Kinase Hog1 and Adr1 Opposingly Regulate Haploid Cell Morphology by Controlling Vacuole Size in Sporisorium scitamineum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Strains Construction
2.3. Nucleic Acid Related Manipulation
2.4. SsHog1 and SsAdr1 Phosphorylation Assays
2.5. Neutral Red Stained and Microscopy
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results
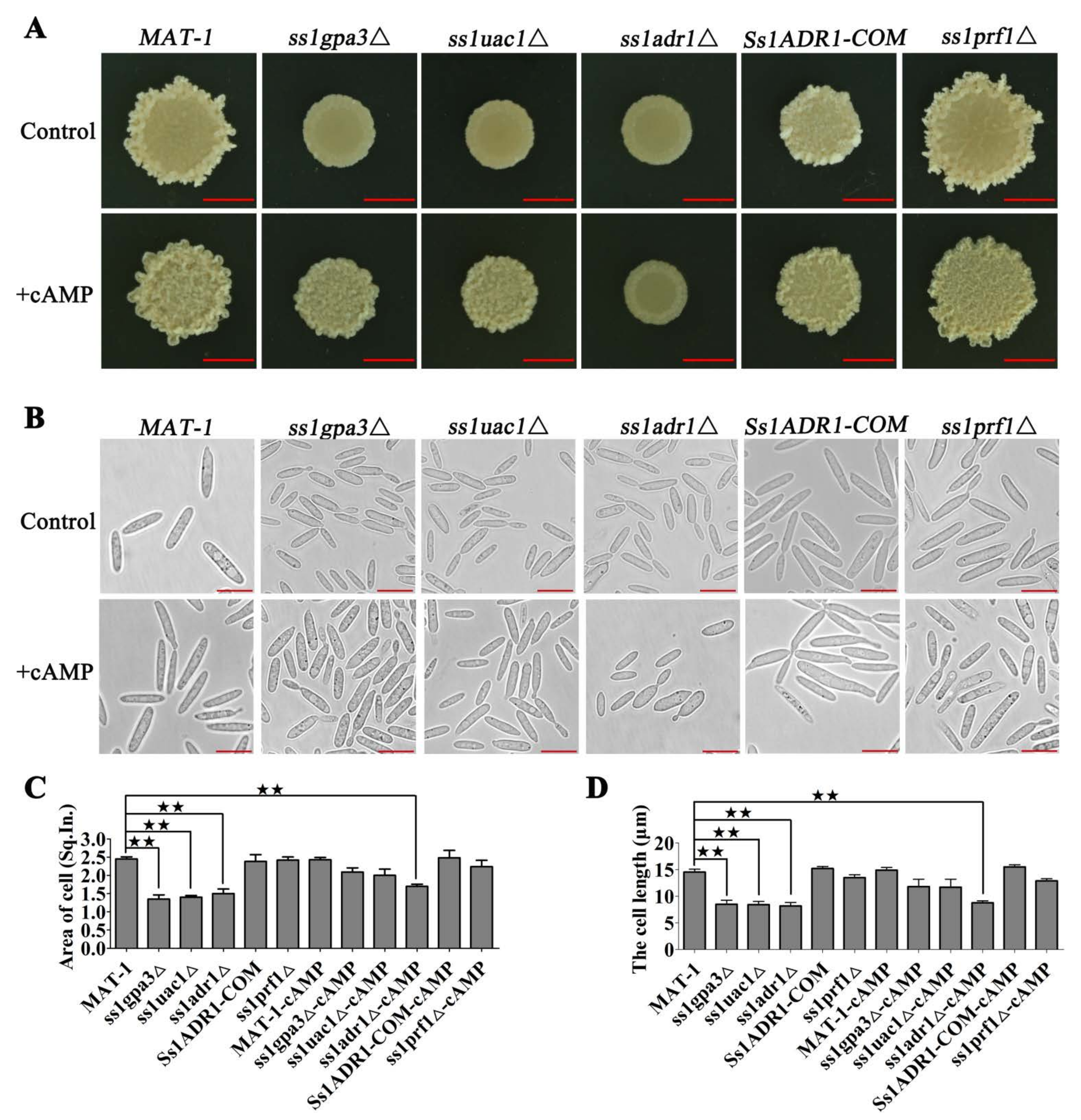
3.1. cAMP/PKA Kinase SsAdr1 Is a Key Factor in Controlling Haploid Cell Size of S. scitamineum
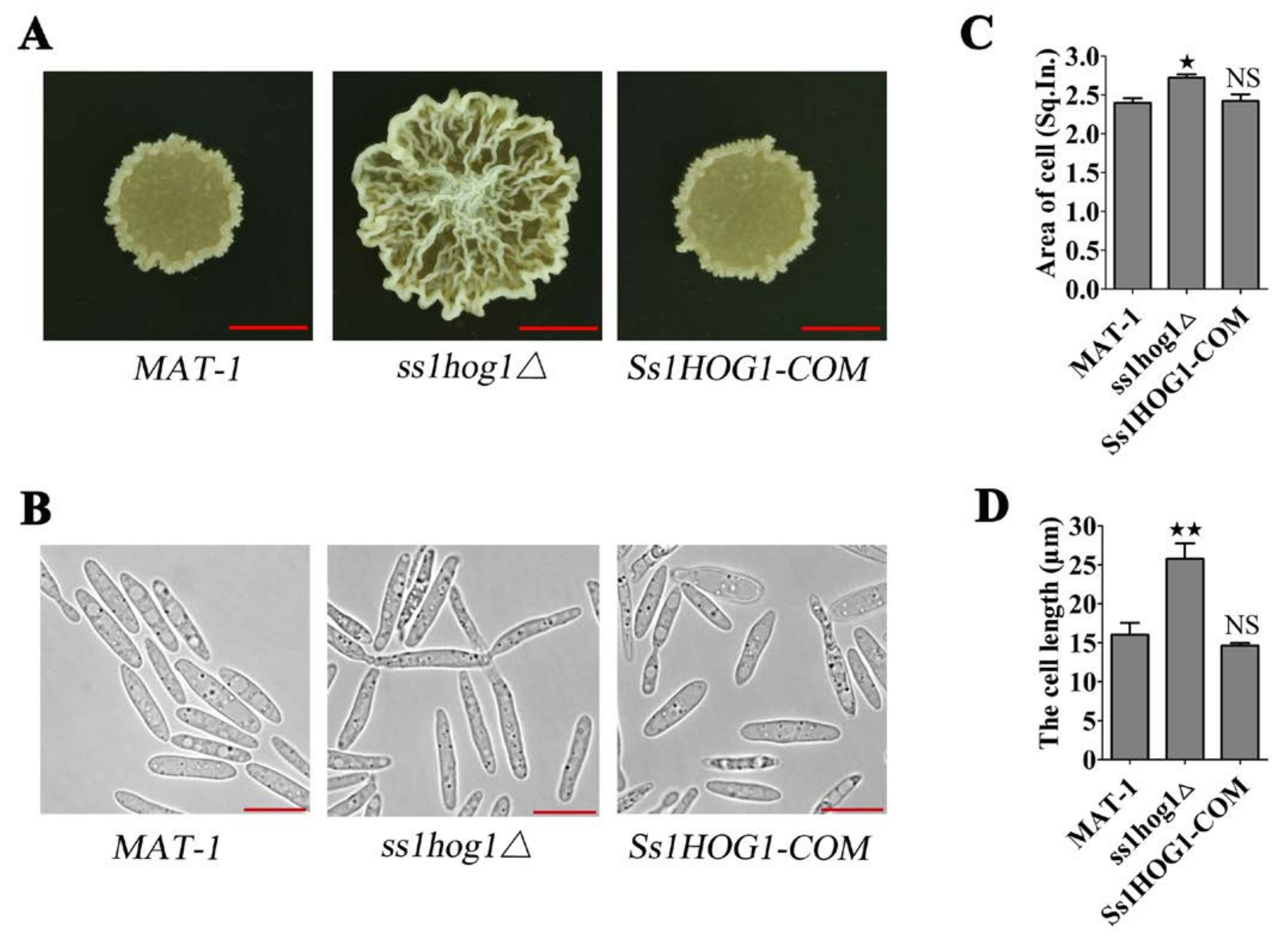
3.2. MAP Kinase SsHog1 Is Required for Maintaining Haploid Cell Morphology of S. scitamineum
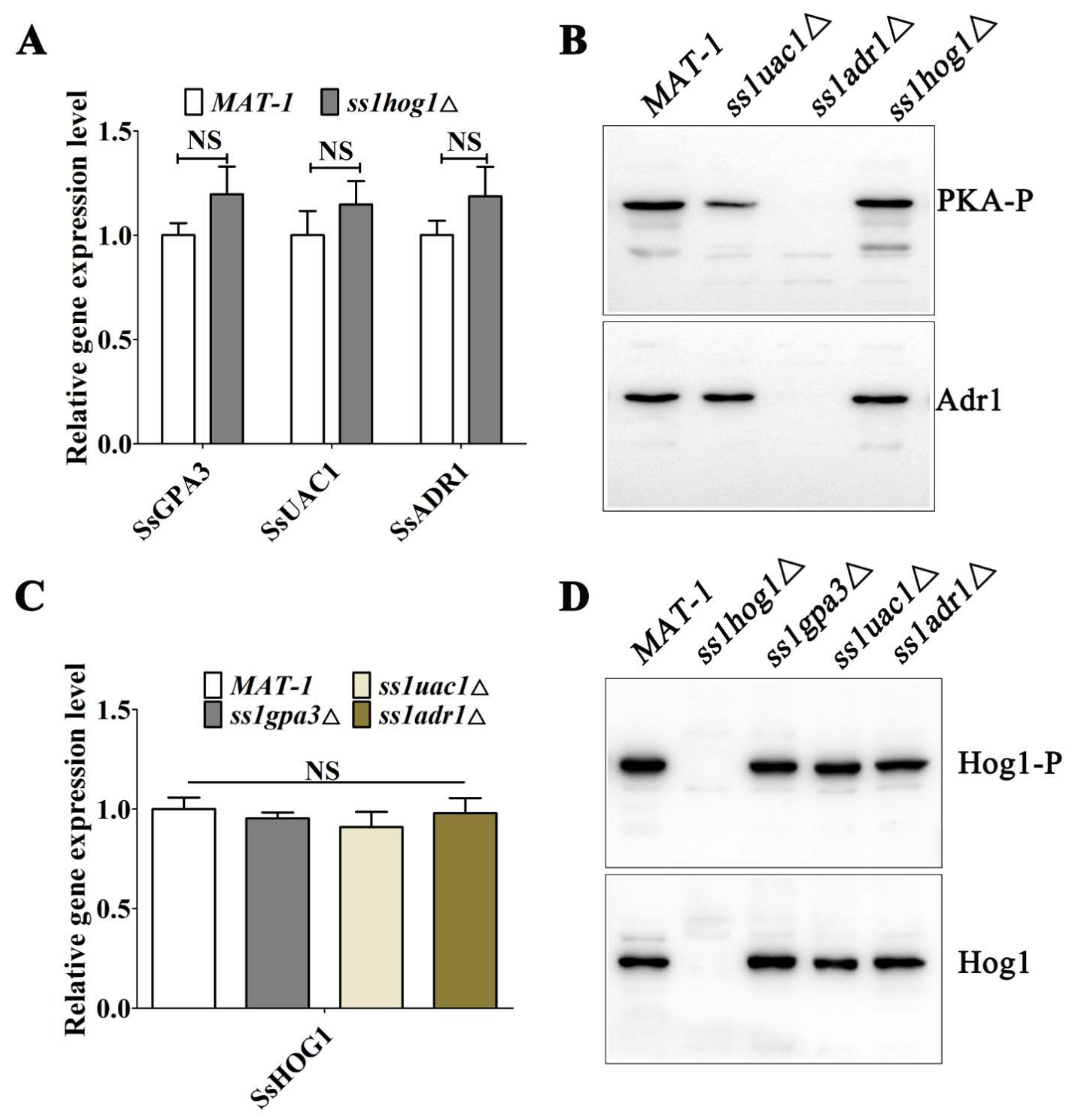
3.3. The Deletions of SsADR1 and SsHOG1 Do Not Affect Expression of Each Other in S. scitamineum
3.4. Deletion of SsHOG1 Recovers the Morphology of cAMP/PKA Defective Mutants
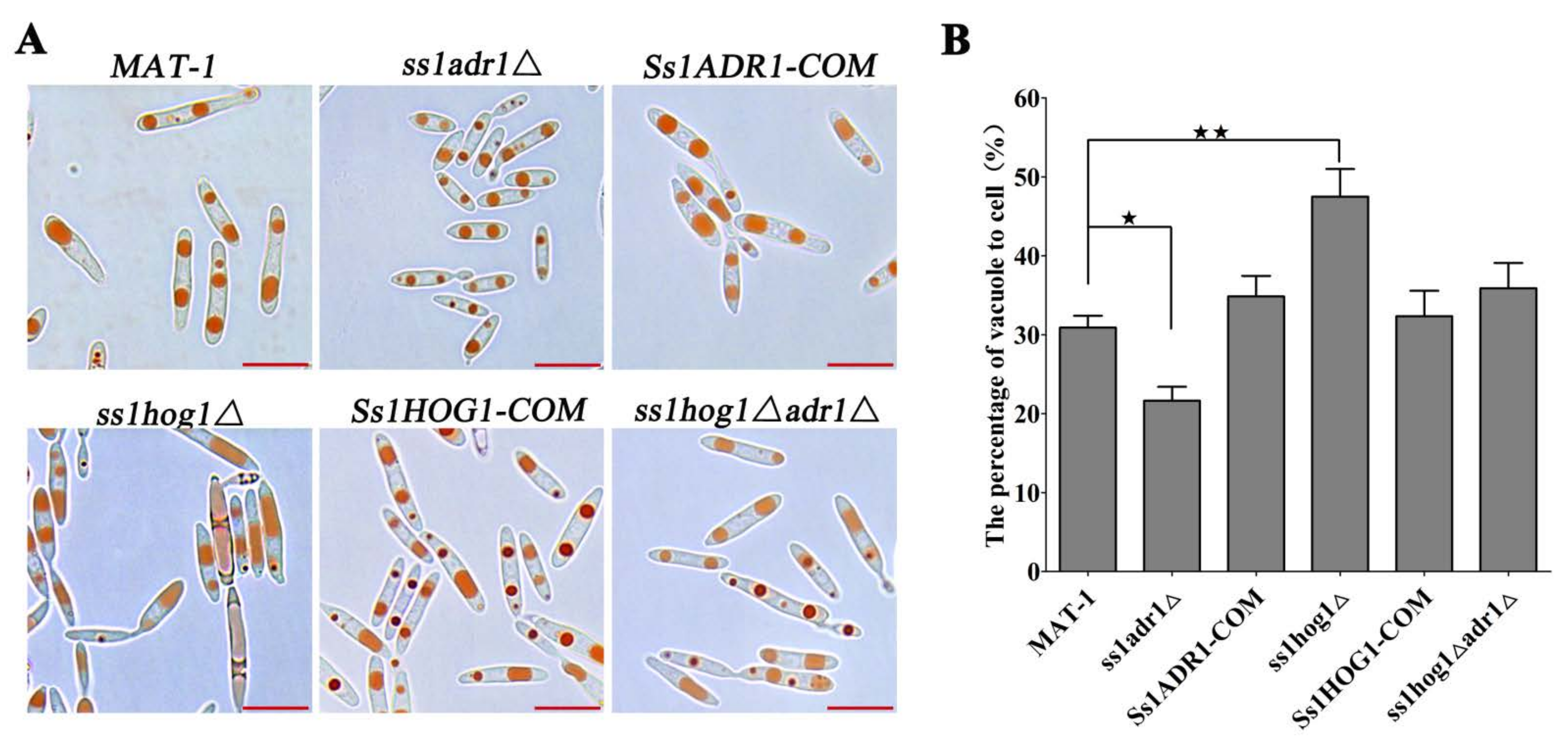
3.5. The SsHog1 and SsAdr1 Collaborate for Haploid Cell Morphology by Controlling Vacuole Size in S. scitamineum
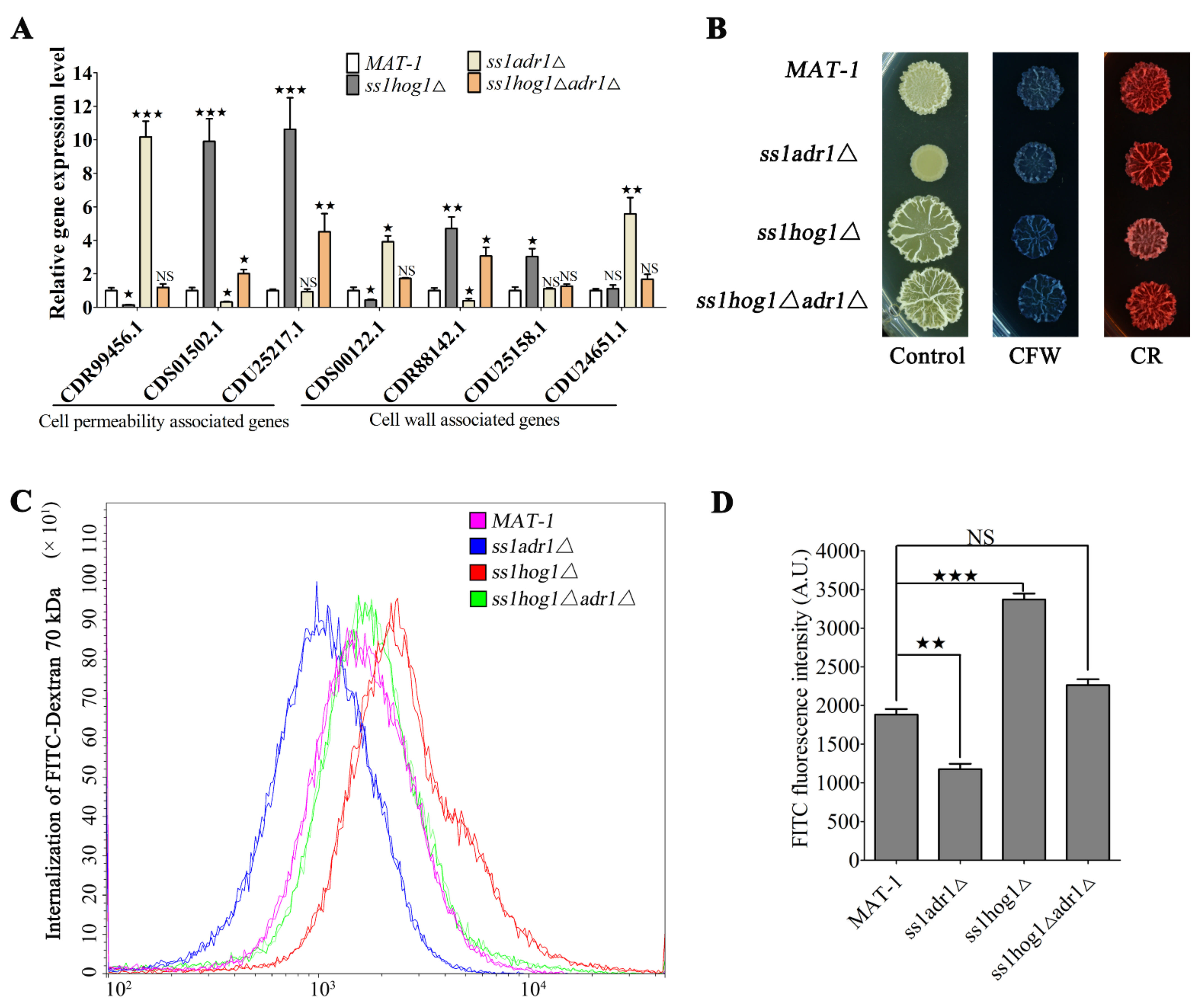
3.6. SsHog1 and SsAdr1 Regulate Cell Wall Permeability to Maintain Cell Size
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dürrenberger, F.; Wong, K.; Kronstad, J.W. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 1998, 95, 5684–5689. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, I.M.; Drake, T.; Chapuis, A.; Zhou, X.; Correia, J.; Taylor-Smith, L.; LeGrave, N.; Rasmussen, T.; Fisher, M.C.; Bicanic, T.; et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog. 2018, 14, e1006978. [Google Scholar] [CrossRef] [PubMed]
- Maidan, M.M.; Thevelein, J.M.; Van Dijck, P. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem. Soc. Trans. 2005, 33, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Maidan, M.M.; De Rop, L.; Serneels, J.; Exler, S.; Rupp, S.; Tournu, H.; Thevelein, J.M.; Van Dijck, P. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 2005, 16, 1971–1986. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, D.; Ortiz-Castellanos, L.; Robledo-Briones, M.; León-Ramírez, C.G. Molecular Mechanisms Involved in the Multicellular Growth of Ustilaginomycetes. Microorganisms 2020, 8, 1072. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/Protein Kinase a Pathway Regulates Virulence and Adaptation to Host Conditions in Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef]
- Pan, X.; Harashima, T.; Heitman, J. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 2000, 3, 567–572. [Google Scholar] [CrossRef]
- Thevelein, J.M.; Cauwenberg, L.; Colombo, S.; De Winde, J.H.; Donation, M.; Dumortier, F.; Kraakman, L.; Lemaire, K.; Ma, P.; Nauwelaers, D.; et al. Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzym. Microb. Technol. 2000, 26, 819–825. [Google Scholar] [CrossRef]
- Amigoni, L.; Colombo, S.; Belotti, F.; Alberghina, L.; Martegani, E. The transcription factor Swi4 is target for PKA regulation of cell size at the G1 to S transition in Saccharomyces cerevisiae. Cell Cycle 2015, 14, 2429–2438. [Google Scholar] [CrossRef]
- Cheetham, J.; Smith, D.A.; da Silva Dantas, A.; Doris, K.S.; Patterson, M.J.; Bruce, C.R.; Quinn, J. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol. Biol. Cell 2007, 18, 4603–4614. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2020, 23, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.L.; Gustin, M.C. Hog1: 20 years of discovery and impact. Sci. Signal. 2014, 7, re7. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, J.; MacCallum, D.M.; Doris, K.S.; da Silva Dantas, A.; Scorfield, S.; Odds, F.; Smith, D.A.; Quinn, J. MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J. Biol. Chem. 2011, 286, 42002–42016. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclère, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; García-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Kojima, K.; Cox, G.M.; Heitman, J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 2005, 16, 2285–2300. [Google Scholar] [CrossRef]
- Gold, S.; Duncan, G.; Barrett, K.; Kronstad, J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994, 8, 2805–2816. [Google Scholar] [CrossRef]
- Regenfelder, E.; Spellig, T.; Hartmann, A.; Lauenstein, S.; Bölker, M.; Kahmann, R. G proteins in Ustilago maydis: Transmission of multiple signals? EMBO J. 1997, 16, 1934–1942. [Google Scholar] [CrossRef]
- Que, Y.; Xu, L.; Wu, Q.; Liu, Y.; Ling, H.; Liu, Y.; Zhang, Y.; Guo, J.; Su, Y.; Chen, J.; et al. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genom. 2014, 15, 996. [Google Scholar] [CrossRef]
- Yan, M.; Dai, W.; Cai, E.; Yi, Z.D.; Chang, C.; Jiang, Z.; Zhang, L.H. Transcriptome analysis of Sporisorium scitamineum reveals critical environmental signals for fungal sexual mating and filamentous growth. BMC Genom. 2016, 17, 354. [Google Scholar] [CrossRef]
- Chang, C.; Cai, E.; Deng, Y.Z.; Mei, D.; Qiu, S.; Chen, B.; Zhang, L.H.; Jiang, Z. cAMP/PKA signalling pathway regulates redox homeostasis essential for Sporisorium scitamineum mating/filamentation and virulence. Environ. Microbiol. 2019, 21, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.; Sun, S.; Deng, Y.; Huang, P.; Sun, X.; Wang, Y.; Chang, C.; Jiang, Z. Histidine Kinase Sln1 and cAMP/PKA Signaling Pathways Antagonistically Regulate Sporisorium scitamineum Mating and Virulence via Transcription Factor Prf1. J. Fungi 2021, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.; Pérez-Martín, J. The crk1 gene encodes an Ime2-related protein that is required for morphogenesis in the plant pathogen Ustilago maydis. Mol. Microbiol. 2003, 47, 729–743. [Google Scholar] [CrossRef]
- Fanning, S.; Xu, W.; Beaurepaire, C.; Suhan, J.P.; Nantel, A.; Mitchell, A.P. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol. Microbiol. 2012, 86, 284–302. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Monge, R.; Navarro-García, F.; Molero, G.; Diez-Orejas, R.; Gustin, M.; Pla, J.; Sánchez, M.; Nombela, C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 1999, 181, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.A.; Selvaggini, S.; de Bruijn, I.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.; Gow, N.A. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007, 63, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.; Li, L.; Deng, Y.; Sun, S.; Jia, H.; Wu, R.; Zhang, L.; Jiang, Z.; Chang, C. MAP kinase Hog1 mediates a cytochrome P450 oxidoreductase to promote the Sporisorium scitamineum cell survival under oxidative stress. Environ. Microbiol. 2021, 23, 3306–3317. [Google Scholar] [CrossRef]
- Bruder Nascimento, A.C.; Dos Reis, T.F.; de Castro, P.A.; Hori, J.I.; Bom, V.L.; de Assis, L.J.; Ramalho, L.N.; Rocha, M.C.; Malavazi, I.; Brown, N.A.; et al. Mitogen activated protein kinases SakA(HOG1) and MpkC collaborate for Aspergillus fumigatus virulence. Mol. Microbiol. 2016, 100, 841–859. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Herman, P.K.; Emr, S.D. The fungal vacuole: Composition, function, and biogenesis. Microbiol. Rev. 1990, 54, 266–292. [Google Scholar] [CrossRef]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. Biochim. Et Biophys. Acta 2009, 1793, 650–663. [Google Scholar] [CrossRef]
- Richards, A.; Veses, V.; Gow, N.A.R. Vacuole dynamics in fungi. Fungal Biol. Rev. 2010, 24, 93–105. [Google Scholar] [CrossRef]
- Veses, V.; Gow, N.A. Vacuolar dynamics during the morphogenetic transition in Candida albicans. FEMS Yeast Res. 2008, 8, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W.S. Vacuoles and the fungal lifestyle. Mycologist 2002, 16, 10–20. [Google Scholar] [CrossRef]
- Löfke, C.; Dünser, K.; Scheuring, D.; Kleine-Vehn, J. Auxin regulates SNARE-dependent vacuolar morphology restricting cell size. eLife 2015, 4, e05868. [Google Scholar] [CrossRef]
- Dünser, K.; Gupta, S.; Herger, A.; Feraru, M.I.; Ringli, C.; Kleine-Vehn, J. Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 2019, 38, e100353. [Google Scholar] [CrossRef]
- Kaiser, S.; Eisa, A.; Kleine-Vehn, J.; Scheuring, D. NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 4752. [Google Scholar] [CrossRef]
- Scheuring, D.; Löfke, C.; Krüger, F.; Kittelmann, M.; Eisa, A.; Hughes, L.; Smith, R.S.; Hawes, C.; Schumacher, K.; Kleine-Vehn, J. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl. Acad. Sci. USA 2016, 113, 452–457. [Google Scholar] [CrossRef]
- Chadwick, W.L.; Biswas, S.K.; Bianco, S.; Chan, Y.M. Non-random distribution of vacuoles in Schizosaccharomyces pombe. Phys. Biol. 2020, 17, 065004. [Google Scholar] [CrossRef]
- Chan, Y.H.; Reyes, L.; Sohail, S.M.; Tran, N.K.; Marshall, W.F. Organelle Size Scaling of the Budding Yeast Vacuole by Relative Growth and Inheritance. Curr. Biol. 2016, 26, 1221–1228. [Google Scholar] [CrossRef]
- Jin, N.; Jin, Y.; Weisman, L.S. Early protection to stress mediated by CDK-dependent PI3,5P(2) signaling from the vacuole/lysosome. J. Cell Biol. 2017, 216, 2075–2090. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, G.; Lin, S.; Xian, X.; Chang, C.; Xi, P.; Shen, W.; Huang, W.; Cai, E.; Jiang, Z. The mating-type locus b of the sugarcane smut Sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genet. Biol. Fg. B 2015, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Deng, Y.; Cai, E.; Yan, M.; Cui, G.; Wang, Z.; Zou, C.; Zhang, B.; Xi, P.; Chang, C.; et al. Identification and Functional Analysis of the Pheromone Response Factor Gene of Sporisorium scitamineum. Front. Microbiol. 2019, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Mei, D.; Zhang, X.; Sun, X.; Li, L.; Wu, R.; Deng, Y.; Jiang, Z.; Chang, C. A gene knockout method based on protoplast transformation with two PCR fragments in Sporisorium scitamineum. Mycosystema 2021, 39, 1–14. [Google Scholar] [CrossRef]
- Góesneto, A.; Loguercioleite, C.; Guerrero, R.T. DNA extraction from frozen field-collected and dehydrated herbarium fungal basidiomata: Performance of SDS and CTAB-based methods. Biotemas 2005, 18, 19–32. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Scheuring, D. To Lead or to Follow: Contribution of the Plant Vacuole to Cell Growth. Front. Plant Sci. 2020, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, I.; Öllinger, K.; Appelqvist, H. Analysis of Lysosomal pH by Flow Cytometry Using FITC-Dextran Loaded Cells. Methods Mol. Biol. 2017, 1594, 179–189. [Google Scholar] [CrossRef]
- Skalhegg, B.S.; Tasken, K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front. Biosci. A J. Virtual Libr. 2000, 5, D678–D693. [Google Scholar]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a Pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef]
- Jones, A.R.; Band, L.R.; Murray, J.A.H. Double or Nothing? Cell Division and Cell Size Control. Trends Plant Sci. 2019, 24, 1083–1093. [Google Scholar] [CrossRef]
- Veitia, R.A. DNA Content, Cell Size, and Cell Senescence. Trends Biochem. Sci. 2019, 44, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Donlin, M.J.; Upadhya, R.; Gerik, K.J.; Lam, W.; VanArendonk, L.G.; Specht, C.A.; Sharma, N.K.; Lodge, J.K. Cross talk between the cell wall integrity and cyclic AMP/protein kinase A pathways in Cryptococcus neoformans. mBio 2014, 5, e01573-14. [Google Scholar] [CrossRef] [PubMed]
- D’Ario, M.; Sablowski, R. Cell Size Control in Plants. Annu. Rev. Genet. 2019, 53, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Bloom, A.L.; Panepinto, J.C. Opposing PKA and Hog1 signals control the post-transcriptional response to glucose availability in Cryptococcus neoformans. Mol. Microbiol. 2016, 102, 306–320. [Google Scholar] [CrossRef]

| Strains | Accession Number for Protein | Genotype (Resistance) | Reference or Source |
|---|---|---|---|
| MAT-1 | wild type | a1, b1 | [41] |
| ss1gpa3Δ | CDU22142.1 | a1, b1 Δgpa3 (HYGR) | [21] |
| ss1uac1Δ | CDU22142.1 | a1, b1 Δuac1 (HYGR) | [21] |
| ss1adr1Δ | CDU22142.1 | a1, b1 Δadr1 (HYGR) | [21] |
| Ss1ADR1-COM | CDU22142.1 | a1, b1 Δadr1 adr1(ZEOR) | [21] |
| ss1prf1Δ | CDU21933.1 | a1, b1 Δprf1 (HYGR) | [42] |
| ss1hog1Δ | CDU21933.1 | a1, b1 Δhog1 (HYGR) | [27] |
| Ss1HOG1-COM | CDU21933.1 | a1, b1 Δhog1 hog1 (ZEOR) | [27] |
| ss1hog1Δgpa3Δ | CDU22378.1 | a1, b1 Δhog1 Δgpa3 (HYGR ZEOR) | This study |
| ss1hog1Δuac1Δ | CDU25762.1 | a1, b1 Δhog1 Δuac1 (HYGR ZEOR) | This study |
| ss1hog1Δadr1Δ | CDU22361.1 | a1, b1 Δhog1 Δadr1 (HYGR ZEOR) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, E.; Yan, M.; Sun, X.; Zeng, R.; Zheng, W.; Deng, Y.; Jiang, Z.; Chang, C. Kinase Hog1 and Adr1 Opposingly Regulate Haploid Cell Morphology by Controlling Vacuole Size in Sporisorium scitamineum. J. Fungi 2022, 8, 865. https://doi.org/10.3390/jof8080865
Cai E, Yan M, Sun X, Zeng R, Zheng W, Deng Y, Jiang Z, Chang C. Kinase Hog1 and Adr1 Opposingly Regulate Haploid Cell Morphology by Controlling Vacuole Size in Sporisorium scitamineum. Journal of Fungi. 2022; 8(8):865. https://doi.org/10.3390/jof8080865
Chicago/Turabian StyleCai, Enping, Meixin Yan, Xian Sun, Rong Zeng, Wenqiang Zheng, Yizhen Deng, Zide Jiang, and Changqing Chang. 2022. "Kinase Hog1 and Adr1 Opposingly Regulate Haploid Cell Morphology by Controlling Vacuole Size in Sporisorium scitamineum" Journal of Fungi 8, no. 8: 865. https://doi.org/10.3390/jof8080865






