Role of Physico-Chemical and Cellular Conditions on the Bone Repair Potential of Plastically Compressed Collagen Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Neutralization Conditions
2.2. Influence of Gel Aging Conditions
2.3. Influence of Cell Density
2.4. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials and Cells
4.2. Plastically Compressed Collagen Hydrogel Preparation
4.3. Cell Culture Conditions
4.4. Structural and Chemical Characterizations
4.4.1. Scanning Electron Microscopy (SEM)/Energy-Dispersive X-ray (EDX) Spectroscopy
4.4.2. Rheological Studies
4.4.3. Fourier Transform Infra-Red Spectroscopy (FTIR)
4.5. Biological Studies
4.5.1. Cell Viability
4.5.2. Metabolic Activity
4.5.3. Histological Studies
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure and Transport. Tissue Eng. Part B Rev. 2014, 220, 683–696. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Villanova, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T.; et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef]
- Parisi, C.; Qin, K.; Fernandes, F.M. Colonization versus encapsulation in cell-laden materials design: Porosity and process biocompatibility determine cellularization pathways. Philos. Trans. R. Soc. A 2021, 379, 20200344. [Google Scholar] [CrossRef]
- Shin, S.-H.; Purevdorj, O.; Castano, O.; Planell, J.A.; Kim, H.-W. A short review: Recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 2012, 3, 2041731412443530. [Google Scholar] [CrossRef]
- Blondeau, M.; Coradin, T. Living materials from sol–gel chemistry: Current challenges and perspectives. J. Mater. Chem. 2012, 22, 22335–22343. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef]
- Hélary, C.; Abed, A.; Mosser, G.; Louedec, L.; Meddahi-Pellé, A.; Giraud-Guille, M.M. Synthesis and in vivo integration of improved concentrated collagen hydrogels. J. Tissue Eng. Regen. Med. 2011, 5, 248–252. [Google Scholar] [CrossRef]
- Cross, V.L.; Zheng, Y.; Choi, N.W.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Wiseman, M.; Chuo, C.B.; Cheema, U.; Nazhat, S.N. Ultrarapid Engineering of Biomimetic Materials and Tissues: Fabrication of Nano- and Microstructures by Plastic Compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006, 2, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbuehl, J.; Schiestl, C.; Meuli, C.M.; Reichmann, E. Modified Plastic Compression of Collagen Hydrogels Provides an Ideal Matrix for Clinically Applicable Skin Substitutes. Tissue Eng. C 2012, 18, 464–474. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Marelli, B.; Muja, N.; Nazhat, S.N. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 2012, 8, 1813–1825. [Google Scholar] [CrossRef]
- Griffanti, G.; Rezabeigi, E.; Li, J.; Murshed, M.; Nazhat, S.N. Rapid Biofabrication of Printable Dense Collagen Bioinks of Tunable Properties. Adv. Funct. Mater. 2020, 30, 1903874. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef]
- Chicatun, F.; Pedraza, C.E.; Ghezzi, C.E.; Marelli, B.; Kaartinen, M.T.; McKee, M.D.; Nazhat, S.N. Osteoid-Mimicking Dense Collagen/Chitosan Hybrid Gels. Biomacromolecules 2011, 12, 2946–2956. [Google Scholar] [CrossRef]
- Anandagoda, N.; Ezra, D.G.; Cheema, U.; Bailly, M.; Brown, R.A. Hyaluronan hydration generates three-dimensional meso-scale structure in engineered collagen tissues. J. R. Soc. Interface 2012, 9, 2680–2887. [Google Scholar] [CrossRef]
- Buxton, P.G.; Bitar, M.; Gellynck, K.; Parkar, M.; Brown, R.A.; Young, A.M.; Knowles, J.C.; Nazhat, S.N. Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone 2008, 43, 377–385. [Google Scholar] [CrossRef]
- East, E.; de Oliveira, D.B.; Golding, J.P.; Phillips, J.B. Alignment of Astrocytes Increases Neuronal Growth in Three-Dimensional Collagen Gels and Is Maintained Following Plastic Compression to Form a Spinal Cord Repair Conduit. Tissue Eng. A 2010, 16, 3173–3184. [Google Scholar] [CrossRef]
- Levis, H.J.; Brown, R.A.; Daniels, J.T. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 2010, 31, 7726–7737. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Chicatun, F.; Nazhat, S.N.; Quinn, T.M. Cartilaginous constructs using primary chondrocytes from continuous expansion culture seeded in dense collagen gels. Acta Biomater. 2013, 9, 9360–9369. [Google Scholar] [CrossRef] [PubMed]
- Vardar, E.; Engelhardt, E.M.; Larsson, H.M.; Mouloungui, E.; Pinnagoda, K.; Hubbell, J.A.; Frey, P. Tubular Compressed Collagen Scaffolds for Ureteral Tissue Engineering in a Flow Bioreactor System. Tissue Eng. A 2015, 21, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Griffanti, G.; Nazhat, S.N. Dense fibrillar collagen-based hydrogels as functional osteoid-mimicking scaffolds. Int. Mater. Rev. 2020, 64, 502–521. [Google Scholar] [CrossRef]
- Coyac, B.R.; Chicatun, F.; Hoac, B.; Nelea, V.; Chaussain, C.; Nazhat, S.N.; McKee, M.D. Mineralization of Dense Collagen Hydrogel Scaffolds by Human Pulp Cells. J. Dent. Res. 2013, 92, 648–654. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef]
- Novais, A.; Lesieur, J.; Sadoine, J.; Slimani, L.; Baroukh, B.; Saubamea, B.; Schmitt, A.; Vital, S.; Poliard, A.; Hélary, C.; et al. Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. Stem Cells Transl. Med. 2019, 8, 844–857. [Google Scholar] [CrossRef]
- Maillard, S.; Sicard, L.; Andrique, C.; Torrens, C.; Lesieur, J.; Baroukh, B.; Coradin, T.; Poliard, A.; Slimani, L.; Chaussain, C. Combining sclerostin neutralization with tissue engineering: An improved strategy for craniofacial bone repair. Acta Biomater. 2022, 140, 178–189. [Google Scholar] [CrossRef]
- Collignon, A.M.; Castillo-Dali, G.; Gomez, E.; Guilbert, T.; Lesieur, J.; Nicoletti, A.; Acuna-Mendoza, S.; Letourneur, D.; Chaussain, C.; Rochefort, G.Y.; et al. Mouse Wnt1-CRE-RosaTomato dental pulp stem cells directly contribute to the calvarial bone regeneration process. Stem Cells 2019, 37, 701–711. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Zhang, Y.L.; Rouiller, I.; Barralet, J.E.; Nazhat, S.N. Fibril formation pH controls intrafibrillar collagen biomineralization in vitro and in vivo. Biomaterials 2015, 37, 252–259. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Nikogeorgos, N.; Ajalloueian, A.; Fossum, M.; Lee, S.; Chronakis, I.S. Compressed collagen constructs with optimized mechanical properties and cell interactions for tissue engineering applications. Int. J. Biol. Macromol. 2018, 108, 158–166. [Google Scholar] [CrossRef]
- Williams, B.R.; Gelman, R.A.; Poppke, D.C.; Piez, K.A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetics results. J. Biol. Chem. 1978, 253, 6578–6585. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile mechanical properties of three-dimensional Type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in dense collagen solutions: A physicochemical study. J. Mol. Biol. 2008, 376, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Hagar, M.N.; Yazid, F.; Luchman, N.A.; Ariffin, S.H.Z.; Wahab, R.M.A.A. Comparative evaluation of osteogenic differentiation potential of stem cells derived from dental pulp and exfoliated deciduous teeth cultured over granular hydroxyapatite based scaffold. BMC Oral Health 2021, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, Q.; Yang, T.; Qi, Y.; Fu, M.; Yang, X.; Qiao, L.; Ling, Q.; Liu, S.; Zhao, Y. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol. Med. Rep. 2018, 17, 6551–6559. [Google Scholar] [CrossRef]
- Sabbagh, J.; Ghassibe-Sabbagh, M.; Fayyad-Kazan, M.; Al-Nemer, F.; Fahed, J.C.; Berberi, A.; Badran, B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020, 101, 103413. [Google Scholar] [CrossRef]
- Goldstein, A.S. Effect of seeding osteoprogenitor cells as dense clusters on cell growth and differentiation. Tissue Eng. 2001, 7, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Comper, W.D.; Veis, A. The mechanism of nucleation for in vitro collagen fibril formation. Biopolymers 1977, 16, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.L.; Huang, E.K.; Silver, F.H. Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000, 19, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Soliakov, A.; Lewis, R.J. In vitro fibrillogenesis of collagen type I in varying ionic and pH conditions. Micron 2013, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, A., Jr.; Gomes Pinheiro, C.C.; Lazzaretti Fernandes, T.; Franco Bueno, D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 2041731417752766. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to cell–hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Jagiełło, A.; Castillo, U.; Botvinick, E. Cell mediated remodeling of stiffness matched collagen and fibrin scaffolds. Sci. Rep. 2022, 12, 11736. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Aydin, H.M.; Hu, B.; Konduru, S.; Kuiper, J.H.; Yang, Y. A facile in vitro model to study rapid mineralization in bone tissues. BioMed. Eng. Online 2014, 13, 136. [Google Scholar] [CrossRef]
- Bitar, M.; Brown, R.A.; Salih, V.; Kidane, A.G.; Knowles, J.C.; Nazhat, S.N. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 2008, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]

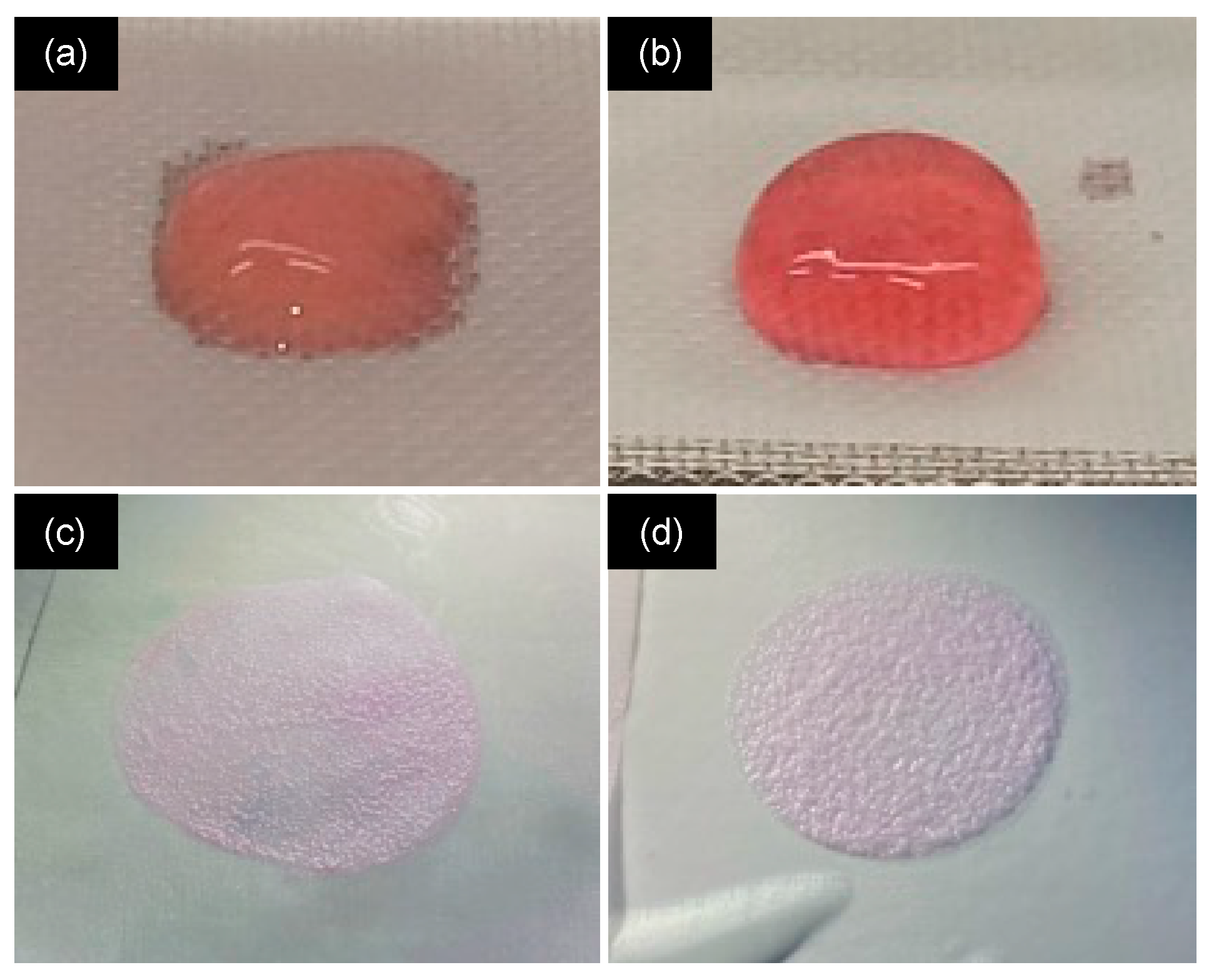
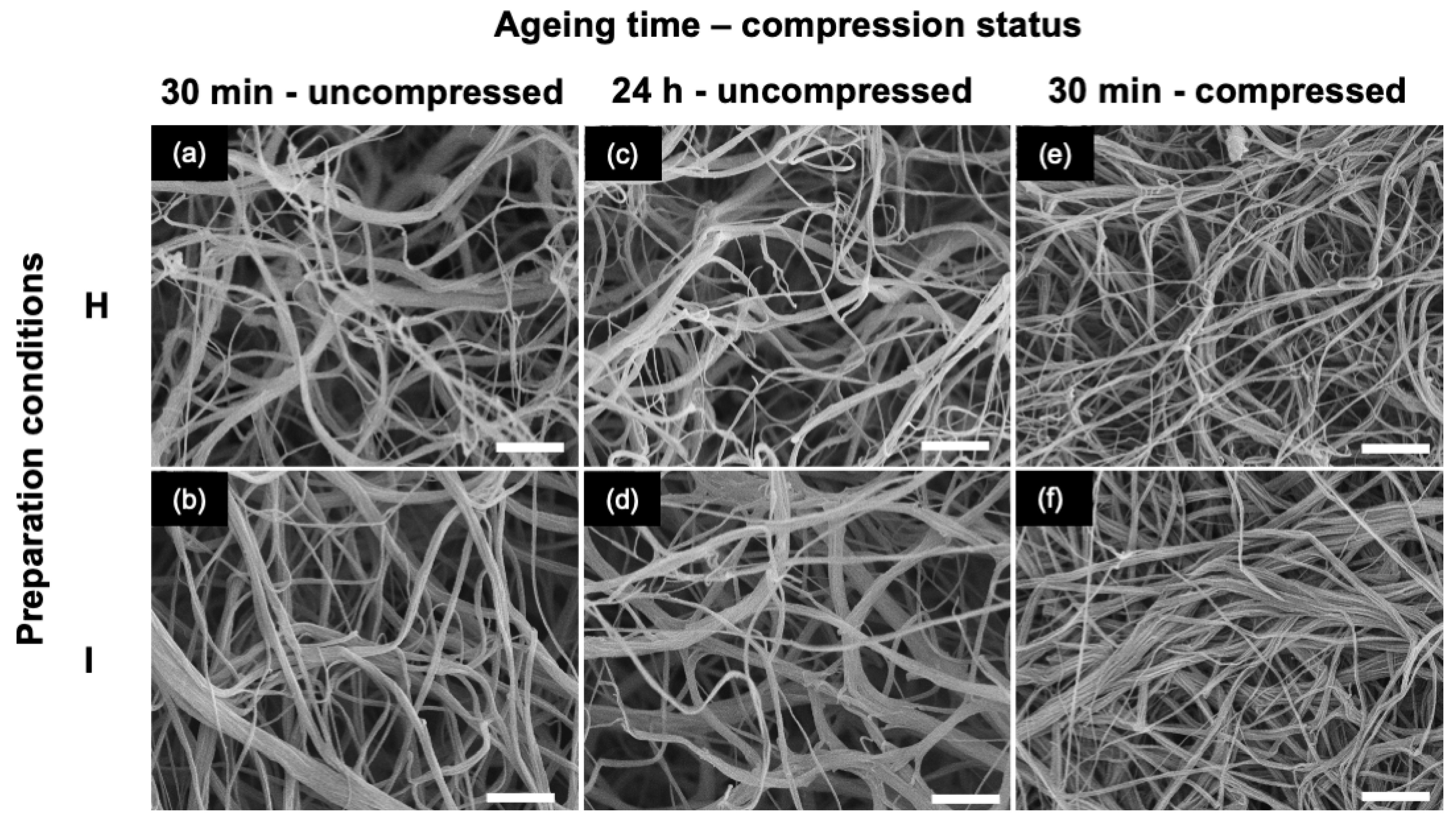

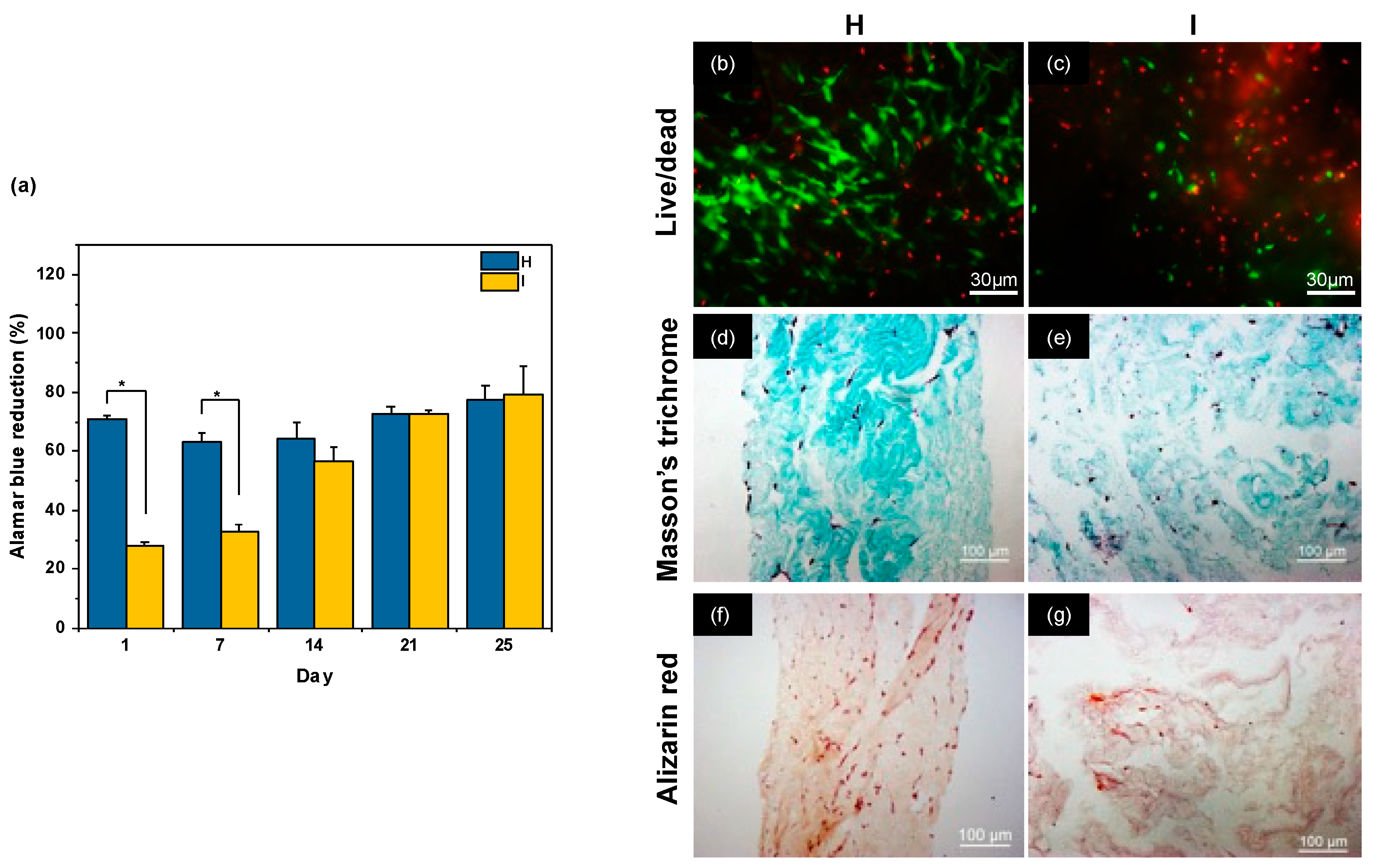
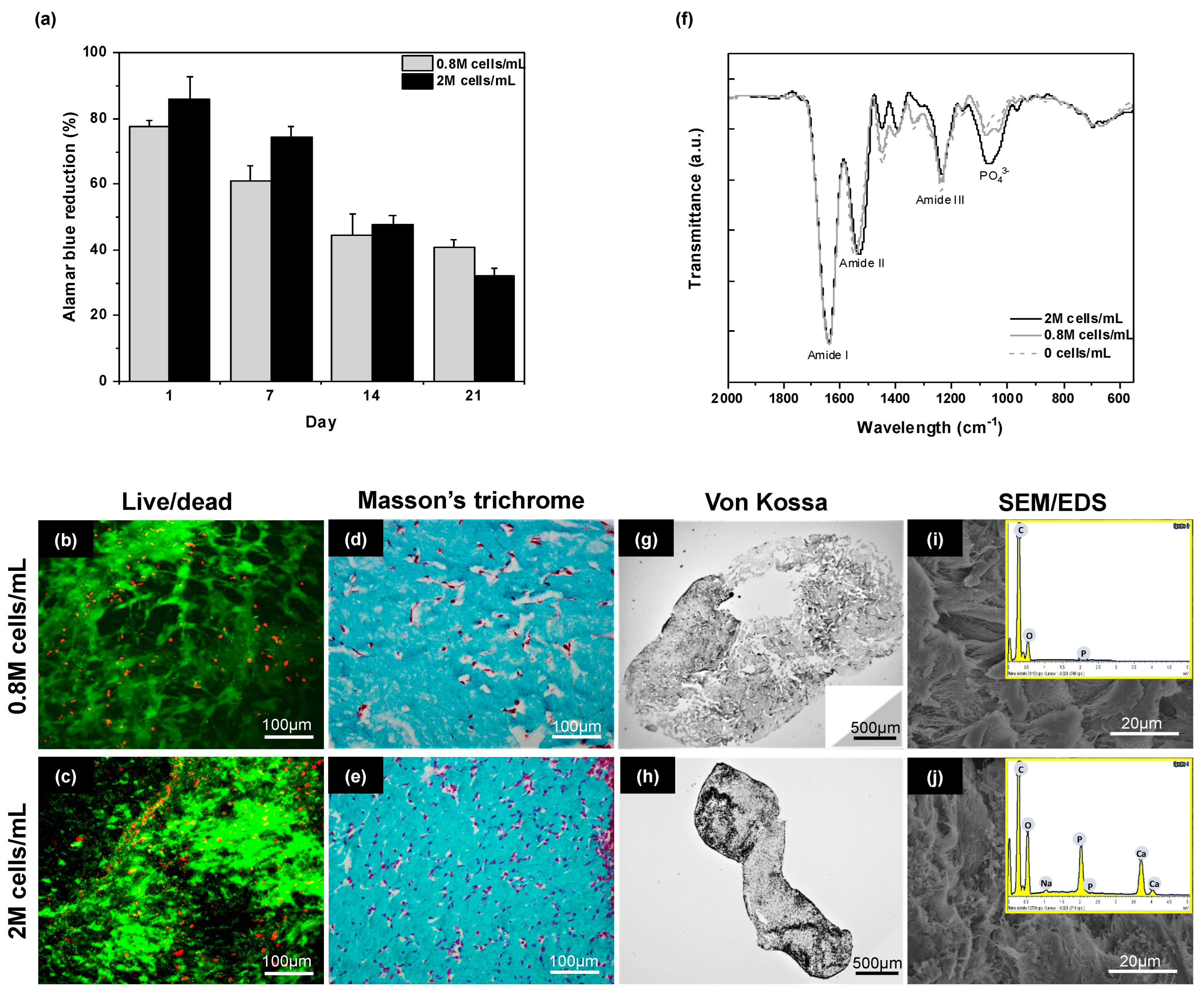
| VDMEM (mL) | [DMEM] | pH | [NaOH] (M) | VNaOH (μL) | Final [DMEM] | Gel before Compression | Gel after Compression |
|---|---|---|---|---|---|---|---|
| 0.5 | 1X | 4.5–5 | 0.1 | 350 | 0.5X | spread and leak | crush |
| 0.5 | 2.5X | 4 | 0.1 | 450 | 0.7X | spread and leak | very thin and fragile |
| 0.5 | 5X | 3.5 | 0.1 | 600 | 0.9X | stable | very thin and stable |
| 0.5 | 10X | 3 | 0.1 | 700 | 1.4X | stable | very thin and stable |
| 1.0 | 1X | 6–7 | 0.1 | 0 | 0.6X | spread and leak | very thin and fragile |
| 1.0 | 2.5X | 3.5 | 0.1 | 600 | 0.8X | stable | very thin and stable |
| 1.0 | 5X | 3 | 0.1 | 700 | 1.3X | stable | thin and stable (H) |
| 1.0 | 10X | <3 | 0.1 | >1000 | - | - | - |
| 1.5 | 1X | 6–7 | 0.1 | 0 | 0.6X | spread and leak | crush |
| 1.5 | 2.5X | 3.5 | 0.1 | 700 | 0.9X | spread and leak | very thin and fragile |
| 1.5 | 5X | 3 | 0.1 | 800 | 1.6X | spread and leak | crush |
| VDMEM (mL) | [DMEM] | pH | [NaOH] (M) | VNaOH (μL) | Final [DMEM] | Gel before Compression | Gel after Compression |
|---|---|---|---|---|---|---|---|
| 1.0 | 1X | 3.5–4 | 5 | 200 | 0.3X | spread and leak | very thin and stable |
| 0.1 | 1000 | ||||||
| 1.0 | 2.5X | 3 | 5 | 205 | 0.7X | stable | thin and stable |
| 0.1 | 640 | ||||||
| 1.0 | 5X | 2–3 | 5 | 210 | 1.2X | spread and leak | very thin and fragile |
| 0.1 | 700 | ||||||
| 1.0 | 10X | 2 | 5 | 215 | 2.3X | no gel formed | - |
| 0.1 | 450 | ||||||
| 1.0 | 1X | 3.5–4 | 5 | 200 | 0.5X | spread and leak | very thin and stable |
| 1 | 30 | ||||||
| 1.0 | 2.5X | 3 | 5 | 205 | 0.8X | stable | thin and stable (I) |
| 1 | 40 | ||||||
| 1.0 | 5X | 2–3 | 5 | 210 | 1.2X | spread and leak | very thin and stable |
| 1 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbitta Akoa, D.; Sicard, L.; Hélary, C.; Torrens, C.; Baroukh, B.; Poliard, A.; Coradin, T. Role of Physico-Chemical and Cellular Conditions on the Bone Repair Potential of Plastically Compressed Collagen Hydrogels. Gels 2024, 10, 130. https://doi.org/10.3390/gels10020130
Mbitta Akoa D, Sicard L, Hélary C, Torrens C, Baroukh B, Poliard A, Coradin T. Role of Physico-Chemical and Cellular Conditions on the Bone Repair Potential of Plastically Compressed Collagen Hydrogels. Gels. 2024; 10(2):130. https://doi.org/10.3390/gels10020130
Chicago/Turabian StyleMbitta Akoa, Daline, Ludovic Sicard, Christophe Hélary, Coralie Torrens, Brigitte Baroukh, Anne Poliard, and Thibaud Coradin. 2024. "Role of Physico-Chemical and Cellular Conditions on the Bone Repair Potential of Plastically Compressed Collagen Hydrogels" Gels 10, no. 2: 130. https://doi.org/10.3390/gels10020130






