Upcycling Low-Quality Cotton Fibers into Mulch Gel Films in a Fast Closed Carbon Cycle
Abstract
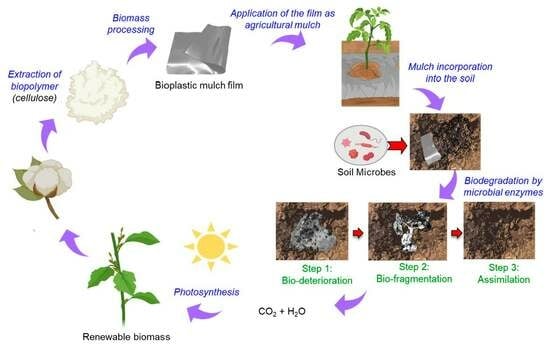
:1. Introduction
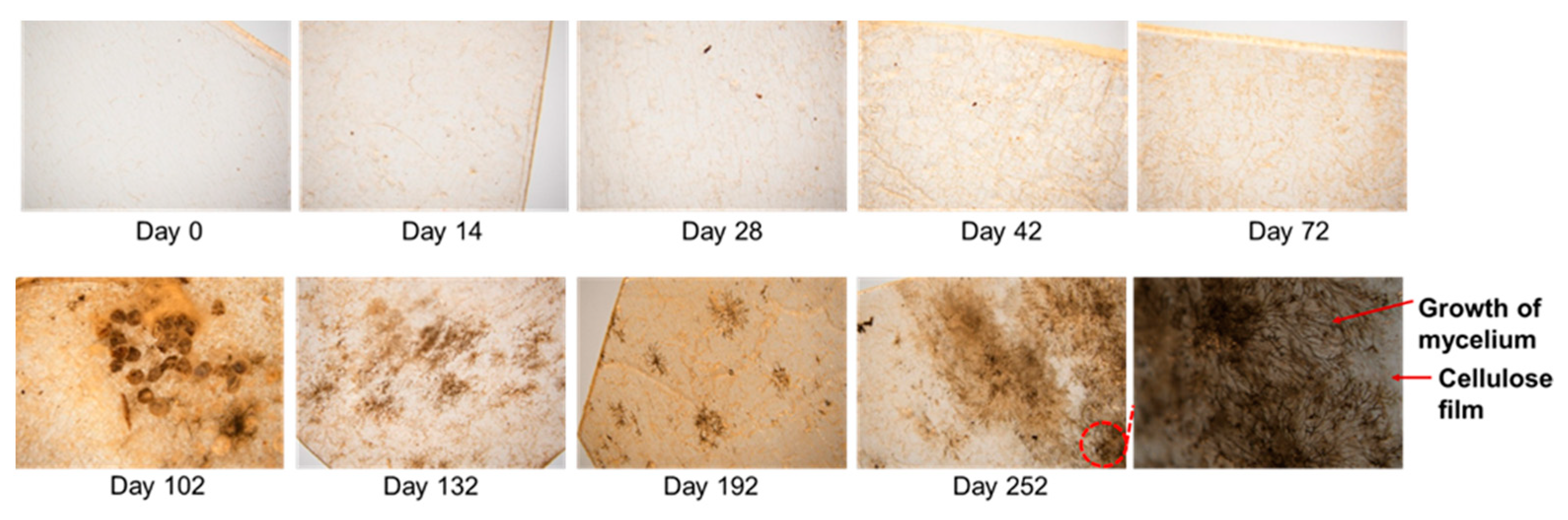
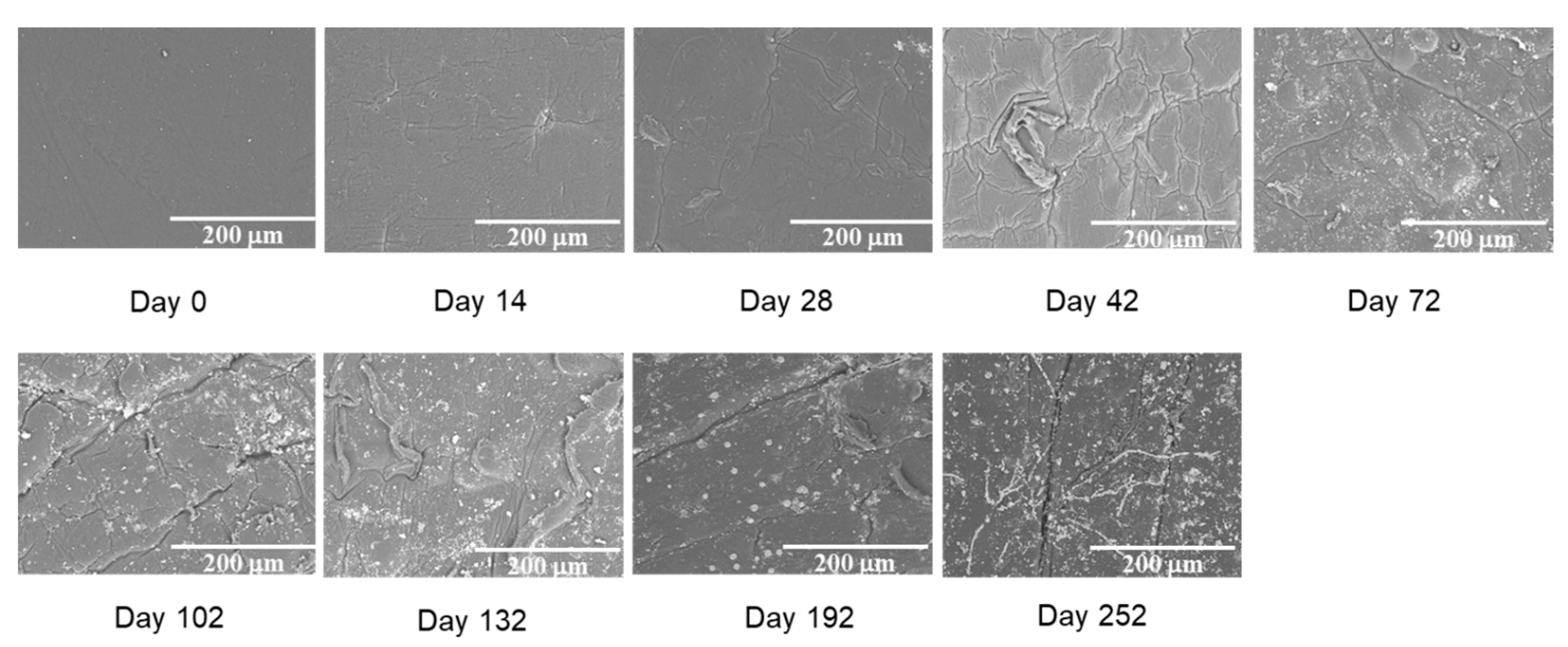
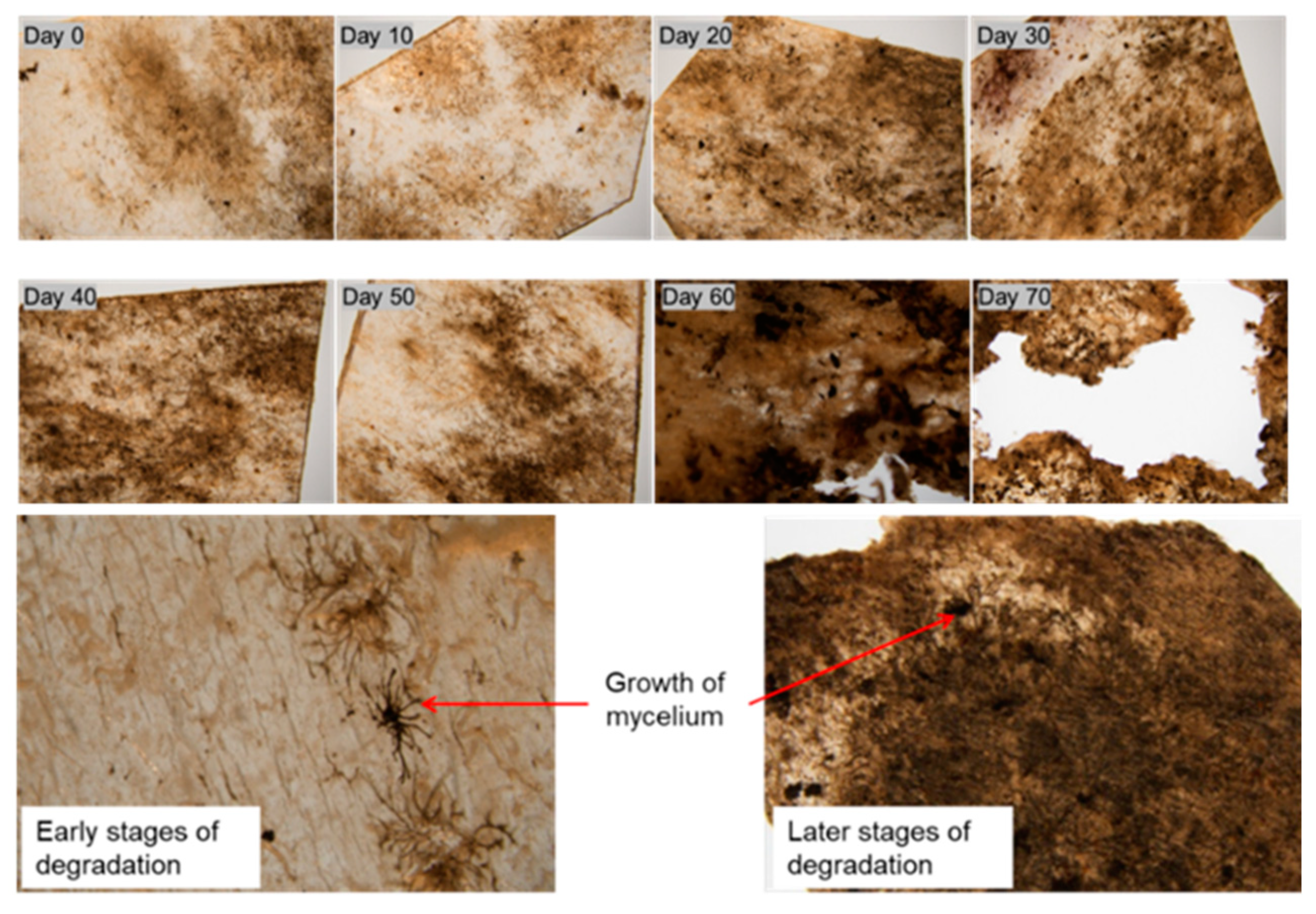
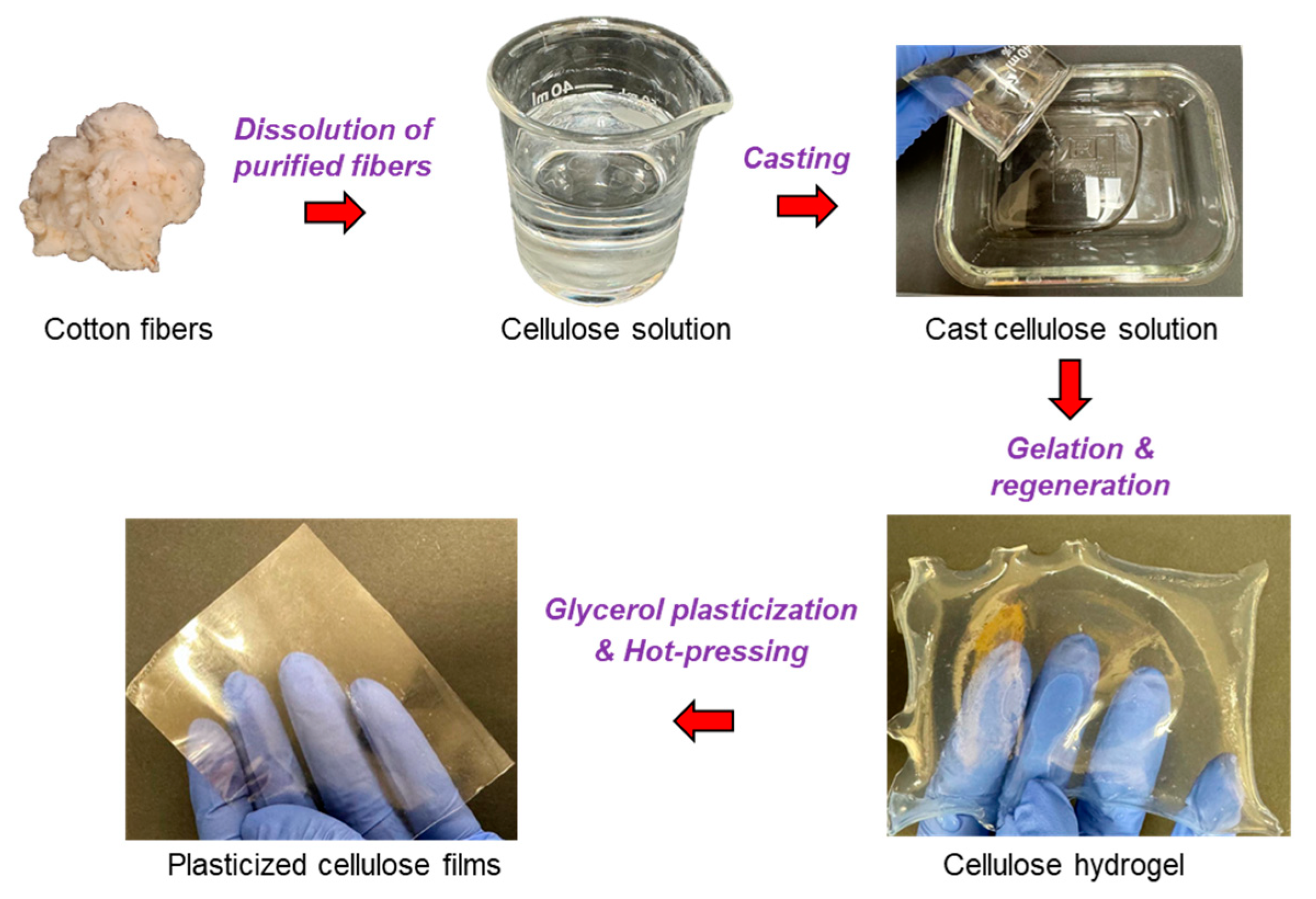
2. Results and Discussion
3. Conclusions
Further Directions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Film Preparation
4.2.2. Soil Cover Experimental Setup
4.3. Characterizations
4.3.1. Weight Change during the Soil Cover Period
4.3.2. Surface Morphology
4.3.3. Thermogravimetric Analysis (TGA)
4.3.4. Fourier Transform Infrared (FTIR) Tests
4.3.5. Tensile Testing
4.3.6. X-Ray Diffraction (XRD) Analysis
4.3.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soley, G. Cotton: World Markets and Trade; U.S. Department of Agriculture: Washington, DC, USA, 2023.
- Zhang, T.; Zhai, Y.; Ma, X.; Shen, X.; Bai, Y.; Zhang, R.; Ji, C.; Hong, J. Towards environmental sustainability: Life cycle assessment-based water footprint analysis on China’s cotton production. J. Clean. Prod. 2021, 313, 127925. [Google Scholar] [CrossRef]
- Kamble, Z.; Behera, B.K. Upcycling textile wastes: Challenges and innovations. Text. Prog. 2021, 53, 65–122. [Google Scholar] [CrossRef]
- Zhang, Z.; Rumi, S.S.; Lucia, L.A.; Abidi, N. Waste treat waste: Alginate calcium versus alginate acid gels in upcycling waste cotton linter as composite biosorbent. Ind. Crops Prod. 2023, 205, 117512. [Google Scholar] [CrossRef]
- Zhang, Z.; Rumi, S.S.; Lucia, L.A.; Abidi, N. Transforming low-quality cotton fibers into dye adsorbents. Environ. Chem. Lett. 2024, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Su, J.; Liu, T.; Ge, S.; Liew, R.K.; Zhang, H.; Naushad, M.; Lam, S.S.; Ng, H.S.; Sonne, C. A sustainable strategy to transform cotton waste into renewable cellulose fiber self-reinforcing composite paper. J. Clean. Prod. 2023, 429, 139567. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Q.; Rong, K.; Shi, Y.; Peng, W.; Li, H.; Guo, Z.; Xu, B.B.; Hou, H.; Algadi, H. MXene enhanced 3D needled waste denim felt for high-performance flexible supercapacitors. Nano-Micro Lett. 2024, 16, 36. [Google Scholar] [CrossRef]
- Zhou, C.; Girouard, F.; O’Brien, B.; Ronholm, J.; Wang, Y. Construction of chevaux-de-frise from cellulose nanocrystals to enable mechano-bactericidal activity on recycled waste cotton films. Green Chem. 2022, 24, 1109–1113. [Google Scholar] [CrossRef]
- Tan, Q.; Yang, L.; Wei, F.; Chen, Y.; Li, J. Comparative life cycle assessment of polyethylene agricultural mulching film and alternative options including different end-of-life routes. Renew. Sustain. Energy Rev. 2023, 178, 113239. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Liquet y González, J.E.; Henderson, K.B.; Anunciado, M.B.; Hayes, D.G.; DeBruyn, J.M. Soil microbial communities associated with biodegradable plastic mulch films. Front. Microbiol. 2020, 11, 587074. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is biodegradable plastic mulch the solution to agriculture’s plastic problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, Y.; Li, J.; Chen, Y.; Li, H. Emission characteristics of PBDEs during flame-retardant plastics extruding process: Field investigation and laboratorial simulation. Environ. Sci. Pollut. Res. 2017, 24, 22450–22457. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N. Analysis of the time course of degradation for fully biobased nonwoven agricultural mulches in compost-enriched soil. Text. Res. J. 2016, 86, 1343–1355. [Google Scholar] [CrossRef]
- Li, C.; Moore-Kucera, J.; Miles, C.; Leonas, K.; Lee, J.; Corbin, A.; Inglis, D. Degradation of potentially biodegradable plastic mulch films at three diverse US locations. Agroecol. Sustain. Food Syst. 2014, 38, 861–889. [Google Scholar] [CrossRef]
- Hayes, D.G.; Dharmalingam, S.; Wadsworth, L.C.; Leonas, K.K.; Miles, C.; Inglis, D.A. Biodegradable agricultural mulches derived from biopolymers. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; ACS: Washington, DC, USA, 2012; pp. 201–223. [Google Scholar]
- Ghimire, S.; Flury, M.; Scheenstra, E.J.; Miles, C.A. Sampling and degradation of biodegradable plastic and paper mulches in field after tillage incorporation. Sci. Total Environ. 2020, 703, 135577. [Google Scholar] [CrossRef] [PubMed]
- Griffin-LaHue, D.; Ghimire, S.; Yu, Y.; Scheenstra, E.J.; Miles, C.A.; Flury, M. In-field degradation of soil-biodegradable plastic mulch films in a Mediterranean climate. Sci. Total Environ. 2022, 806, 150238. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Tu, Q.; Wang, P.; Zeng, J.; Li, J.; Wang, B.; Xu, J.; Chen, K.; Zhang, Z.; Abidi, N. Conductive Polymer/Nanocellulose Composites as a Functional Platform for Electronic Devices: A Mini-Review. Polym. Rev. 2023, 64, 162–191. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, D.; Sun, J.; Zhou, Z.; Jia, Y.; Pang, B.; Ma, Z.; Du, X. Effects of fiber wax and cellulose content on colored cotton fiber quality. Euphytica 2010, 173, 141–149. [Google Scholar] [CrossRef]
- Rumi, S.S.; Liyanage, S.; Abidi, N. Conversion of low-quality cotton to bioplastics. Cellulose 2021, 28, 2021–2038. [Google Scholar] [CrossRef]
- Erdal, N.B.; Hakkarainen, M. Degradation of cellulose derivatives in laboratory, man-made, and natural environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, C.; Funakawa, S.; Fujii, K.; Kadono, A.; Kosaki, T. Effects of climatic and soil properties on cellulose decomposition rates in temperate and tropical forests. Biol. Fertil. Soils 2014, 50, 633–643. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Ductile nanocellulose-based films with high stretchability and tear resistance. Eur. Polym. J. 2015, 69, 328–340. [Google Scholar] [CrossRef]
- Towey, J.; Dougan, L. Structural examination of the impact of glycerol on water structure. J. Phys. Chem. B 2012, 116, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, S.; Lee, B.-K. Microfibrillated cellulose film with enhanced mechanical and water-resistant properties by glycerol and hot-pressing treatment. Cellulose 2021, 28, 5693–5705. [Google Scholar] [CrossRef]
- Seale, T. Life Cycle of Neurospora crassa Viewed by Scanning Electron Microscopy. J. Bacteriol. 1973, 113, 1015–1025. [Google Scholar] [CrossRef]
- Carlile, M. The success of the hypha and mycelium. In The Growing Fungus; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–19. [Google Scholar]
- Raridon, R.J.; Kraus, K.A. Properties of organic-water mixtures. IV. The effect of various salts on the miscibility gap of the glycerol triacetate-water system. J. Colloid Sci. 1965, 20, 1000–1013. [Google Scholar] [CrossRef]
- Primc, G.; Tomšič, B.; Vesel, A.; Mozetič, M.; Ražić, S.E.; Gorjanc, M. Biodegradability of oxygen-plasma treated cellulose textile functionalized with ZnO nanoparticles as antibacterial treatment. J. Phys. D Appl. Phys. 2016, 49, 324002. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.-C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L.A.; Yu, S. A “bird nest” bioinspired strategy deployed for inducing cellulose gelation without concomitant dissolution. Adv. Compos. Hybrid Mater. 2023, 6, 178. [Google Scholar] [CrossRef]
- Hamou, K.B.; Kaddami, H.; Elisabete, F.; Erchiqui, F. Synergistic association of wood/hemp fibers reinforcements on mechanical, physical and thermal properties of polypropylene-based hybrid composites. Ind. Crops Prod. 2023, 192, 116052. [Google Scholar] [CrossRef]
- Radu, E.-R.; Panaitescu, D.M.; Nicolae, C.-A.; Gabor, R.A.; Rădiţoiu, V.; Stoian, S.; Alexandrescu, E.; Fierăscu, R.; Chiulan, I. The soil biodegradability of structured composites based on cellulose cardboard and blends of polylactic acid and polyhydroxybutyrate. J. Polym. Environ. 2021, 29, 2310–2320. [Google Scholar] [CrossRef]
- Mostafa, H.M.; Sourell, H.; Bockisch, F. Mechanical properties of some bioplastics under different soil types used as biodegradable drip tubes. Agric. Eng. Int. CIGR J. 2010, 12, 12–21. [Google Scholar]
- Bilck, A.P.; Grossmann, M.V.; Yamashita, F. Biodegradable mulch films for strawberry production. Polym. Test. 2010, 29, 471–476. [Google Scholar] [CrossRef]
- Mtibe, A.; Linganiso, L.Z.; Mathew, A.P.; Oksman, K.; John, M.J.; Anandjiwala, R.D. A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohydr. Polym. 2015, 118, 1–8. [Google Scholar] [CrossRef]
- Park, C.H.; Kang, Y.K.; Im, S.S. Biodegradability of cellulose fabrics. J. Appl. Polym. Sci. 2004, 94, 248–253. [Google Scholar] [CrossRef]
- Shakoor, N.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Abbas, A.; Hussain, M.; Jiang, Y.; Zhou, P.; Li, Y. Interplay of higher plants with lithium pollution: Global trends, meta-analysis, and perspectives. Chemosphere 2023, 310, 136663. [Google Scholar] [CrossRef]
- Kalinowska, M.; Hawrylak-Nowak, B.; Szymańska, M. The influence of two lithium forms on the growth, L-ascorbic acid content and lithium accumulation in lettuce plants. Biol. Trace Elem. Res. 2013, 152, 251–257. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Tang, J.; Busso, C.A.; Jiang, D.; Wang, Y.; Wu, D.; Musa, A.; Miao, R.; Miao, C. Seed burial depth and soil water content affect seedling emergence and growth of Ulmus pumila var. sabulosa in the Horqin Sandy Land. Sustainability 2016, 8, 68. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumi, S.S.; Liyanage, S.; Zhang, Z.; Abidi, N. Upcycling Low-Quality Cotton Fibers into Mulch Gel Films in a Fast Closed Carbon Cycle. Gels 2024, 10, 218. https://doi.org/10.3390/gels10040218
Rumi SS, Liyanage S, Zhang Z, Abidi N. Upcycling Low-Quality Cotton Fibers into Mulch Gel Films in a Fast Closed Carbon Cycle. Gels. 2024; 10(4):218. https://doi.org/10.3390/gels10040218
Chicago/Turabian StyleRumi, Shaida S., Sumedha Liyanage, Zhen Zhang, and Noureddine Abidi. 2024. "Upcycling Low-Quality Cotton Fibers into Mulch Gel Films in a Fast Closed Carbon Cycle" Gels 10, no. 4: 218. https://doi.org/10.3390/gels10040218