. [Google Scholar]
- Çakir, E.; Vinyard, C.J.; Essick, G.; Daubert, C.R.; Drake, M.; Foegeding, A.E. Interrelations among physical characteristics, sensory perception and oral processing of protein-based soft-solid structures. Food Hydrocoll. 2012, 29, 234–245. [Google Scholar] [CrossRef]
- Everard, C.D.; O’Callaghan, D.J.; Howard, T.V.; O’Donnell, C.P.; Sheehan, E.M.; Delahunty, C.M. Relationships between sensory and rheological measurements of texture in maturing commercial cheddar cheese over a range of moisture and pH at the point of manufacture. J. Texture Stud. 2006, 37, 361–382. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Drake, M.A. Invited Review: Sensory and Mechanical Properties of Cheese Texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, A.R.; Mulvihill, D.M.; Auty, M.E.A. Combined microscopic and dynamic rheological methods for studying the structural breakdown properties of whey protein gels and emulsion filled gels. Food Hydrocoll. 2011, 25, 275–282. [Google Scholar] [CrossRef]
- Abhyankar, A.R.; Mulvihill, D.M.; Auty, M.E.A. Combined confocal microscopy and large deformation analysis of emulsion filled gels and stirred acid milk gels. Food Struct. 2014, 1, 127–136. [Google Scholar] [CrossRef]
| References | Effect Studied | Principal Results |
|---|---|---|
| [48] | Droplet size and emulsifier agent type on the rheology of WPI emulsion-filled gels | Gel strength of emulsion stabilized by WPI was higher than gel strength of emulsion stabilized by nonionic surfactants and gel strength of emulsion stabilized by sodium dodecyl sulfate (SDS). For droplets stabilized by WPI, the gel strength increased as the droplet size decreased. A relatively insensitive effect on the gel strength was observed for droplets stabilized by small-molecule surfactants. |
| [49] | Influence of nonionic emulsifier (Tween 20) on rheological behavior of β-lactoglobulin emulsion gels | G′ (storage modulus) demonstrated increase at low emulsifier content, decrease at intermediate emulsifier content, and then at high emulsifier contents it either increased again or remained low, depending on the protein content. |
| [23] | Volume fraction of oil droplets in soybean protein gels | Increasing oil volume fraction, storage and loss moduli (G′ and G″) of the gels increased. Higher compressive stresses of the gels containing smaller oil droplets were obtained in comparison to those containing larger droplets, especially at higher volume fractions of oil droplets. |
| [33] | The influence of oil and calcium concentrations on the rheological properties of cold gelation of β-lactoglobulin emulsion gels | The oil content affected G′ (storage modulus) of gels, the effect of which was higher than that of calcium concentration. |
| [50] | Emulsion droplets size influence and fat content influence in preheated emulsions stabilized by whey protein produced by cold gelation | The storage modulus (G′) of the emulsion-filled gels increased with decreasing emulsion droplets size and increasing fat content. |
| [51] | Different types of oils in GDL-induced SPI gels | The SPI gels filled by palm stearin emulsion were harder than the SPI gels filled by soy and sunflower oil emulsions as well as the SPI gel without oil. Gels containing the two liquid oils were softer than the control (SPI gel without oil). |
| [52] | Emulsions of vegetable oils (olive and peanut) of various particle sizes in composite gels with 2% myofibrillar protein | Increases in storage modulus (G′) of myofibrillar protein sols/gels with the addition of emulsions. G′ increases with smaller emulsion droplet size. Hardness of gels containing olive oil emulsion was higher than that containing peanut oil emulsion. Myofibrillar protein as an emulsifier agent promoted stronger reinforcement of the gels than the oil droplets stabilized by Tween 80-stablized oil droplets. |
| [53] | Presence of emulsified olive oil effect on G′ of gelatin-starch phase-separated gels | The dispersed oil phase behaved as an active filler within the phase-separated gel matrix due to increase of G′ after oil incorporation in the gels. |
| [54] | Different oil fractions in SPI-stabilized emulsion gels | Increase in oil fraction progressively increased the storage modulus (G′) (gel strength). |
| [10] | Oil and gellan gum concentrations on the viscoelastic behavior of high acyl gellan gumemulsion-filled gels | Stronger gels were obtained with increasing gellan concentration, but oil fraction had a small effect on the elastic behavior of the emulsions. |
| [55] | Droplet–matrix interactions in gelatin gels filled by O/W animal fat or oil emulsions stabilized with different emulsifiers | The mechanical properties of the gels (Young’s modulus (E) and fracture properties) were affected by droplet–matrix interaction, fat content, and solid fat content. |
| [56] | The volume fraction of soybean oil the rheological behavior of acid- and salt-induced soft tofu-type gels | A lower gel point temperature and a reduction in gelation time were observed for a high oil volume fraction, while an increase was observed in the storage modulus at the gel point when the oil volume fraction in the gels increased. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremias-Andrade, I.M.; Souki, N.P.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. https://doi.org/10.3390/gels2030022
Geremias-Andrade IM, Souki NPBG, Moraes ICF, Pinho SC. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels. 2016; 2(3):22. https://doi.org/10.3390/gels2030022
Chicago/Turabian StyleGeremias-Andrade, Ivana M., Nayla P.B.G. Souki, Izabel C.F. Moraes, and Samantha C. Pinho. 2016. "Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials" Gels 2, no. 3: 22. https://doi.org/10.3390/gels2030022