Enhancing Water Resistance in Foam Cement through MTES-Based Aerogel Impregnation
Abstract
:1. Introduction
2. Results and Discussion
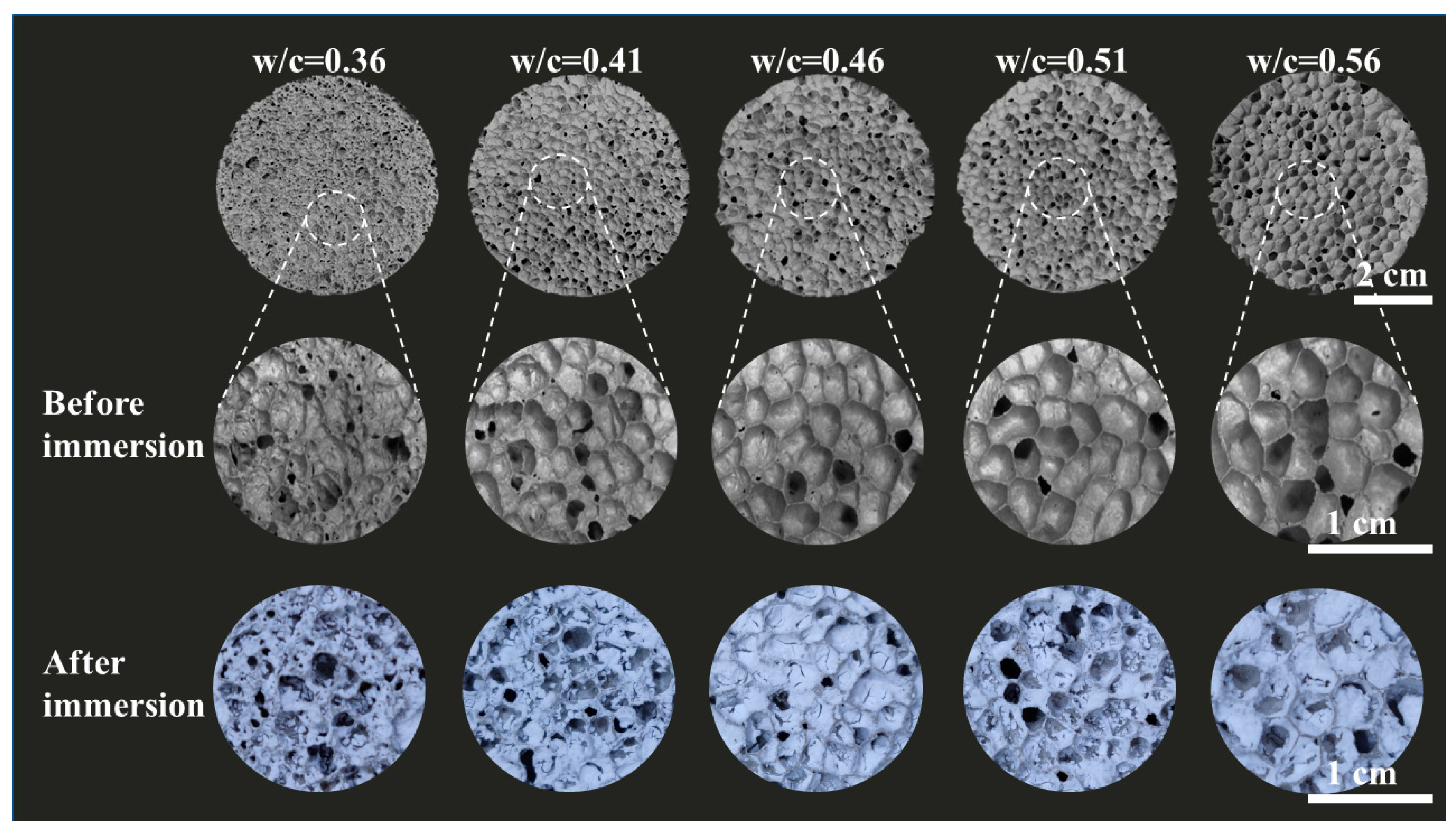
2.1. Microstructure
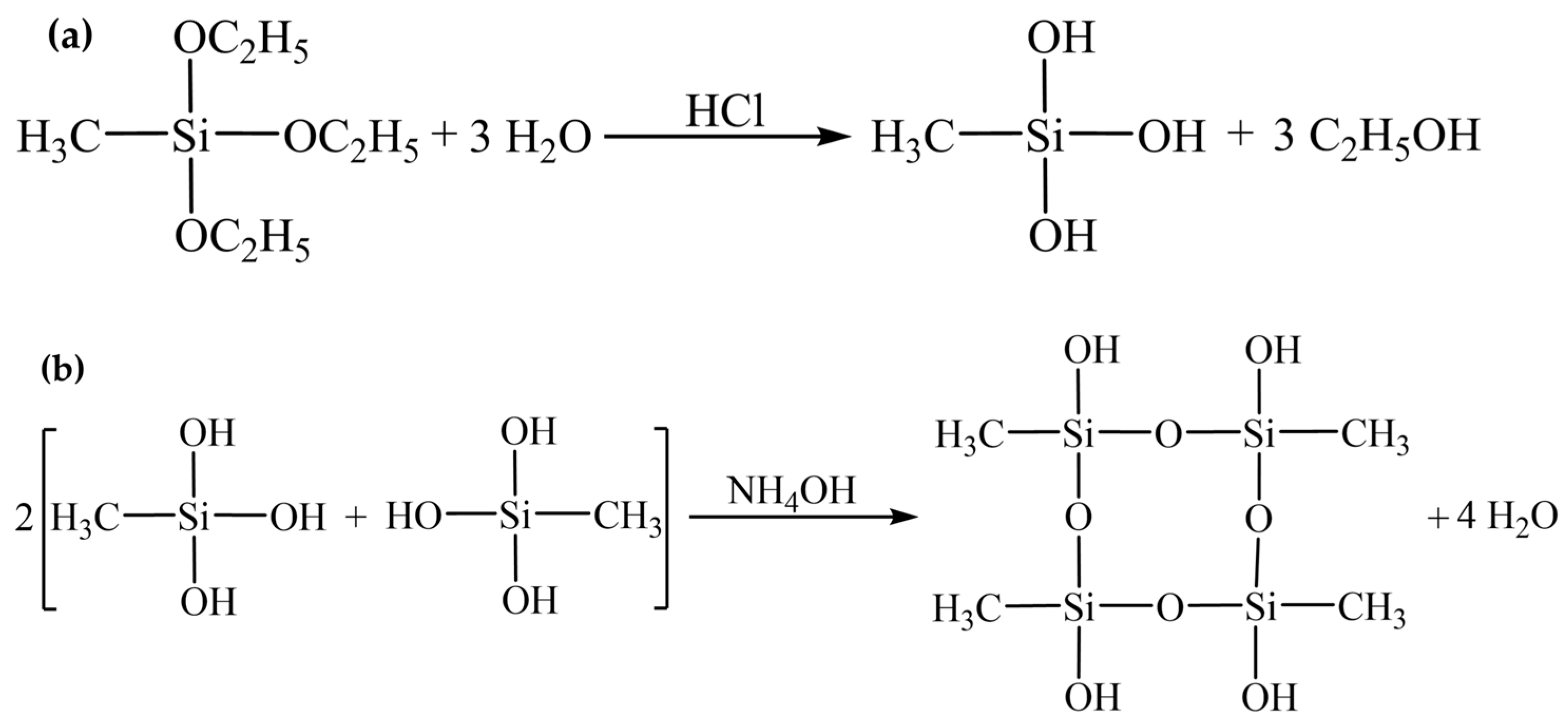
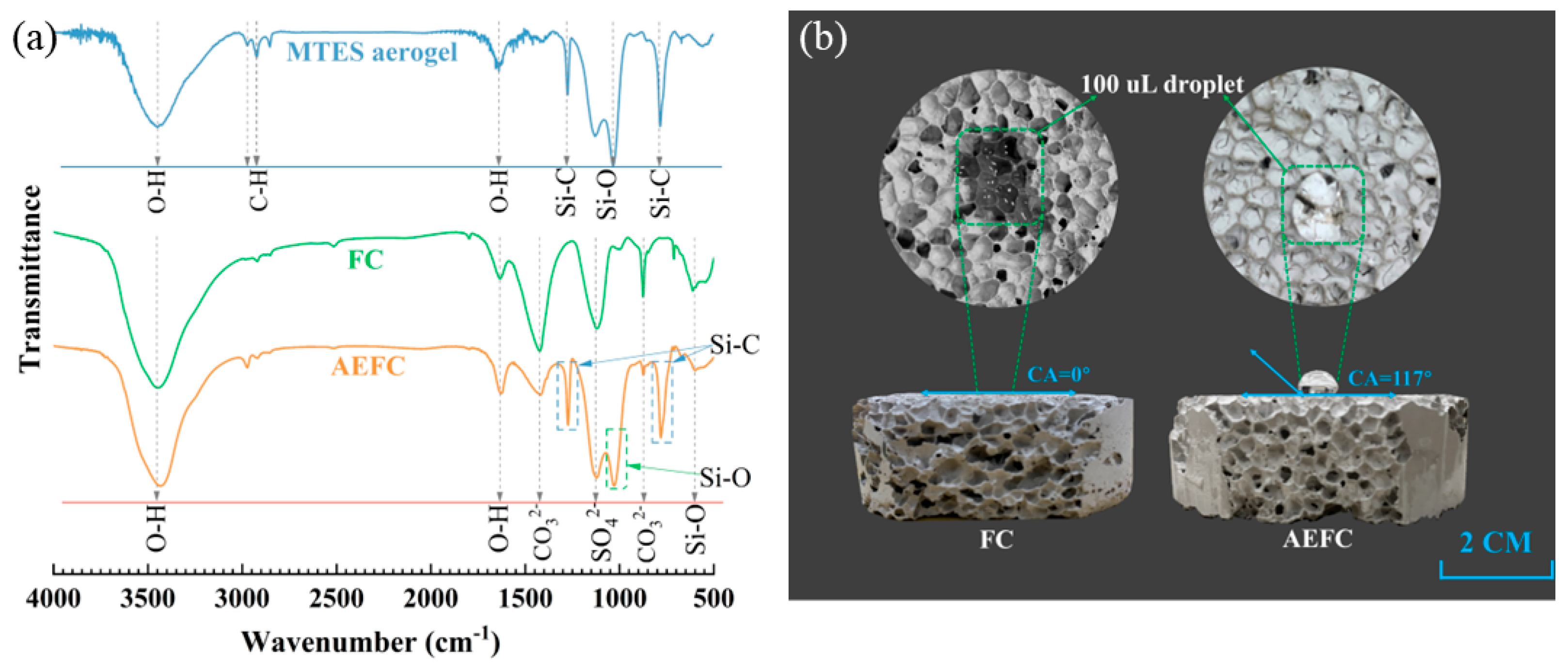
2.2. Chemical Composition and Hydrophobic Mechanism
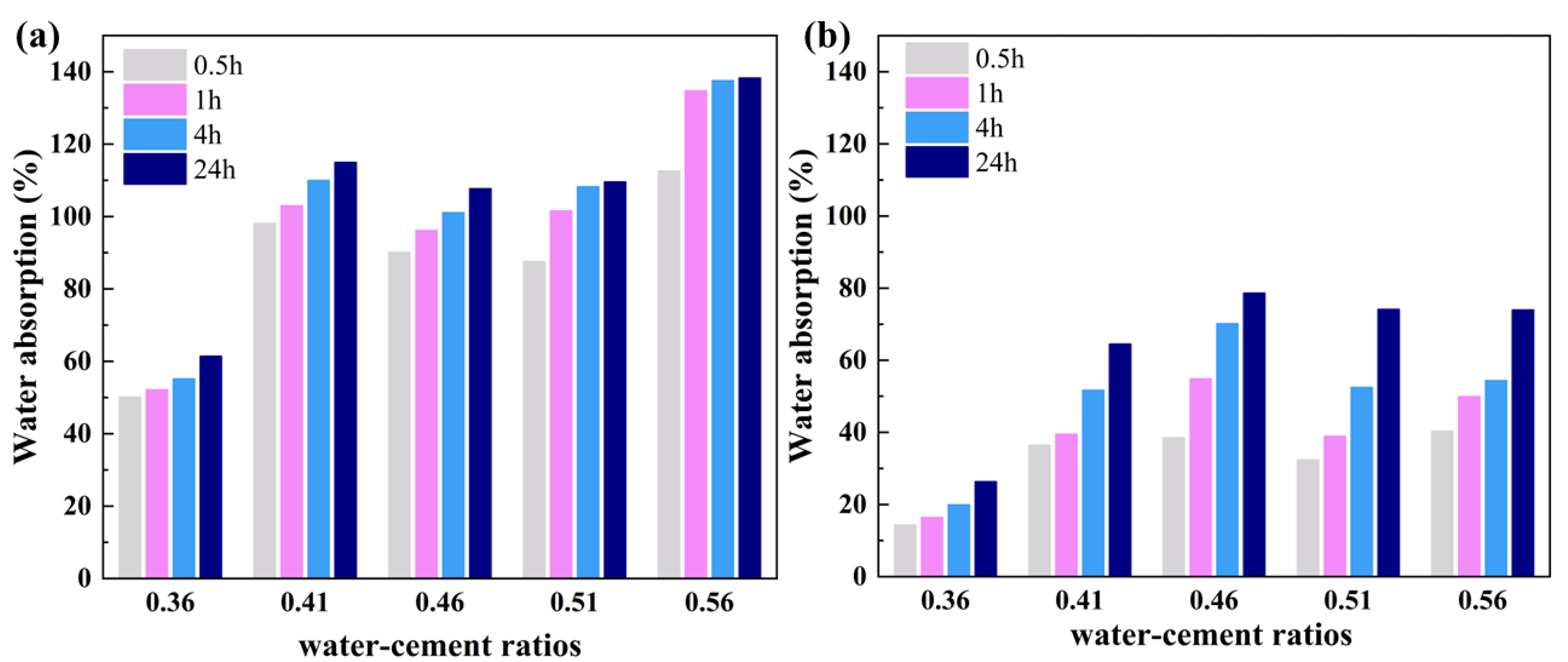
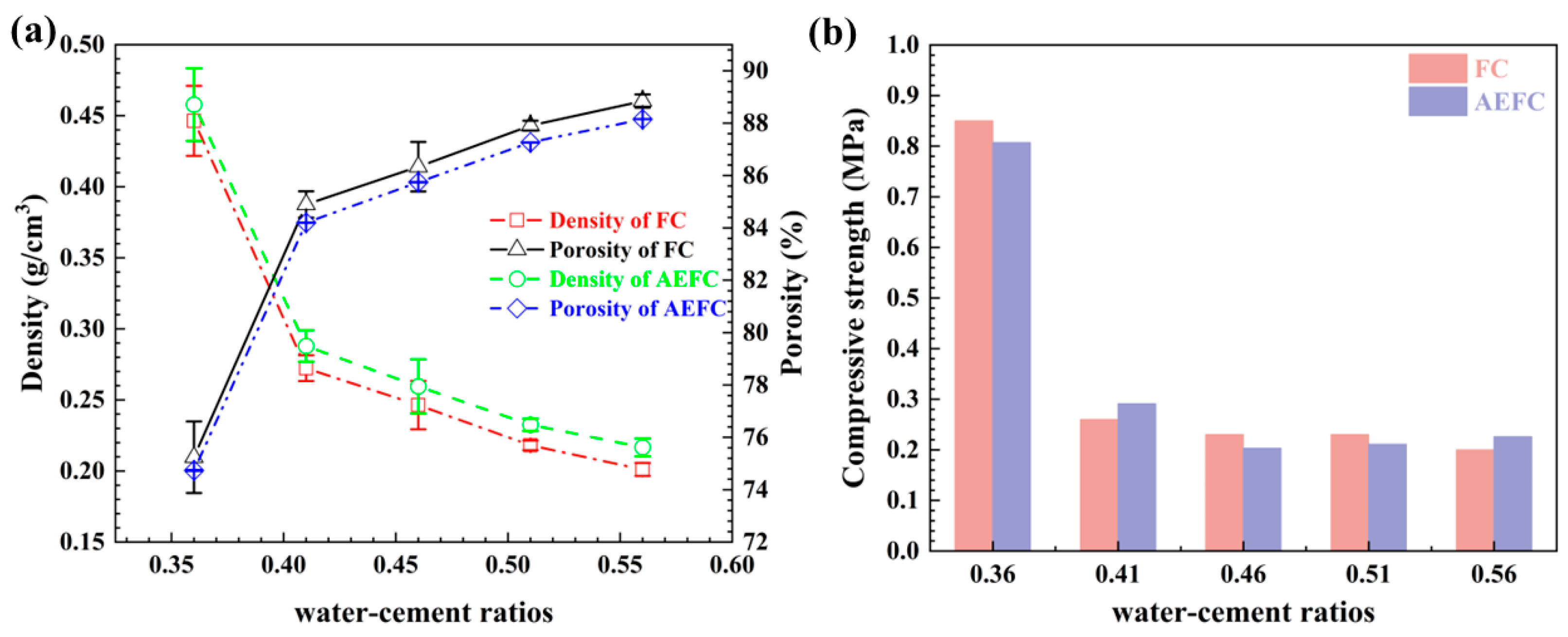
2.3. Water Resistance
2.4. Compressive Strength
2.5. Thermal Insulation Property
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
4.2. Sample Preparation
4.2.1. Preparing Foamed Cement
4.2.2. Preparing MTES-Based Aerogel
4.2.3. Embedding of MTES-Based Aerogel
4.3. Methods of Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A Review on Buildings Energy Consumption Information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R.; Seeniraj, R.V. Phase Change Material-Based Building Architecture for Thermal Management in Residential and Commercial Establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Berardi, U. Aerogel-Enhanced Systems for Building Energy Retrofits: Insights from a Case Study. Energy Build. 2018, 159, 370–381. [Google Scholar] [CrossRef]
- Pacheco Torgal, F.; Buratti, C.; Kalaiselvam, S.; Granqvist, C.-G.; Ivanov, V. (Eds.) Nano and Biotech Based Materials for Energy Building Efficiency; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-27503-1. [Google Scholar]
- Zhang, Z.; Li, B.; Wang, Z.; Liu, W.; Liu, X. Development of Reduced Thermal Conductivity Ductile Cement-Based Composite Material by Using Silica Aerogel and Silane. J. Build. Eng. 2023, 65, 105698. [Google Scholar] [CrossRef]
- Mehta, A.; Ashish, D.K. Silica Fume and Waste Glass in Cement Concrete Production: A Review. J. Build. Eng. 2020, 29, 100888. [Google Scholar] [CrossRef]
- Rudžionis, Ž.; Adhikary, S.K.; Manhanga, F.C.; Ashish, D.K.; Ivanauskas, R.; Stelmokaitis, G.; Navickas, A.A. Natural Zeolite Powder in Cementitious Composites and Its Application as Heavy Metal Absorbents. J. Build. Eng. 2021, 43, 103085. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K. Turning Waste Expanded Polystyrene into Lightweight Aggregate: Towards Sustainable Construction Industry. Sci. Total Environ. 2022, 837, 155852. [Google Scholar] [CrossRef]
- Berardi, U. A Cross-Country Comparison of the Building Energy Consumptions and Their Trends. Resour. Conserv. Recycl. 2017, 123, 230–241. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-Based Thermal Superinsulation: An Overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Optimizing Insulation Thickness and Analysing Environmental Impacts of Aerogel-Based Thermal Superinsulation in Buildings. Energy Build. 2014, 77, 28–39. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward Aerogel Based Thermal Superinsulation in Buildings: A Comprehensive Review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Yan, Q.; Meng, Z.; Luo, J.; Wu, Z. Experimental Study on Improving the Properties of Rock Wool and Glass Wool by Silica Aerogel. Energy Build. 2021, 247, 111146. [Google Scholar] [CrossRef]
- Yan, Q.; Feng, Z.; Luo, J.; Xia, W. Preparation and Characterization of Building Insulation Material Based on SiO2 Aerogel and Its Composite with Expanded Perlite. Energy Build. 2022, 255, 111661. [Google Scholar] [CrossRef]
- Fickler, S.; Milow, B.; Ratke, L.; Schnellenbach-Held, M.; Welsch, T. Development of High Performance Aerogel Concrete. Energy Procedia 2015, 78, 406–411. [Google Scholar] [CrossRef]
- Ng, S.; Sandberg, L.I.C.; Jelle, B.P. Insulating and Strength Properties of an Aerogel-Incorporated Mortar Based an UHPC Formulations. Key Eng. Mater. 2014, 629–630, 43–48. [Google Scholar] [CrossRef]
- Sobolev, K.; Flores, I.; Torres-Martinez, L.M.; Valdez, P.L.; Zarazua, E.; Cuellar, E.L. Engineering of SiO2 Nanoparticles for Optimal Performance in Nano Cement-Based Materials. In Nanotechnology in Construction 3; Bittnar, Z., Bartos, P.J.M., Němeček, J., Šmilauer, V., Zeman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 139–148. ISBN 978-3-642-00979-2. [Google Scholar]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Liu, Z.; Ng, S. Thermal and Mechanical Properties of SiO2 Aerogel–Incorporated Geopolymer Insulation Materials. J. Mater. Civ. Eng. 2019, 31, 04019099. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel Based Thermal Insulating Cementitious Composites: A Review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Z.; Niu, Y.; Li, J.; Zhang, Y. Study on the Preparation and Properties of High-Porosity Foamed Concretes Based on Ordinary Portland Cement. Mater. Des. 2016, 92, 949–959. [Google Scholar] [CrossRef]
- Meng, T.; Wei, H.; Yang, X.; Zhang, B.; Zhang, Y.; Zhang, C. Effect of Mixed Recycled Aggregate on the Mechanical Strength and Microstructure of Concrete under Different Water Cement Ratios. Materials 2021, 14, 2631. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, L.; Deng, Y.; Huang, S.; Sun, M.; Wang, X.; Liu, Q.; Li, M.; Li, Z. Effects of Precursor Concentration on the Physicochemical Properties of Ambient-Pressure-Dried MTES Based Aerogels with Using Pure Water as the Only Solvent. J. Sol-Gel Sci. Technol. 2021, 100, 477–488. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R″Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Hirashima, H.; Rao, A.V. Studies on Rheological Properties of Methyltriethoxysilane (MTES) Based Flexible Superhydrophobic Silica Aerogels. Microporous Mesoporous Mater. 2009, 117, 617–626. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Koebel, M.M.; Malfait, W.J. Silica Aerogels with Tailored Chemical Functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H. Characteristics of Ambient-Pressure-Dried Aerogels Synthesized via Different Surface Modification Methods. J. Sol-Gel Sci. Technol. 2015, 76, 138–149. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Li, Z.; Shi, L.; Zhang, Y.; Liu, Q. Rapid Synthesis and Characterization of Monolithic Ambient Pressure Dried MTMS Aerogels in Pure Water. J. Porous Mater. 2020, 27, 1241–1251. [Google Scholar] [CrossRef]
- El-Alfi, E.A.; Gado, R.A. Preparation of Calcium Sulfoaluminate-Belite Cement from Marble Sludge Waste. Constr. Build. Mater. 2016, 113, 764–772. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of Calcium Sulfate Source on the Hydration of Calcium Sulfoaluminate Eco-Cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early Hydration and Setting of Portland Cement Monitored by IR, SEM and Vicat Techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Scrivener, K.L. Backscattered Electron Imaging of Cementitious Microstructures: Understanding and Quantification. Cem. Concr. Compos. 2004, 26, 935–945. [Google Scholar] [CrossRef]
- Li, T.; Huang, F.; Li, L.; Zhu, J.; Jiang, X.; Huang, Y. Preparation and Properties of Sulphoaluminate Cement-Based Foamed Concrete with High Performance. Constr. Build. Mater. 2020, 263, 120945. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Lim, T.-K.; Jeong, S.-M.; Yang, K.-H. Thermal Transfer and Moisture Resistances of Nano-Aerogel-Embedded Foam Concrete. Constr. Build. Mater. 2020, 236, 117575. [Google Scholar] [CrossRef]
- Iwański, M.M.; Chomicz-Kowalska, A.; Maciejewski, K. Resistance to Moisture-Induced Damage of Half-Warm-Mix Asphalt Concrete with Foamed Bitumen. Materials 2020, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kim, H.; Lee, N.K.; Park, J.-J.; Jang, J.G. Microstructural Characteristics and CO2 Uptake of Calcium Sulfoaluminate Cement by Carbonation Curing at Different Water-to-Cement Ratios. Cem. Concr. Res. 2023, 163, 107012. [Google Scholar] [CrossRef]
- Haruehansapong, S.; Pulngern, T.; Chucheepsakul, S. Effect of the Particle Size of Nanosilica on the Compressive Strength and the Optimum Replacement Content of Cement Mortar Containing Nano-SiO2. Constr. Build. Mater. 2014, 50, 471–477. [Google Scholar] [CrossRef]
- Gavela, S.; Nikoloutsopoulos, N.; Papadakos, G.; Passa, D.; Sotiropoulou, A. Multifactorial Experimental Analysis of Concrete Compressive Strength as a Function of Time and Water-to-Cement Ratio. Procedia Struct. Integr. 2018, 10, 135–140. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Liu, Y. Optimal Water-Cement Ratio of Cement-Stabilized Soil. Constr. Build. Mater. 2022, 320, 126211. [Google Scholar] [CrossRef]
- Rahmani, K.; Rahmanzadeh, B.; Piroti, S. Experimental Study of the Effect of Water-Cement Ratio on Compressive Strength, Abrasion Resistance, Porosity and Permeability of Nano Silica Concrete. Frat. Integrità Strutt. 2018, 12, 16–24. [Google Scholar] [CrossRef]
- Yan, Q.; Shen, X.; Luo, J.; Yan, H.; Gao, H. Experimental Study on Effective Thermal Conductivity of Building Insulation Materials. Meas. Sci. Technol. 2019, 30, 105602. [Google Scholar] [CrossRef]
- Sang, G.; Zhu, Y.; Yang, G.; Zhang, H. Preparation and Characterization of High Porosity Cement-Based Foam Material. Constr. Build. Mater. 2015, 91, 133–137. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Hu, C.; Tang, Y. Effect of Water-Cement Ratio on Pore Structure and Strength of Foam Concrete. Adv. Mater. Sci. Eng. 2016, 2016, e9520294. [Google Scholar] [CrossRef]
- Wang, L.; Liu, P.; Jing, Q.; Liu, Y.; Wang, W.; Zhang, Y.; Li, Z. Strength Properties and Thermal Conductivity of Concrete with the Addition of Expanded Perlite Filled with Aerogel. Constr. Build. Mater. 2018, 188, 747–757. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-H.; Choi, C.; Jang, S.; Choi, S. A Review of Thermal Conductivity Data, Mechanisms and Models for Nanofluids. Int. J. Micro-Nano Scale Transp. 2010, 1, 269–322. [Google Scholar] [CrossRef]
- Kaddouri, W.; El Moumen, A.; Kanit, T.; Madani, S.; Imad, A. On the Effect of Inclusion Shape on Effective Thermal Conductivity of Heterogeneous Materials. Mech. Mater. 2016, 92, 28–41. [Google Scholar] [CrossRef]
- Chauhan, D.; Singhvi, N.; Singh, R. Dependence of Effective Thermal Conductivity of Composite Materials on the Size of Filler Particles. J. Reinf. Plast. Compos. 2013, 32, 1323–1330. [Google Scholar] [CrossRef]
- Khan, K.A.; Khan, S.Z.; Khan, M.A. Effective Thermal Conductivity of Two-Phase Composites Containing Highly Conductive Inclusions. J. Reinf. Plast. Compos. 2016, 35, 1586–1599. [Google Scholar] [CrossRef]
- Pal, R. New Models for Thermal Conductivity of Particulate Composites. J. Reinf. Plast. Compos. 2007, 26, 643–651. [Google Scholar] [CrossRef]
- Wang, J.; Carson, J.K.; North, M.F.; Cleland, D.J. A New Approach to Modelling the Effective Thermal Conductivity of Heterogeneous Materials. Int. J. Heat Mass Transf. 2006, 49, 3075–3083. [Google Scholar] [CrossRef]
- Chu, Z. Combination of the Unifying Model for the Effective Thermal Conductivity of Isotropic, Porous and Composite Geomaterials. Int. J. Rock Mech. Min. Sci. 2023, 164, 105342. [Google Scholar] [CrossRef]
- Levy, F.L. A Modified Maxwell-Eucken Equation for Calculating the Thermal Conductivity of Two-Component Solutions or Mixtures. Int. J. Refrig. 1981, 4, 223–225. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zhang, W.; Yan, H.; Wang, Y.; Li, M.; Liu, Q. Rapid Synthesis and Characterization of Ambient Pressure Dried Monolithic Silica Aerogels in Ethanol/Water Co-Solvent System. J. Non-Cryst. Solids 2019, 503–504, 214–223. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H.; Zhang, H. Tailoring Thermal Properties of Ambient Pressure Dried MTMS/TEOS Co-Precursor Aerogels. Mater. Lett. 2016, 171, 91–94. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded-Aerogels. J. Phys. Chem. 2002, 36, 52–64. [Google Scholar] [CrossRef]




| Element | Weight% | Atomic % | Weight Error % | Net Int. | K Ratio |
|---|---|---|---|---|---|
| Si | 6.76 | 9.37 | 7.66 | 59.17 | 0.0649 |
| Ca | 93.24 | 90.63 | 3.87 | 245.70 | 0.9245 |
| Element | Weight % | Atomic % | Weight Error % | Net Int. | K Ratio |
|---|---|---|---|---|---|
| Si | 96.16 | 97.27 | 2.74 | 639.21 | 0.9599 |
| Ca | 3.84 | 2.73 | 25.75 | 6.65 | 0.0343 |
| Oxide Constituents | SO3 | CaO | Al2O3 | SiO2 | Fe2O3 | MgO | Na2O | K2O | TiO2 | Else |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 16.95 | 59.90 | 13.04 | 4.00 | 2.43 | 0.93 | 0.24 | 0.45 | 0.85 | 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yao, S.; Wang, G.; Deng, X.; Zhou, F.; Wu, X.; Liu, Q. Enhancing Water Resistance in Foam Cement through MTES-Based Aerogel Impregnation. Gels 2024, 10, 118. https://doi.org/10.3390/gels10020118
Li Z, Yao S, Wang G, Deng X, Zhou F, Wu X, Liu Q. Enhancing Water Resistance in Foam Cement through MTES-Based Aerogel Impregnation. Gels. 2024; 10(2):118. https://doi.org/10.3390/gels10020118
Chicago/Turabian StyleLi, Zhi, Shengjie Yao, Guichao Wang, Xi Deng, Fang Zhou, Xiaoxu Wu, and Qiong Liu. 2024. "Enhancing Water Resistance in Foam Cement through MTES-Based Aerogel Impregnation" Gels 10, no. 2: 118. https://doi.org/10.3390/gels10020118






