Exploring Cellulose Triacetate Nanofibers as Sustainable Structuring Agent for Castor Oil: Formulation Design and Rheological Insights
Abstract
:1. Introduction
2. Results and Discussion
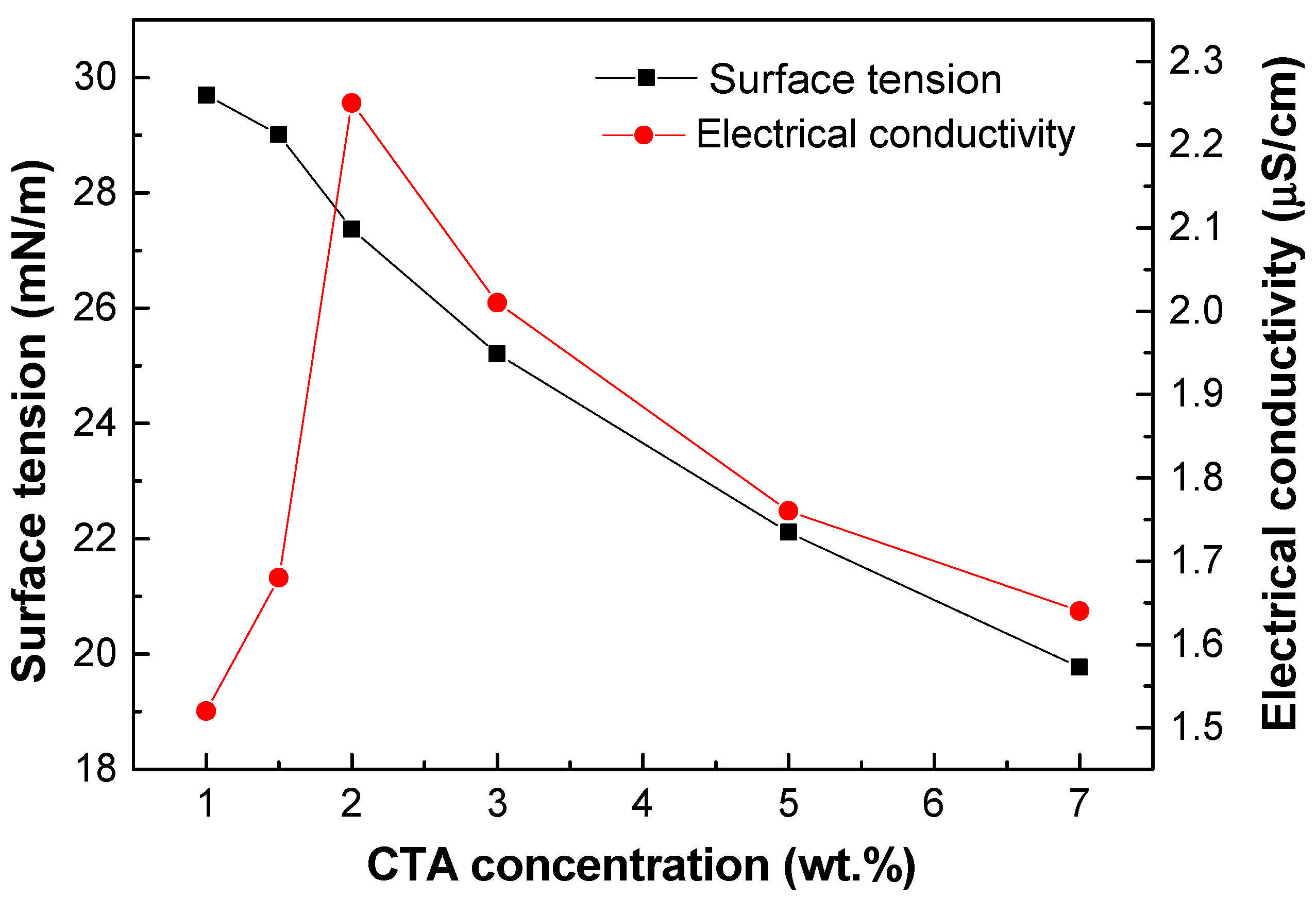
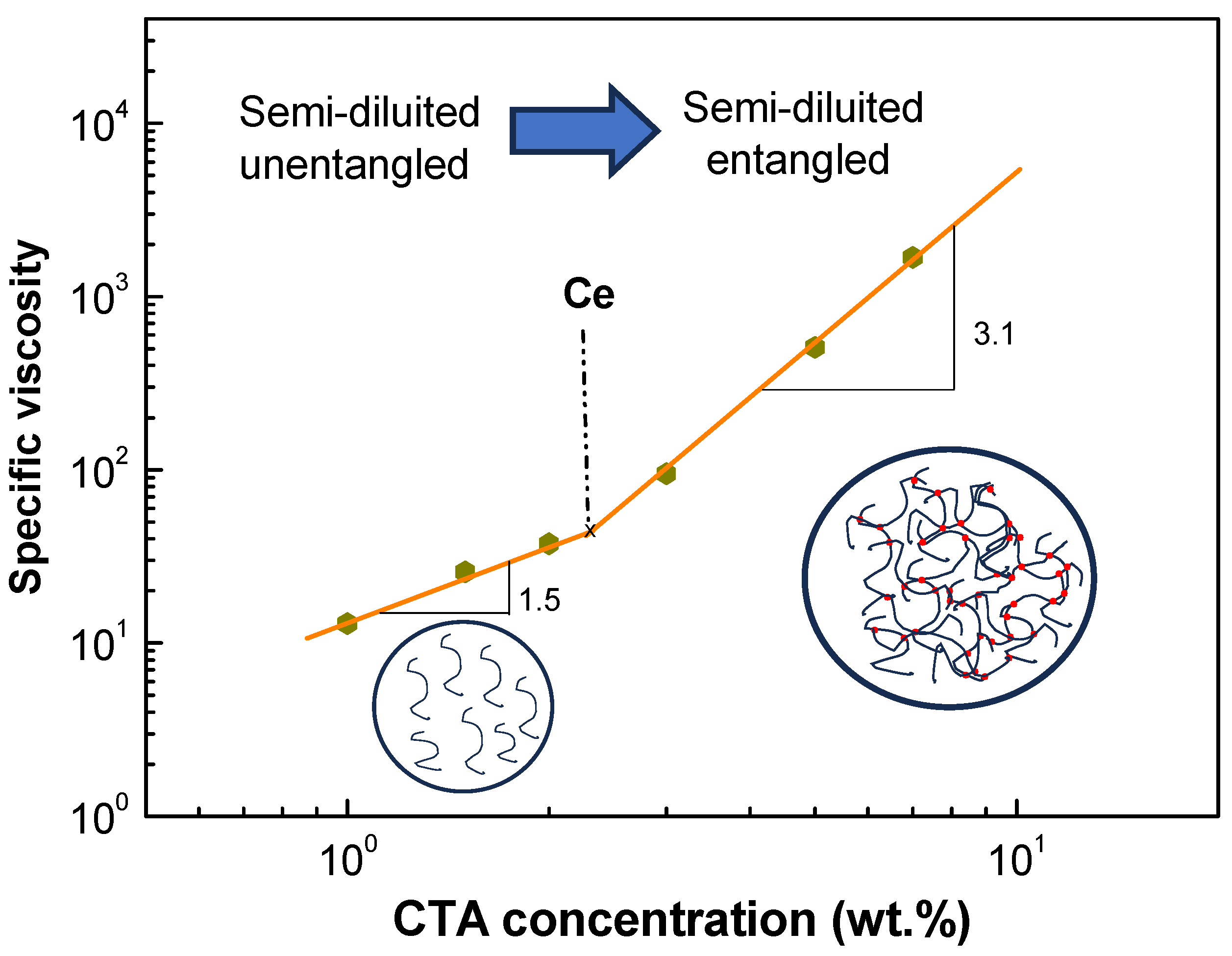
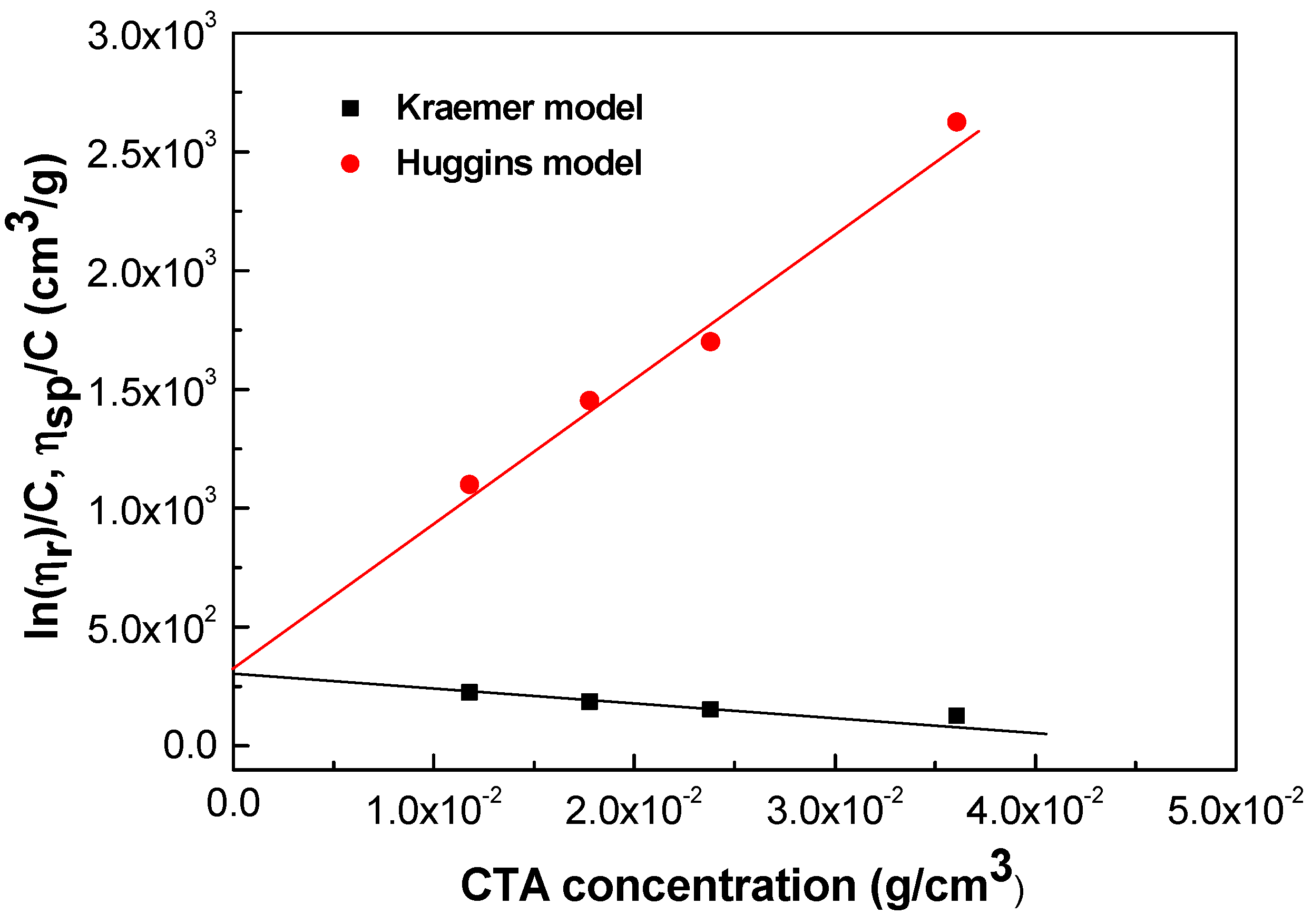
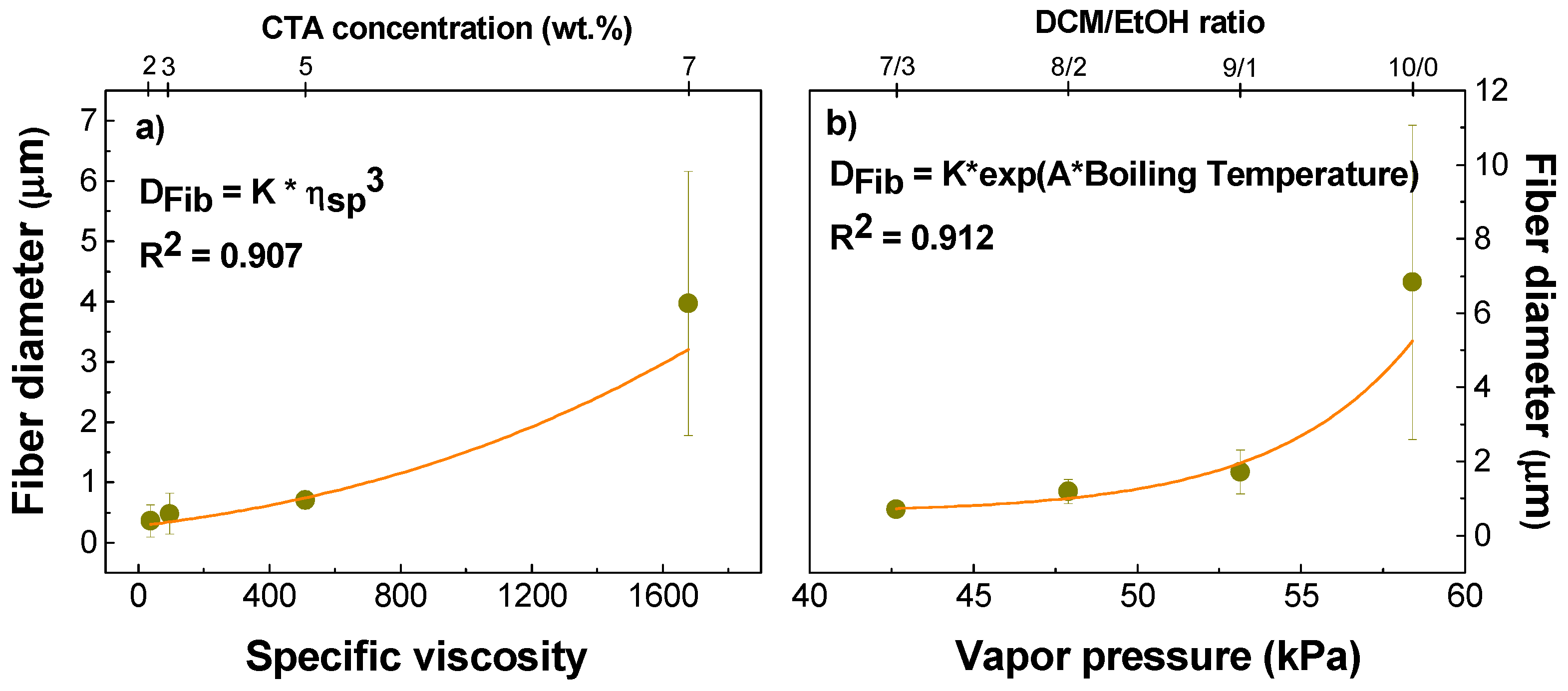
2.1. Physico-Chemical Properties of Cellulose Triacetate Solutions
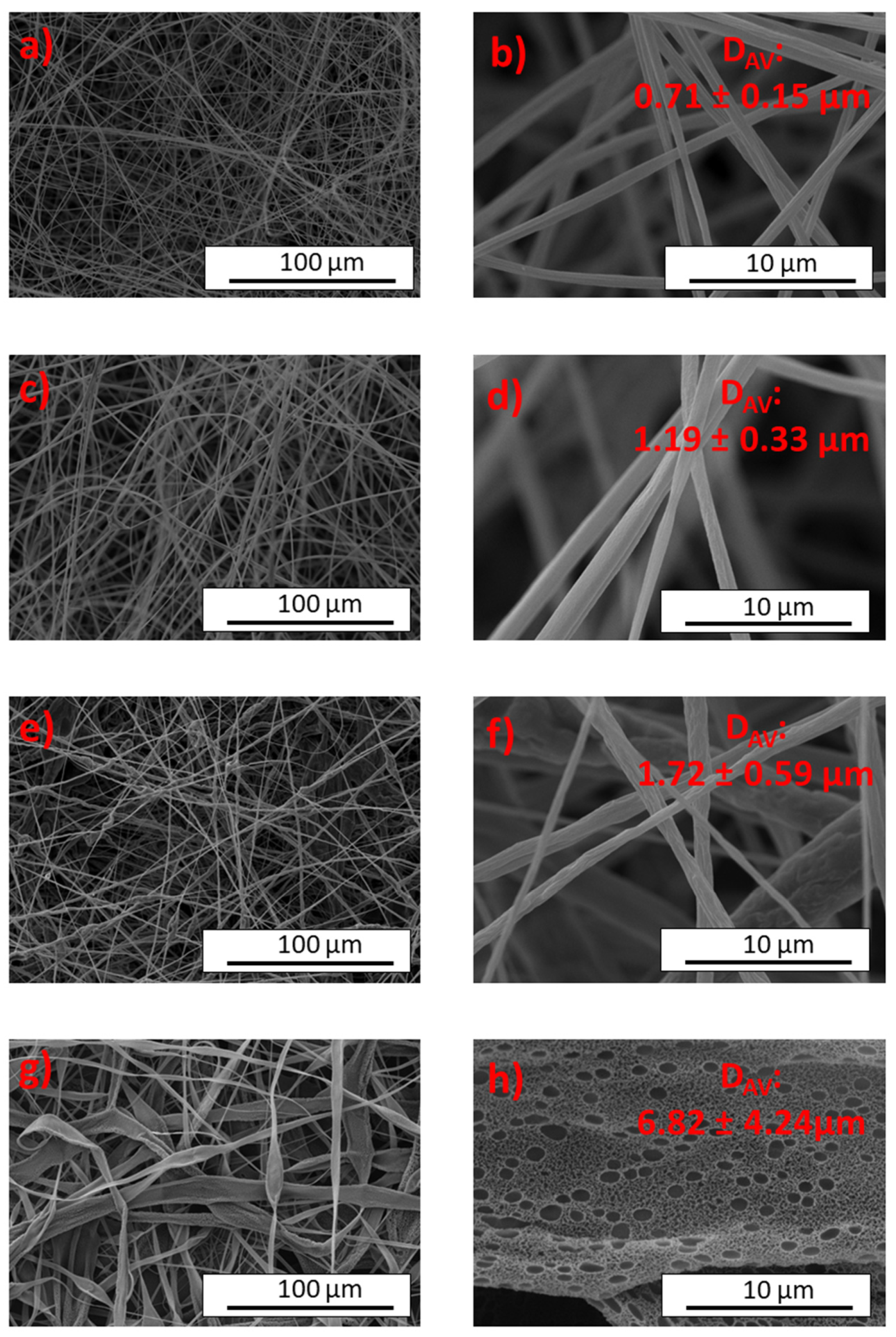
2.2. Characterization of Cellulose Triacetate Electrospun Nanostructures
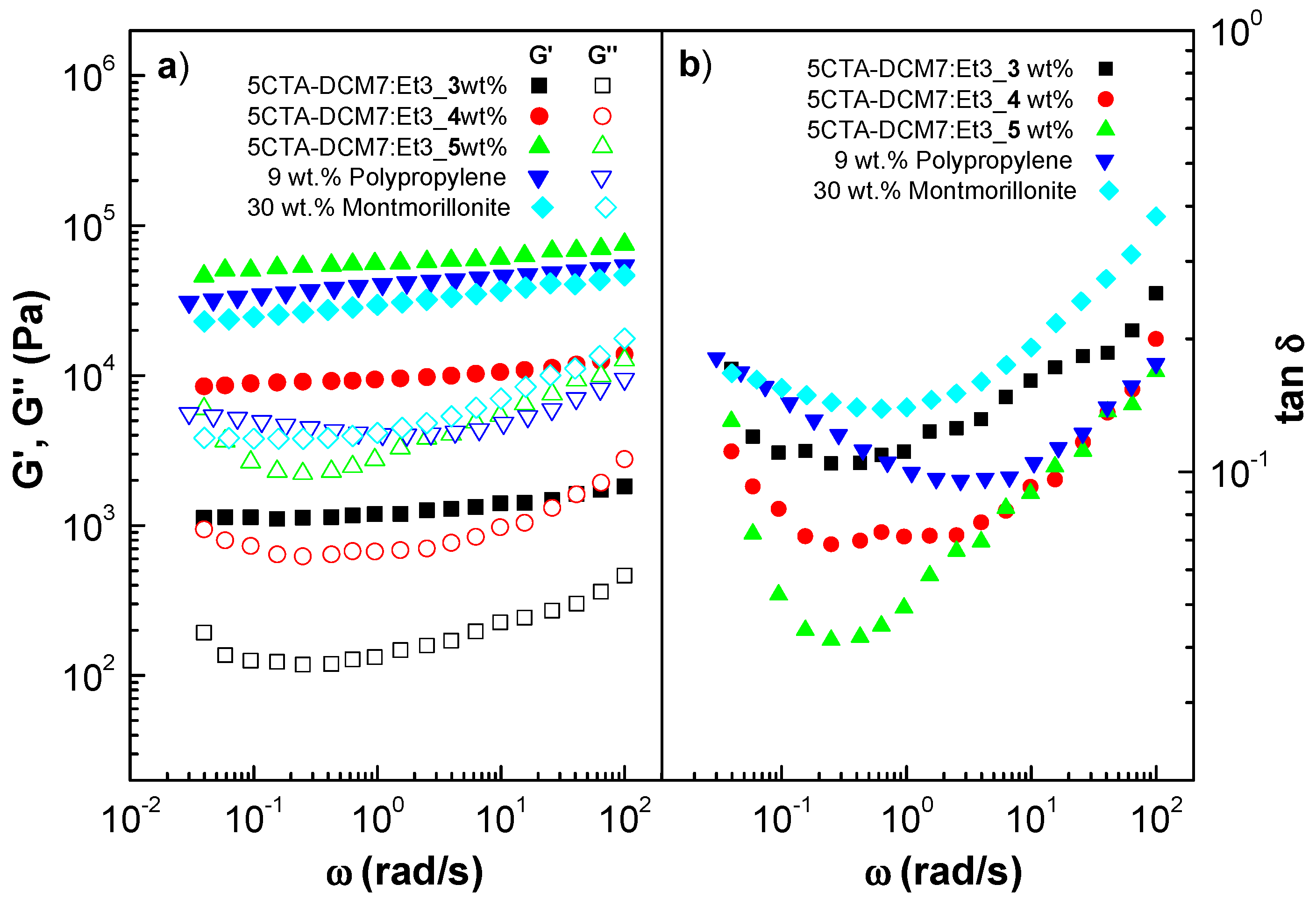
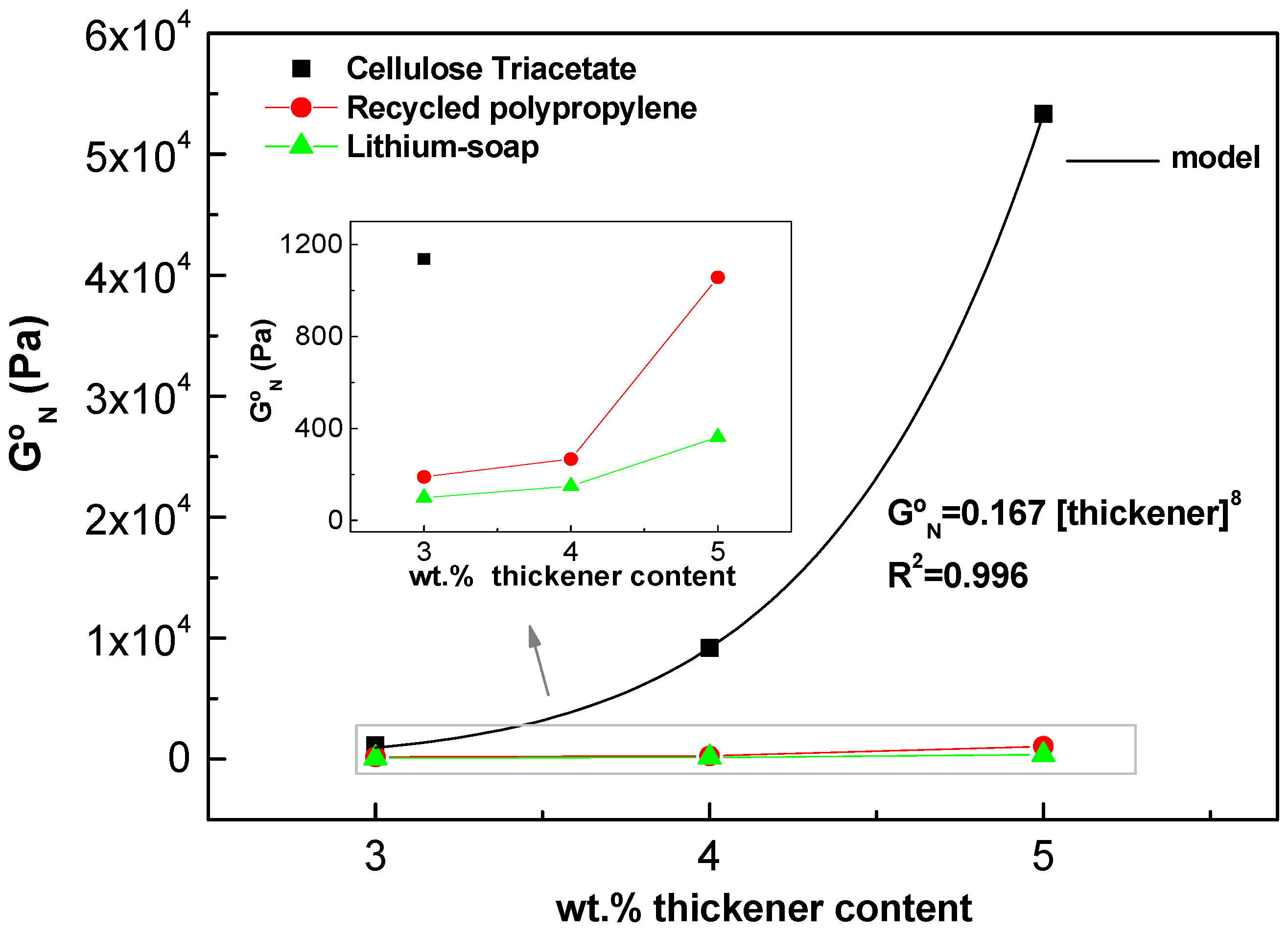
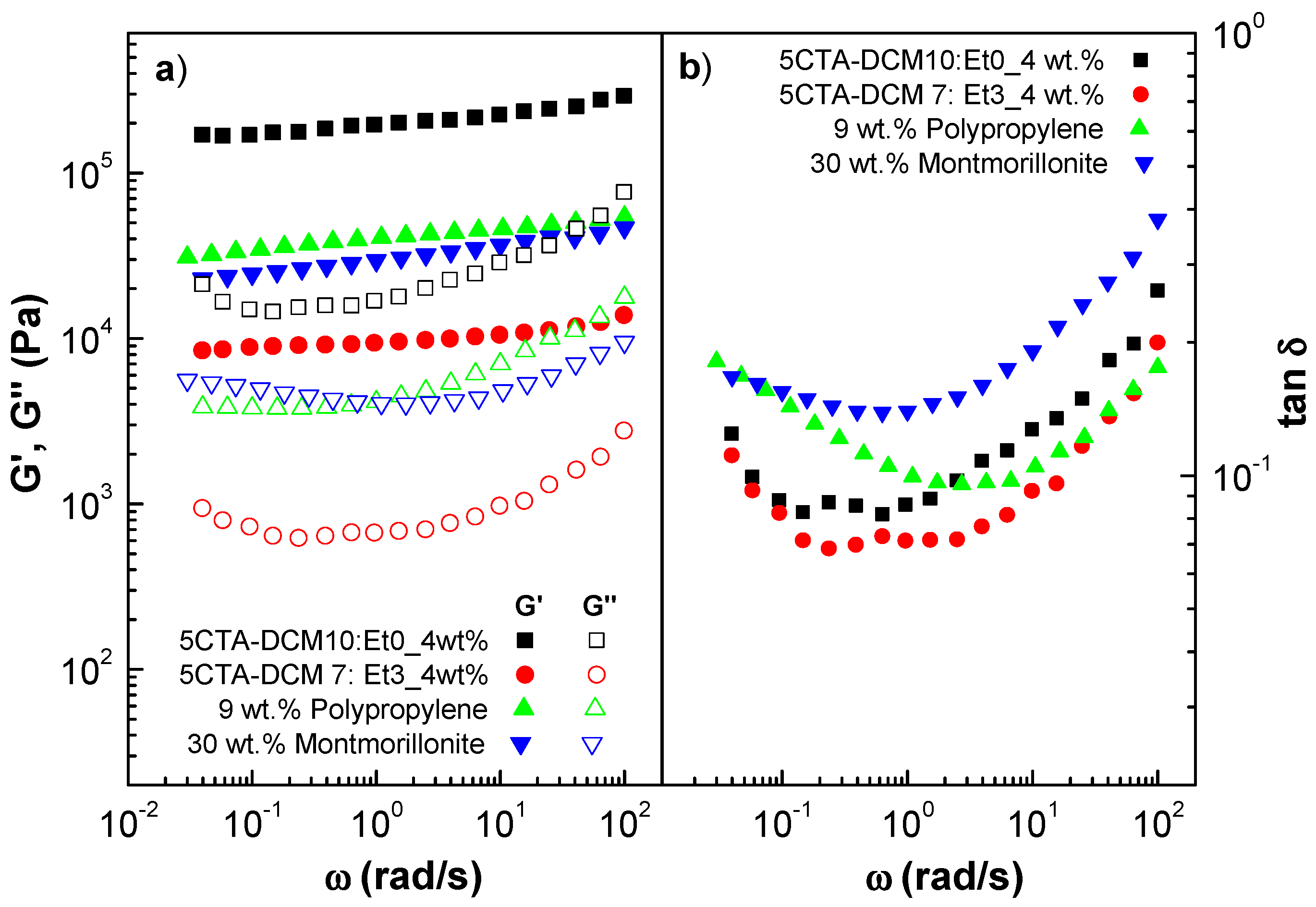
2.3. Ability of Cellulose Triacetate Nanostructures to form Gel-like Dispersions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of CTA Solutions
4.3. Electrospinning Process and Characterization of CTA Electrospun Fibers
4.4. Gel-like Dispersions Preparation
4.5. Rheological Characterization of Gel-like Dispersions
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Li, C.; Liang, H.; Chen, J.; Yu, S. Ultralight, Flexible, and Fire-Resistant Carbon Nanofiber Aerogels from Bacterial Cellulose. Angew. Chemie Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A Review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Production of a Novel Poly(Ɛ-caprolactone)-methylcellulose Electrospun Wound Dressing by Incorporating Bioactive Glass and Manuka Honey. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wang, F.; Lan, T.; Zhang, Y.; Shao, Z. Convenient Fabrication of Carboxymethyl Cellulose Electrospun Nanofibers Functionalized with Silver Nanoparticles. Cellulose 2016, 23, 1899–1909. [Google Scholar] [CrossRef]
- Gouda, M.; Abu-Abdeen, M. Highly Conductive Cellulosic Nanofibers for Efficient Water Desalination. Fibers Polym. 2017, 18, 2111–2117. [Google Scholar] [CrossRef]
- Naseem, S.; Wu, C.-M.; Xu, T.-Z.; Lai, C.-C.; Rwei, S.-P. Oil-Water Separation of Electrospun Cellulose Triacetate Nanofiber Membranes Modified by Electrophoretically Deposited TiO2/Graphene Oxide. Polymers 2018, 10, 746. [Google Scholar] [CrossRef]
- Wu, C.M.; Danh, K.S.; Nakagaito, A.N. Effects of Cellulose Nanofiber on the Thermal, Mechanical, and Optical Properties of Triacetate Cellulose Nanocomposites. Express Polym. Lett. 2020, 14, 467–476. [Google Scholar] [CrossRef]
- Motora, K.G.; Wu, C.-M.; Xu, T.-Z.; Chala, T.F.; Lai, C.-C. Photocatalytic, Antibacterial, and Deodorization Activity of Recycled Triacetate Cellulose Nanocomposites. Mater. Chem. Phys. 2020, 240, 122260. [Google Scholar] [CrossRef]
- Rubio-Valle, J.F.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Electrospun Nanofibres with Antimicrobial Activities. In Antimicrobial Textiles from Natural Resources; Elsevier: Amsterdam, The Netherlands, 2021; pp. 589–618. [Google Scholar]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J.M. The Influence of Electrospinning Parameters and Solvent Selection on the Morphology and Diameter of Polyimide Nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Rubio-Valle, J.F.; Sánchez, M.C.; Valencia, C.; Martín-Alfonso, J.E.; Franco, J.M. Electrohydrodynamic Processing of PVP-Doped Kraft Lignin Micro- and Nano-Structures and Application of Electrospun Nanofiber Templates to Produce Oleogels. Polymers 2021, 13, 2206. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mussa Farkhani, S. Electrospinning and Electrospun Nanofibres. IET Nanobiotechnology 2014, 8, 83–92. [Google Scholar] [CrossRef]
- Han, S.O.; Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Ultrafine Porous Fibers Electrospun from Cellulose Triacetate. Mater. Lett. 2005, 59, 2998–3001. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.; Wang, W.; Wang, F.; Zhang, D.; Wang, J.; Liu, Y.; Kong, L. Ultrafine Cellulose Triacetate Mats Electrospun by Using Co-solvent of DMSO/Chloroform System. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible Oleogels: An Opportunity for Fat Replacement in Foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The Physiological Functions and Pharmaceutical Applications of Inulin: A Review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Franco, J.M. Ethylene-Vinyl Acetate Copolymer (EVA)/Sunflower Vegetable Oil Polymer Gels: Influence of Vinyl Acetate Content. Polym. Test. 2014, 37, 78–85. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Franco, J.M. Influence of Polymer Reprocessing Cycles on the Microstructure and Rheological Behavior of Polypropylene/Mineral Oil Oleogels. Polym. Test. 2015, 45, 12–19. [Google Scholar] [CrossRef]
- Patel, A.R. A Colloidal Gel Perspective for Understanding Oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Suzuki, M.; Hanabusa, K. Polymer Organogelators That Make Supramolecular Organogels through Physical Cross-Linking and Self-Assembly. Chem. Soc. Rev. 2010, 39, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alfonso, M.A.; Martín-Alfonso, J.E.; Rubio-Valle, J.F.; Hinestroza, J.P.; Franco, J.M. Tunable Architectures of Electrospun Cellulose Acetate Phthalate Applied as Thickeners in Green Semisolid Lubricants. Appl. Mater. Today 2024, 36, 102030. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Martín-Alfonso, J.E.; Franco, J.M. Oleo-Dispersions of Electrospun Cellulose Acetate Butyrate Nanostructures: Toward Renewable Semisolid Lubricants. Adv. Sustain. Syst. 2024. [Google Scholar] [CrossRef]
- Borrego, M.; Martín-Alfonso, J.E.; Valencia, C.; Sánchez Carrillo, M. del C.; Franco, J.M. Developing Electrospun Ethylcellulose Nanofibrous Webs: An Alternative Approach for Structuring Castor Oil. ACS Appl. Polym. Mater. 2022, 4, 7217–7227. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.; Klee, D.; Möller, M. Electrospinning of Polymer Melts: Phenomenological Observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Nie, H.; He, A.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Pérez-Puyana, V.; Romero, A. Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning. Polymers 2022, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Valle, J.F.; Sánchez, M.C.; Valencia, C.; Martín-Alfonso, J.E.; Franco, J.M. Production of Lignin/Cellulose Acetate Fiber-Bead Structures by Electrospinning and Exploration of Their Potential as Green Structuring Agents for Vegetable Lubricating Oils. Ind. Crops Prod. 2022, 188, 115579. [Google Scholar] [CrossRef]
- Zoccola, M.; Montarsolo, A.; Aluigi, A.; Varesano, A.; Vineis, C.; Tonin, C. Electrospinning of Polyamide 6/Modified-Keratin Blends. e-Polymers 2007, 7, 105. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Liu, K.; Wang, W.; Peng, W.; Ma, H.; Wang, Q.; Shi, X.; Sun, H.; Duan, X. Electrospun Fe3O4-Chitosan/Polyvinyl Alcohol Nanofibrous Film for Improved Capture and Elimination of Foodborne Pathogens. Int. J. Biol. Macromol. 2023, 253, 126692. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, J.; Dong, Y.; Sun, F. Investigation of the Rheological Response of a Bio-Liquefied Formaldehyde Resin-Based Precursor for Electrospinning. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 661, 130950. [Google Scholar] [CrossRef]
- Han, Y.; Shi, C.; Cui, F.; Chen, Q.; Tao, Y.; Li, Y. Solution Properties and Electrospinning of Polyacrylamide and ε-Polylysine Complexes. Polymer 2020, 204, 122806. [Google Scholar] [CrossRef]
- Kol, R.; Nachtergaele, P.; De Somer, T.; D’hooge, D.R.; Achilias, D.S.; De Meester, S. Toward More Universal Prediction of Polymer Solution Viscosity for Solvent-Based Recycling. Ind. Eng. Chem. Res. 2022, 61, 10999–11011. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Zhang, S.; Xu, G.; Fu, K.; Lee, H.; Zhang, X. Parameter Study and Characterization for Polyacrylonitrile Nanofibers Fabricated via Centrifugal Spinning Process. Eur. Polym. J. 2013, 49, 3834–3845. [Google Scholar] [CrossRef]
- Rogalski, J.; Bastiaansen, C.; Peijs, T. PA6 Nanofibre Production: A Comparison between Rotary Jet Spinning and Electrospinning. Fibers 2018, 6, 37. [Google Scholar] [CrossRef]
- Zhang, E.; Dai, X.; Dong, Z.; Qiu, X.; Ji, X. Critical Concentration and Scaling Exponents of One Soluble Polyimide—From Dilute to Semidilute Entangled Solutions. Polymer 2016, 84, 275–285. [Google Scholar] [CrossRef]
- Colby, R.H. Structure and Linear Viscoelasticity of Flexible Polymer Solutions: Comparison of Polyelectrolyte and Neutral Polymer Solutions. Rheol. Acta 2010, 49, 425–442. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Role of Molecular Entanglements in Starch Fiber Formation by Electrospinning. Biomacromolecules 2012, 13, 2247–2253. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of Chain Entanglements on Fiber Formation during Electrospinning of Polymer Solutions: Good Solvent, Non-Specific Polymer–Polymer Interaction Limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Johannessen, M.; Henriksen, A. Chemistry of Snow Meltwater: Changes in Concentration during Melting. Water Resour. Res. 1978, 14, 615–619. [Google Scholar] [CrossRef]
- Maron, S.H.; Reznik, R.B. A New Method for Determination of Intrinsic Viscosity. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 309–324. [Google Scholar] [CrossRef]
- Pamies, R.; Hernández Cifre, J.G.; del Carmen López Martínez, M.; García de la Torre, J. Determination of Intrinsic Viscosities of Macromolecules and Nanoparticles. Comparison of Single-Point and Dilution Procedures. Colloid Polym. Sci. 2008, 286, 1223–1231. [Google Scholar] [CrossRef]
- Abdel-Azim, A.-A.A.; Atta, A.M.; Farahat, M.S.; Boutros, W.Y. Determination of Intrinsic Viscosity of Polymeric Compounds through a Single Specific Viscosity Measurement. Polymer 1998, 39, 6827–6833. [Google Scholar] [CrossRef]
- Eich, A.; Wolf, B.A. Intrinsic Viscosities of Polyelectrolytes: Determination and Modeling of the Effects of Extra Salt. ChemPhysChem 2011, 12, 2786–2790. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, A.V.; Sayko, R.; Colby, R.H. Viscosity of Polymer Solutions and Molecular Weight Characterization. ACS Macro Lett. 2023, 12, 773–779. [Google Scholar] [CrossRef]
- Wagner, H.L. The Mark–Houwink–Sakurada Equation for the Viscosity of Linear Polyethylene. J. Phys. Chem. Ref. Data 1985, 14, 611–617. [Google Scholar] [CrossRef]
- Kamide, K.; Miyazaki, Y.; Abe, T. Dilute Solution Properties and Unperturbed Chain Dimension of Cellulose Triacetate. Polym. J. 1979, 11, 523–538. [Google Scholar] [CrossRef]
- Graessley, W. Polymer Chain Dimensions and the Dependence of Viscoelastic Properties on Concentration, Molecular Weight and Solvent Power. Polymer 1980, 21, 258–262. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.; Liu, X.; Sutti, A.; Ke, Q.; Mo, X.; Wang, X.; Morsi, Y.; Lin, T. Electrospinning of Nanofibres with Parallel Line Surface Texture for Improvement of Nerve Cell Growth. Soft Matter 2011, 7, 10812. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium Alginate Solutions: Correlation between Rheological Properties and Spinnability. J. Mater. Sci. 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Borrego, M.; Martín-Alfonso, J.E.; Sánchez, M.C.; Valencia, C.; Franco, J.M. Electrospun Lignin-PVP Nanofibers and Their Ability for Structuring Oil. Int. J. Biol. Macromol. 2021, 180, 212–221. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Tanvir, A.; Ting, V.P.; Eichhorn, S.J. Nanoporous Electrospun Cellulose Acetate Butyrate Nanofibres for Oil Sorption. Mater. Lett. 2020, 261, 127116. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat Polymer Ribbons and Other Shapes by Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol Loaded Electrospun Fibrous Films from Zein and Poly(Lactic Acid) for Active Food Packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun Porous Cellulose Acetate Fibers from Volatile Solvent Mixture. Mater. Lett. 2011, 65, 2291–2294. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Martín-Alfonso, M.J.; Valencia, C.; Cuberes, M.T. Rheological and Tribological Approaches as a Tool for the Development of Sustainable Lubricating Greases Based on Nano-Montmorillonite and Castor Oil. Friction 2021, 9, 415–428. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Franco, J.M.; Valencia, C.; Gallegos, C.; Urquiola, F.; Urchegui, R. Atomic Force Microscopy and Thermo-Rheological Characterisation of Lubricating Greases. Tribol. Lett. 2011, 41, 463–470. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C.; Arteaga, J.F.; Díaz, M.J.; Franco, J.M. Design of Lubricating Grease Formulations Using Recycled Polypropylene from Postconsumer Films as Thickener Agent. J. Appl. Polym. Sci. 2013, 127, 1369–1376. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Moreno, G.; Valencia, C.; Sánchez, M.C.; Franco, J.M.; Gallegos, C. Influence of Soap/Polymer Concentration Ratio on the Rheological Properties of Lithium Lubricating Greases Modified with Virgin LDPE. J. Ind. Eng. Chem. 2009, 15, 687–693. [Google Scholar] [CrossRef]
- Wu, S. Chain Structure and Entanglement. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 723–741. [Google Scholar] [CrossRef]
- Larson, R.G.; Sridhar, T.; Leal, L.G.; McKinley, G.H.; Likhtman, A.E.; McLeish, T.C.B. Definitions of Entanglement Spacing and Time Constants in the Tube Model. J. Rheol. (N. Y. N. Y). 2003, 47, 809–818. [Google Scholar] [CrossRef]
- Gomez-Hermoso-de-Mendoza, J.; Kortaberria, G.; Gutierrez, J.; Tercjak, A. Competition between Polycrystalline Morphology and Microphase Separation in Blends Based on Cellulose Triacetate. Polym. Degrad. Stab. 2022, 204, 110093. [Google Scholar] [CrossRef]
- Gomez-Hermoso-de-Mendoza, J.; Gutierrez, J.; Tercjak, A. Comparative Study of Nano and Macro Mechanical Properties of Cellulose Triacetate Based Nanocomposites by Mean of Quantitative Nanomechanical Mapping and Mechanical Testing. Compos. Sci. Technol. 2021, 211, 108851. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor Oil as a Potential Renewable Resource for the Production of Functional Materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E. Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels. Gels 2022, 8, 504. [Google Scholar] [CrossRef] [PubMed]



| Spinning Solutions | η (Pa∙s) | η0 (Pa∙s) | η∞ (Pa∙s) | c (1/s) | p (-) |
|---|---|---|---|---|---|
| 1 wt.% CTA | 0.0072 | - | - | - | - |
| 1.5 wt.% CTA | 0.0141 | - | - | - | - |
| 2 wt.% CTA | - | 0.031 | 0.001 | 0.021 | 0.072 |
| 3 wt.% CTA | - | 0.055 | 0.004 | 7.682 | 0.132 |
| 5 wt.% CTA | - | 0.265 | 0.036 | 9.482 | 0.539 |
| 7 wt.% CTA | - | 0.862 | 0.065 | 11.871 | 0.716 |
| Property | Castor Oil |
|---|---|
| Dynamic viscosity at 40 °C (mPa s) | 230.0 |
| Density at 15 °C (g/cm3) | 0.9630 |
| Myristic 1 14:0 2 | - |
| Palmitic 16:0 | 1.70 |
| Stearic 18:0 | 1.96 |
| Oleic 18:1 | 5.34 |
| Ricinoleic (18:1 -OH) | 82.48 |
| Linoleic 18:2 | 7.01 |
| Linoleic 18:3 | 1.51 |
| Saturated (SFAs) | 3.66 |
| Monounsaturated (MUFAs) | 87.82 |
| Polyunsaturated (PUFAs) | 8.52 |
| Unsaturated/saturated ratio | 26.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Estrada-Villegas, G.M.; Sánchez-Domínguez, M.; Martín-Alfonso, J.E. Exploring Cellulose Triacetate Nanofibers as Sustainable Structuring Agent for Castor Oil: Formulation Design and Rheological Insights. Gels 2024, 10, 221. https://doi.org/10.3390/gels10040221
Martín-Alfonso MA, Rubio-Valle JF, Estrada-Villegas GM, Sánchez-Domínguez M, Martín-Alfonso JE. Exploring Cellulose Triacetate Nanofibers as Sustainable Structuring Agent for Castor Oil: Formulation Design and Rheological Insights. Gels. 2024; 10(4):221. https://doi.org/10.3390/gels10040221
Chicago/Turabian StyleMartín-Alfonso, M. A., José F. Rubio-Valle, Gethzemani M. Estrada-Villegas, Margarita Sánchez-Domínguez, and José E. Martín-Alfonso. 2024. "Exploring Cellulose Triacetate Nanofibers as Sustainable Structuring Agent for Castor Oil: Formulation Design and Rheological Insights" Gels 10, no. 4: 221. https://doi.org/10.3390/gels10040221