A Novel, Controllable, and Efficient Method for Building Highly Hydrophobic Aerogels
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
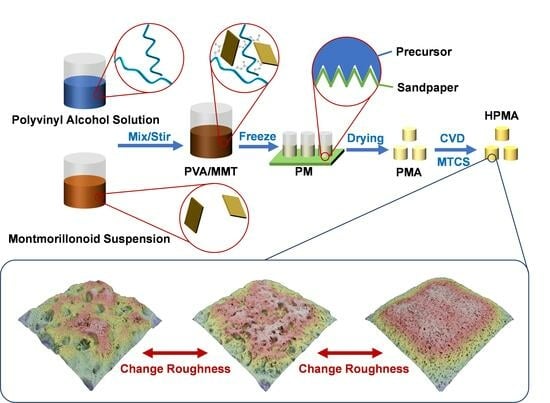
4.1. Preparation of PVA/MMT Precursor
4.2. Preparation of Aerogels by Template
4.3. Preparation of Hydrophobic Aerogels
4.4. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Yang, L.; Chen, Z.; Yin, L.; Yang, M.; Liu, T.; Li, M.; Cui, S. A flexible silica aerogel paper with temperature-switch opacifier for thermal insulation. J. Mater. Res. Technol. 2023, 24, 4037–4046. [Google Scholar]
- Wu, Z.-H.; Feng, X.-L.; Qu, Y.-X.; Gong, L.-X.; Cao, K.; Zhang, G.-D.; Shi, Y.; Gao, J.-F.; Song, P.; Tang, L.-C. Silane modified MXene/polybenzazole nanocomposite aerogels with exceptional surface hydrophobicity, flame retardance and thermal insulation. Compos. Commun. 2023, 37, 101402. [Google Scholar]
- Xue, T.; Yu, Y.; Fu, Z.; Wang, Q.; Hu, Z.; Fan, W.; Liu, T. Double-network polyimide/silica aerogel fiber for thermal insulation under extremely hot and humid environment. Compos. Sci. Technol. 2023, 242, 110196. [Google Scholar]
- Pérez-Moreno, A.; Piñero, M.; Fernández-Montesinos, R.; Pinaglia-Tobaruela, G.; Reyes-Peces, M.V.; Mesa-Díaz, M.d.M.; Vilches-Pérez, J.I.; Esquivias, L.; de la Rosa-Fox, N.; Salido, M. Chitosan-Silica Hybrid Biomaterials for Bone Tissue Engineering: A Comparative Study of Xerogels and Aerogels. Gels 2023, 9, 383. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, Y.; Lu, K.; Wang, Z.; Wang, J.; Lai, L.; Xin, W.; Xiao, Y.; Xiong, S.; Ding, F. Chitosan/silica hybrid aerogels with synergistic capability for superior hydrophobicity and mechanical robustness. Carbohydr. Polym. 2023, 121245. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.C.; Zhang, H.R.; Li, S.C.; Chen, W.S.; Wu, Z.Y.; Liang, H.W.; Yu, H.P.; Yu, S.H. Robust Carbonaceous Nanofiber Aerogels from All Biomass Precursors. Adv. Funct. Mater. 2023, 33, 2207532. [Google Scholar]
- Kurmysheva, A.Y.; Yanushevich, O.; Krikheli, N.; Kramar, O.; Vedenyapina, M.D.; Podrabinnik, P.; Solís Pinargote, N.W.; Smirnov, A.; Kuznetsova, E.; Malyavin, V.V. Adsorption Ability of Graphene Aerogel and Reduced Graphene Aerogel toward 2, 4-D Herbicide and Salicylic Acid. Gels 2023, 9, 680. [Google Scholar] [CrossRef]
- Liu, B.-W.; Cao, M.; Zhang, Y.-Y.; Wang, Y.-Z.; Zhao, H.-B. Multifunctional protective aerogel with superelasticity over− 196 to 500 °C. Nano Res. 2022, 15, 7797–7805. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, Y.; Peng, F.; Feng, J.; Li, L.; Feng, J. Fiber-reinforced alumina-carbon core-shell aerogel composite with heat-induced gradient structure for thermal protection up to 1800 °C. Chem. Eng. J. 2023, 461, 141721. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Jha, K.; Yahya, E.B.; Panchal, S.; Patel, N.; Garai, A.; Kumari, S.; Jameel, M. Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems. Gels 2023, 9, 666. [Google Scholar]
- Cui, H.; Wu, N.; Ma, X.; Niu, F. Superior intrinsic flame-retardant phosphorylated chitosan aerogel as fully sustainable thermal insulation bio-based material. Polym. Degrad. Stab. 2023, 207, 110213. [Google Scholar]
- Dong, X.; Liu, Q.; Zhao, Y.; Zhai, W. Highly robust and hydrophobic aerogel beads with dandelion-like structure for water treatment. Chem. Eng. J. 2023, 456, 141050. [Google Scholar] [CrossRef]
- Fan, B.; Wu, L.; Ming, A.; Liu, Y.; Yu, Y.; Cui, L.; Zhou, M.; Wang, Q.; Wang, P. Highly compressible and hydrophobic nanofibrillated cellulose aerogels for cyclic oil/water separation. Int. J. Biol. Macromol. 2023, 125066. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Qiao, D.; Dong, L.; Chen, X.; Wang, Z.; Li, Y. Efficient recovery of highly viscous crude oil spill by superhydrophobic ocean biomass-based aerogel assisted with solar energy. Chem. Eng. J. 2023, 467, 143532. [Google Scholar]
- Huang, Y.; Yang, H.; Yu, Y.; Li, H.; Li, H.; Bai, J.; Shi, F.; Liu, J. Bacterial cellulose biomass aerogels for oil-water separation and thermal insulation. J. Environ. Chem. Eng. 2023, 11, 110403. [Google Scholar] [CrossRef]
- Li, S.-L.; Wang, J.; Zhao, H.-B.; Cheng, J.-B.; Zhang, A.-N.; Wang, T.; Cao, M.; Fu, T.; Wang, Y.-Z. Ultralight biomass aerogels with multifunctionality and superelasticity under extreme conditions. ACS Appl. Mater. Interfaces 2021, 13, 59231–59242. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ke, Y.; Shang, Q.; Yang, X.; Wang, D.; Liao, G. Fabrication of multifunctional biomass-based aerogel with 3D hierarchical porous structure from waste reed for the synergetic adsorption of dyes and heavy metal ions. Chem. Eng. J. 2023, 451, 138934. [Google Scholar]
- Wang, W.; Lin, J.-H.; Guo, J.; Sun, R.; Han, G.; Peng, F.; Chi, S.; Dong, T. Biomass Chitosan-Based Tubular/Sheet Superhydrophobic Aerogels Enable Efficient Oil/Water Separation. Gels 2023, 9, 346. [Google Scholar] [PubMed]
- Wang, Y.-T.; Zhao, H.-B.; Guo, M.-L.; Degracia, K.; Sun, H.; Sun, M.-Z.; Zhao, Z.-Y.; Schiraldi, D.A.; Wang, Y.-Z. Rigid and fire-resistant all-biomass aerogels. ACS Sustain. Chem. Eng. 2022, 10, 12117–12126. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Jia, X.; Yang, J.; Miao, X.; Shao, D.; Song, H.; Li, Y. Full biomass-derived multifunctional aerogel for solar-driven interfacial evaporation. Chem. Eng. J. 2023, 471, 144684. [Google Scholar] [CrossRef]
- Ye, J.; Cai, P.; Huang, Z.; Pan, Y. Highly Hydrophobic All-Biomass Aerogels with Advanced Oil Absorption and Recyclability. ACS Appl. Polym. Mater. 2023, 5, 3632–3642. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Pan, D.; Hou, Y. Robust, Fire-Retardant, and Water-Resistant Wood/Polyimide Composite Aerogels with a Hierarchical Pore Structure for Thermal Insulation. Gels 2023, 9, 467. [Google Scholar] [CrossRef]
- Peng, D.; Zhao, J.; Liang, X.; Guo, X.; Li, H. Corn stalk pith-based hydrophobic aerogel for efficient oil sorption. J. Hazard. Mater. 2023, 448, 130954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xue, T.; Ma, Z.; Fan, W.; Liu, T. Mechanically Strong and Thermally Insulating Polyimide Aerogel Fibers Reinforced by Prefabricated Long Polyimide Fibers. ACS Appl. Mater. Interfaces 2023, 15, 12443–12452. [Google Scholar] [CrossRef]
- Schinazi, G.; Price, E.J.; Schiraldi, D.A. Fire testing methods of bio-based flame-retardant polymeric materials. In Bio-Based Flame-Retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–95. [Google Scholar]
- Zeng, F.; Men, X.; Chen, M.; Liu, B.; Han, Q.; Huang, S.; Zhao, H.; Wang, Y. Molecular-micron multiscale toughening and flame retarding for polyurethane foams. Chem. Eng. J. 2023, 454, 140023. [Google Scholar]
- Li, S.-L.; Wang, Y.-T.; Liu, B.-W.; Shi, H.-G.; Zhao, H.-B.; Wang, Y.-Z. Fe3O4@ PANI/chitosan composite aerogel with electromagnetic induction heating capacity toward efficient removing viscous oil. Compos. Commun. 2022, 36, 101367. [Google Scholar] [CrossRef]
- Lin, X.C.; Li, S.L.; Li, W.X.; Wang, Z.H.; Zhang, J.Y.; Liu, B.W.; Fu, T.; Zhao, H.B.; Wang, Y.Z. Thermo-Responsive Self-Ceramifiable Robust Aerogel with Exceptional Strengthening and Thermal Insulating Performance at Ultrahigh Temperatures. Adv. Funct. Mater. 2023, 23, 2214913. [Google Scholar] [CrossRef]
- Wu, F.; Hu, P.; Hu, F.; Tian, Z.; Tang, J.; Zhang, P.; Pan, L.; Barsoum, M.W.; Cai, L.; Sun, Z. Multifunctional MXene/C Aerogels for Enhanced Microwave Absorption and Thermal Insulation. Nano-Micro Lett. 2023, 15, 194. [Google Scholar]
- Zhao, X.; Liu, Y.; Zhao, L.; Yazdkhasti, A.; Mao, Y.; Siciliano, A.P.; Dai, J.; Jing, S.; Xie, H.; Li, Z. A scalable high-porosity wood for sound absorption and thermal insulation. Nat. Sustain. 2023, 6, 306–315. [Google Scholar]
- Ahmadi Heidari, N.; Fathi, M.; Hamdami, N.; Taheri, H.; Siqueira, G.; Nystrom, G. Thermally Insulating Cellulose Nanofiber Aerogels from Brewery Residues. ACS Sustain. Chem. Eng. 2023, 11, 10698–10708. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, B.-W.; Zeng, F.-R.; Lin, X.-C.; Zhang, J.-Y.; Wang, X.-L.; Wang, Y.-Z.; Zhao, H.-B. Fully recyclable multifunctional adhesive with high durability, transparency, flame retardancy, and harsh-environment resistance. Sci. Adv. 2022, 8, eadd8527. [Google Scholar]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.-T.; Wang, Y.-Z.; Schiraldi, D.A. Nonflammable alginate nanocomposite aerogels prepared by a simple freeze-drying and post-cross-linking method. ACS Appl. Mater. Interfaces 2016, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; He, J.-H.; Li, Z.; Lu, J.-H.; Liu, B.-W.; Fu, T.; Zhao, H.-B.; Wang, Y.-Z. A sponge heated by electromagnetic induction and solar energy for quick, efficient, and safe cleanup of high-viscosity crude oil spills. J. Hazard. Mater. 2022, 436, 129272. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Sun, M.; Wang, G.; Liu, Q.; Li, M.; Shulga, Y.M.; Li, Z. Aramid Pulp Reinforced Clay Aerogel Composites: Mechanical, Thermal and Combustion Behavior. Gels 2022, 8, 654. [Google Scholar] [CrossRef]
- Wu, N.; Niu, F.; Lang, W.; Xia, M. Highly efficient flame-retardant and low-smoke-toxicity poly (vinyl alcohol)/alginate/montmorillonite composite aerogels by two-step crosslinking strategy. Carbohydr. Polym. 2019, 221, 221–230. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Shang, K.; Wang, Y.-T.; Ye, D.-D.; Kang, A.-H.; Liao, W.; Wang, Y.-Z. Ultrasoft gelatin aerogels for oil contaminant removal. J. Mater. Chem. A 2016, 4, 9381–9389. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Liao, S.-F.; Shang, K.; Chen, M.-J.; Huang, J.-Q.; Wang, Y.-Z.; Schiraldi, D.A. Efficient approach to improving the flame retardancy of poly (vinyl alcohol)/clay aerogels: Incorporating piperazine-modified ammonium polyphosphate. ACS Appl. Mater. Interfaces 2015, 7, 1780–1786. [Google Scholar] [PubMed]
- Wang, Y.-T.; Zhao, H.-B.; Degracia, K.; Han, L.-X.; Sun, H.; Sun, M.; Wang, Y.-Z.; Schiraldi, D.A. Green approach to improving the strength and flame retardancy of poly (vinyl alcohol)/clay aerogels: Incorporating biobased gelatin. ACS Appl. Mater. Interfaces 2017, 9, 42258–42265. [Google Scholar]
- Chen, H.Y.; Chen, Z.Y.; Mao, M.; Wu, Y.Y.; Yang, F.; Gong, L.X.; Zhao, L.; Cao, C.F.; Song, P.; Gao, J.F. Self-Adhesive polydimethylsiloxane foam materials decorated with MXene/Cellulose nanofiber interconnected network for versatile functionalities. Adv. Funct. Mater. 2023, 33, 2304927. [Google Scholar] [CrossRef]
- Shao, L.; Xu, J.; Ma, J.; Zhai, B.; Li, Y.; Xu, R.; Ma, Z.; Zhang, G.; Wang, C.; Qiu, J. MXene/RGO composite aerogels with light and high-strength for supercapacitor electrode materials. Compos. Commun. 2020, 19, 108–113. [Google Scholar] [CrossRef]
- Wang, D.; Peng, H.; Yu, B.; Zhou, K.; Pan, H.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Biomimetic structural cellulose nanofiber aerogels with exceptional mechanical, flame-retardant and thermal-insulating properties. Chem. Eng. J. 2020, 389, 124449. [Google Scholar]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, H.; Hostler, S.; Schiraldi, D.A. Effects of feather-fiber reinforcement on poly (vinyl alcohol)/clay aerogels: Structure, property and applications. Polymer 2018, 137, 201–208. [Google Scholar] [CrossRef]
- Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D.A. The relation between the rheological properties of gels and the mechanical properties of their corresponding aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef]
- Shang, K.; Ye, D.-D.; Kang, A.-H.; Wang, Y.-T.; Liao, W.; Xu, S.; Wang, Y.-Z. Robust and fire retardant borate-crosslinked poly (vinyl alcohol)/montmorillonite aerogel via melt-crosslink. Polymer 2017, 131, 111–119. [Google Scholar]
- Ye, D.-D.; Wang, T.; Liao, W.; Wang, H.; Zhao, H.-B.; Wang, Y.-T.; Xu, S.; Wang, Y.-Z. Ultrahigh-temperature insulating and fire-resistant aerogels from cationic amylopectin and clay via a facile route. ACS Sustain. Chem. Eng. 2019, 7, 11582–11592. [Google Scholar] [CrossRef]
- Lou, F.; Dong, S.; Zhu, K.; Chen, X.; Ma, Y. Thermal Insulation Performance of Aerogel Nano-Porous Materials: Characterization and Test Methods. Gels 2023, 9, 220. [Google Scholar]
- Sun, M.; Schiraldi, D.A. Clay-Based Aerogels. In Springer Handbook of Aerogels; Springer: Berlin/Heidelberg, Germany, 2023; pp. 883–917. [Google Scholar]








| Sample | Modulus (MPa) | Density (g/cm3) | Specific Modulus (×103 m) |
|---|---|---|---|
| PVA | 2.9 ± 0.5 | 0.0754 ± 0.003 | ~38.6 |
| PMA | 5.5 ± 0.4 | 0.0972 ± 0.0004 | ~56.6 |
| Sample | t = 0 s | t = 180 s | t = 360 s |
|---|---|---|---|
| PMA | 0° | 0° | 0° |
| HPMA | 125.3 ± 1.4° | 120.6 ± 2.1° | 121.7 ± 3.7° |
| Sample | t = 0 s | t = 180 s | t = 360 s |
|---|---|---|---|
| HPMA-240 | 128.5 ± 1.8° | 122.9 ± 3.2° | 121.3 ± 3.7° |
| HPMA-400 | 130.8 ± 2.1° | 124.4 ± 3.3° | 121.7 ± 3.7° |
| HPMA-600 | 130.3 ± 0.9° | 128.5 ± 0.9° | 125.6 ± 1.8° |
| HPMA-1000 | 135.9 ± 1.2° | 133.9 ± 0.8° | 132.3 ± 0.1° |
| HPMA-2000 | 140.3 ± 1.4° | 139.2 ± 0.6° | 137.6 ± 0.6° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-L.; Wang, Y.-T.; Zhang, S.-J.; Sun, M.-Z.; Li, J.; Chu, L.-Q.; Hu, C.-X.; Huang, Y.-L.; Gao, D.-L.; Schiraldi, D.A. A Novel, Controllable, and Efficient Method for Building Highly Hydrophobic Aerogels. Gels 2024, 10, 121. https://doi.org/10.3390/gels10020121
Li S-L, Wang Y-T, Zhang S-J, Sun M-Z, Li J, Chu L-Q, Hu C-X, Huang Y-L, Gao D-L, Schiraldi DA. A Novel, Controllable, and Efficient Method for Building Highly Hydrophobic Aerogels. Gels. 2024; 10(2):121. https://doi.org/10.3390/gels10020121
Chicago/Turabian StyleLi, Shu-Liang, Yu-Tao Wang, Shi-Jun Zhang, Ming-Ze Sun, Jie Li, Li-Qiu Chu, Chen-Xi Hu, Yi-Lun Huang, Da-Li Gao, and David A. Schiraldi. 2024. "A Novel, Controllable, and Efficient Method for Building Highly Hydrophobic Aerogels" Gels 10, no. 2: 121. https://doi.org/10.3390/gels10020121