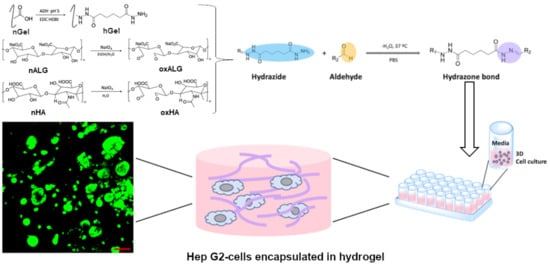
Functionalized Gelatin/Polysaccharide Hydrogels for Encapsulation of Hepatocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degree of Functionalization of Modified Gelatin and oxPS
2.2. Mechanical Properties and Crosslinking Kinetics of Hydrogels
2.3. Stability of Hydrogels
2.4. Cell Culture Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrazide-Functionalized Gelatin
4.3. Synthesis of Oxidized Hyaluronate (oxHA) and Oxidized Alginate (oxALG)
4.4. Hydrogel Formation and Characterization
4.4.1. Studies on Gel Stability
4.4.2. Mechanical Properties of Hydrogels
4.4.3. Rheological Studies
4.5. Biological Studies
4.5.1. Cell-Viability and Proliferation Studies
4.5.2. Cytotoxicity Studies by Coverage of HepG2 Cells with Hydrogel Components and Hydrogels
4.5.3. Encapsulation of HepG2 Cells in Hydrogels
4.6. Statistical Calculations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef]
- Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers 2021, 13, 2970. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodríguez-Fernández, J.; Gallego-Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides-Towards Biocompatible Hydrogels for Biomedical Applications. Chemistry 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Willems, C.; Trutschel, M.-L.; Mazaikina, V.; Strätz, J.; Mäder, K.; Fischer, S.; Groth, T. Hydrogels Based on Oxidized Cellulose Sulfates and Carboxymethyl Chitosan: Studies on Intrinsic Gel Properties, Stability, and Biocompatibility. Macromol. Biosci. 2021, 21, e2100098. [Google Scholar] [CrossRef]
- Köwitsch, A.; Chhalotre, A.; Groth, T. Effect of thiolated glycosaminoglycans on the behavior of breast cancer cells: Toward the development of in vitro models of cancer. Int. J. Artif. Organs 2017, 40, 31–39. [Google Scholar] [CrossRef]
- Alkhoury, H.; Hautmann, A.; Fuhrmann, B.; Syrowatka, F.; Erdmann, F.; Zhou, G.; Stojanović, S.; Najman, S.; Groth, T. Studies on the Mechanisms of Anti-Inflammatory Activity of Heparin- and Hyaluronan-Containing Multilayer Coatings-Targeting NF-κB Signalling Pathway. Int. J. Mol. Sci. 2020, 21, 3724. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Gao, X.; Huang, R.; Jiao, Y.; Groth, T.; Yang, W.; Tu, C.; Li, H.; Gong, F.; Chu, J.; Zhao, M. Enhanced wound healing in diabetic mice by hyaluronan/chitosan multilayer-coated PLLA nanofibrous mats with sustained release of insulin. Appl. Surf. Sci. 2022, 576, 151825. [Google Scholar] [CrossRef]
- Harrer, D.; Sanchez Armengol, E.; Friedl, J.D.; Jalil, A.; Jelkmann, M.; Leichner, C.; Laffleur, F. Is hyaluronic acid the perfect excipient for the pharmaceutical need? Int. J. Pharm. 2021, 601, 120589. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef]
- Rowley, J.A.; Mooney, D.J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef]
- de Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, e2005476. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Ulubayram, K.; Aksu, E.; Gurhan, S.I.D.; Serbetci, K.; Hasirci, N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. J. Biomater. Sci. Polym. Ed. 2002, 13, 1203–1219. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Cailliez, V.O.; Grady, J.G.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Legallais, C.; Kim, D.; Mihaila, S.M.; Mihajlovic, M.; Figliuzzi, M.; Bonandrini, B.; Salerno, S.; Yousef Yengej, F.A.; Rookmaaker, M.B.; Sanchez Romero, N.; et al. Bioengineering Organs for Blood Detoxification. Adv. Healthc. Mater. 2018, 7, e1800430. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Paradis, V. Liver extracellular matrix in health and disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef]
- Dunn, J.C.; Tompkins, R.G.; Yarmush, M.L. Hepatocytes in collagen sandwich: Evidence for transcriptional and translational regulation. J. Cell Biol. 1992, 116, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fernandez, J.; Garcia-Legler, E.; Villanueva-Badenas, E.; Donato, M.T.; Gomez-Ribelles, J.L.; Salmeron-Sanchez, M.; Gallego-Ferrer, G.; Tolosa, L. Primary human hepatocytes-laden scaffolds for the treatment of acute liver failure. Biomater. Adv. 2023, 153, 213576. [Google Scholar] [CrossRef] [PubMed]
- Ijima, H.; Nakamura, S.; Bual, R.P.; Yoshida, K. Liver-specific extracellular matrix hydrogel promotes liver-specific functions of hepatocytes in vitro and survival of transplanted hepatocytes in vivo. J. Biosci. Bioeng. 2019, 128, 365–372. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Donato, M.T.; Castell, J.V.; Jover, R. Human hepatocytes in primary culture: The choice to investigate drug metabolism in man. Curr. Drug Metab. 2004, 5, 443–462. [Google Scholar] [CrossRef]
- Sauer, V.; Roy-Chowdhury, N.; Guha, C.; Roy-Chowdhury, J. Induced pluripotent stem cells as a source of hepatocytes. Curr. Pathobiol. Rep. 2014, 2, 11–20. [Google Scholar] [CrossRef]
- Thompson, J.; Jones, N.; Al-Khafaji, A.; Malik, S.; Reich, D.; Munoz, S.; MacNicholas, R.; Hassanein, T.; Teperman, L.; Stein, L.; et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 2018, 24, 380–393. [Google Scholar] [CrossRef]
- Krasteva, N.; Harms, U.; Albrecht, W.; Seifert, B.; Hopp, M.; Altankov, G.; Groth, T. Membranes for biohybrid liver support systems--investigations on hepatocyte attachment, morphology and growth. Biomaterials 2002, 23, 2467–2478. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Sreedevi Madhavikutty, A.; Okada, S.; Qiming, Z.; Inagaki, N.F.; Ohta, S.; Ito, T. Injectable, shear-thinning, photocrosslinkable, and tissue-adhesive hydrogels composed of diazirine-modified hyaluronan and dendritic polyethyleneimine. Biomater. Sci. 2024, 12, 1454–1464. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed Engl. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Kamedani, M.; Okawa, M.; Madhavikutty, A.S.; Tsai, C.-C.; Singh Chandel, A.K.; Fujiyabu, T.; Inagaki, N.F.; Ito, T. Injectable Extracellular Matrix-Inspired Hemostatic Hydrogel Composed of Hyaluronan and Gelatin with Shear-Thinning and Self-Healing. Biomacromolecules 2024, 25, 1790–1799. [Google Scholar] [CrossRef]
- Cimen, Z.; Babadag, S.; Odabas, S.; Altuntas, S.; Demirel, G.; Demirel, G.B. Injectable and Self-Healable pH-Responsive Gelatin–PEG/Laponite Hybrid Hydrogels as Long-Acting Implants for Local Cancer Treatment. ACS Appl. Polym. Mater. 2021, 3, 3504–3518. [Google Scholar] [CrossRef]
- Sakai, S.; Yamaguchi, S.; Takei, T.; Kawakami, K. Oxidized alginate-cross-linked alginate/gelatin hydrogel fibers for fabricating tubular constructs with layered smooth muscle cells and endothelial cells in collagen gels. Biomacromolecules 2008, 9, 2036–2041. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728–744. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Mechanical properties of whole-body soft human tissues: A review. Biomed. Mater. 2021, 16, 062004. [Google Scholar] [CrossRef]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic principle and good practices of rheology for polymers for teachers and beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Porter, R.S.; Johnson, J.F. The Entanglement Concept in Polymer Systems. Chem. Rev. 1966, 66, 1–27. [Google Scholar] [CrossRef]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and Enzymatically Dual Cross-Linked Oxidized Alginate Gelatin Hydrogels with Tunable Stiffness and Degradation Behavior for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Ayyildiz, M.; Aktas, R.G.; Basdogan, C. Effect of Solution and Post-Mortem Time on Mechanical and Histological Properties of Liver during Cold Preservation. Biorheology 2014, 51, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Ruoß, M.; Häussling, V.; Schügner, F.; Olde Damink, L.H.H.; Lee, S.M.L.; Ge, L.; Ehnert, S.; Nussler, A.K. A standardized collagen-based scaffold improves human hepatocyte shipment and allows metabolic studies over 10 days. Bioengineering 2018, 5, 86. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and Its Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Collins, M.N.; Birkinshaw, C. Comparison of the effectiveness of four different crosslinking agents with hyaluronic acid hydrogel films for tissue-culture applications. J. Appl. Polym. Sci. 2007, 104, 3183–3191. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef]
- Timperio, A.M.; Rinalducci, S.; Zolla, L. Hydrazide derivatives produce active oxygen species as hydrazine. Bioorg. Chem. 2005, 33, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Genç, H.; Hazur, J.; Karakaya, E.; Dietel, B.; Bider, F.; Groll, J.; Alexiou, C.; Boccaccini, A.R.; Detsch, R.; Cicha, I. Differential Responses to Bioink-Induced Oxidative Stress in Endothelial Cells and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 2358. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular mechanisms of aldehyde toxicity: A chemical perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Alarake, N.Z.; Frohberg, P.; Groth, T.; Pietzsch, M. Mechanical properties and biocompatibility of in situ enzymatically cross-linked gelatin hydrogels. Int. J. Artif. Organs 2017, 40, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bednarzig, V.; Karakaya, E.; Egaña, A.L.; Teßmar, J.; Boccaccini, A.R.; Detsch, R. Advanced ADA-GEL bioink for bioprinted artificial cancer models. Bioprinting 2021, 23, e00145. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of encapsulated MG-63 cells in RGD and gelatine-modified alginate hydrogels. Tissue Eng. Part A 2014, 20, 2140–2150. [Google Scholar] [CrossRef] [PubMed]







| Sample | Degree of Functionalization |
|---|---|
| hGel | 48.6% |
| oxHA * | 12.5% |
| oxALG * | 20.7% |
| Oxidized Polysaccharides | Gelation Time (min) | G’ (Pa) * | G” (Pa) * |
|---|---|---|---|
| oxALG | 39.85 ± 3.7 | 414.78 ± 55.8 | 9.0 ± 2.2 |
| oxHA | 23.08 ± 6.6 | 521.37 ± 59.9 | 22.70 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, C.; Qi, F.; Trutschel, M.-L.; Groth, T. Functionalized Gelatin/Polysaccharide Hydrogels for Encapsulation of Hepatocytes. Gels 2024, 10, 231. https://doi.org/10.3390/gels10040231
Willems C, Qi F, Trutschel M-L, Groth T. Functionalized Gelatin/Polysaccharide Hydrogels for Encapsulation of Hepatocytes. Gels. 2024; 10(4):231. https://doi.org/10.3390/gels10040231
Chicago/Turabian StyleWillems, Christian, Fangdi Qi, Marie-Luise Trutschel, and Thomas Groth. 2024. "Functionalized Gelatin/Polysaccharide Hydrogels for Encapsulation of Hepatocytes" Gels 10, no. 4: 231. https://doi.org/10.3390/gels10040231