Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance
Abstract
:1. Introduction
2. S. chartarum Enzymes and Their Possible Applications
2.1. Laccases
2.2. Mannanases
2.3. β-Glucanases
2.4. Fucoidanases and Alginate Lyases
3. Secondary Metabolites from S. chartarum
3.1. Phenylspirodrimanes
3.2. Trichothecenes
3.3. Isoindoline derivatives
3.4. Atranones and Dolabellane Diterpenoids
3.5. Chromenes
3.6. Cochlioquinones
3.7. Xanthones
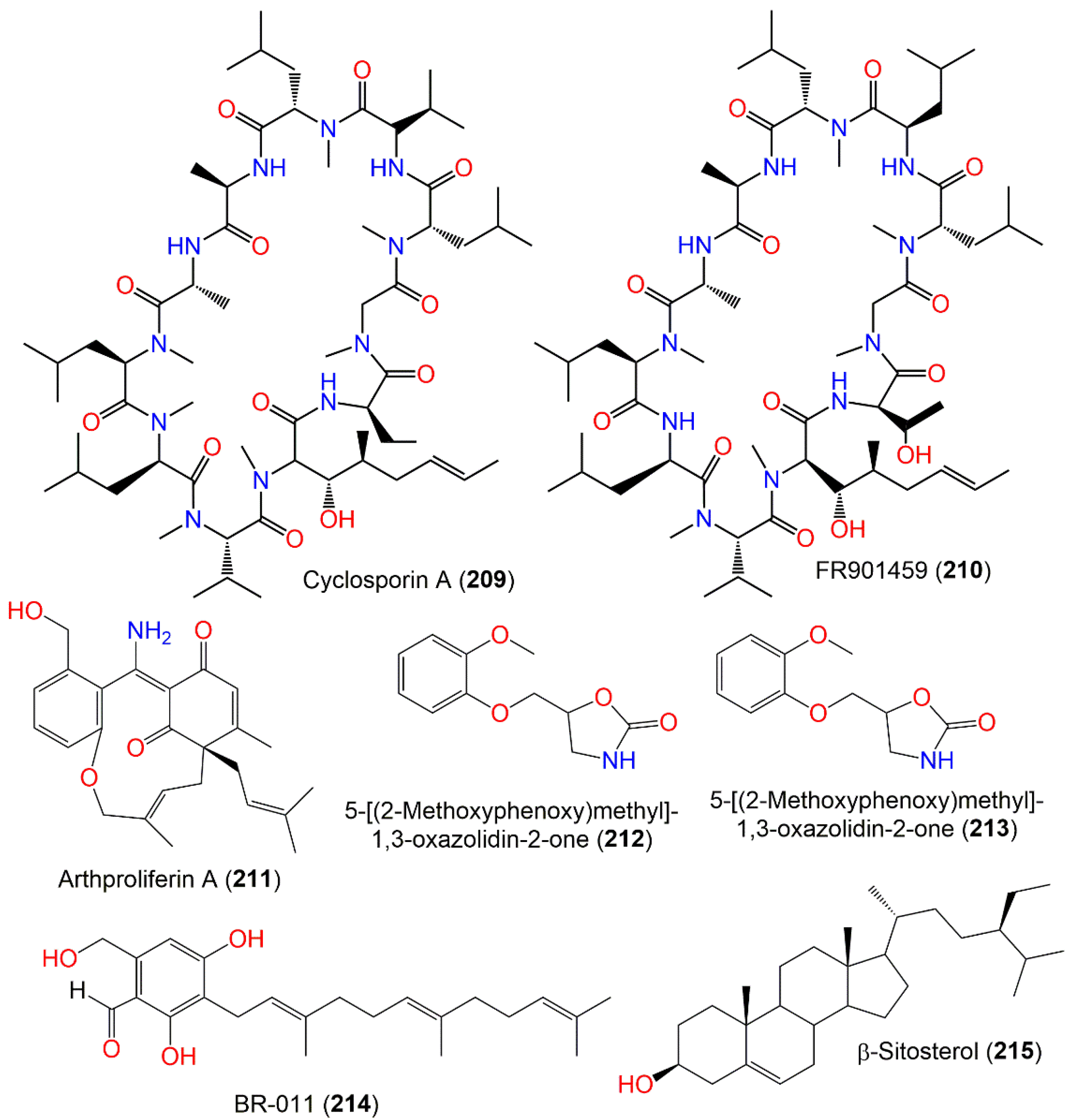
3.8. Cyclosporins
3.9. Other metabolites
4. Extract Bioactivities and Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-549 | Human lung cancer cell line |
| A2780 | Human ovarian cancer cell line |
| BGC823 | Human gastric carcinoma cell line |
| BV2 | Microglia cell line |
| C4-2B | Human prostatic carcinoma cell line |
| CCR5 | Chemokine (C-C motif) receptor 5 |
| CCK-8 | Cell counting Kit 8 |
| CD | Circular dichroism |
| ConA | Concanavalin A |
| CH2Cl2 | Dichloromethane |
| CHO | Chinese hamster ovary cell line |
| CsA | Cyclosporin A |
| DRG | Dorsal root ganglia |
| EC50 | The concentration (or dose) effective in producing 50% of the maximal response |
| ECD | Electronic circular dichroism |
| EtOAc | Ethyl acetate |
| FGFR3 | Fibroblast growth factor receptor 3 |
| GC-MS | Gas chromatography–mass spectrometry |
| HCT-116 | Human colorectal carcinoma cell line |
| HepG2 | Human hepatic cancer cell line |
| HIV | human immunodeficiency virus |
| HL-60 | Human myeloid leukemia cell line: |
| HPLC | High performance liquid chromatography |
| IFN-γ | Interferon γ |
| IGF1R | Insulin-like growth factor 1 receptor |
| IL-2 | Interleukin 2 |
| MCF-7 | Human breast cancer cell line |
| MDAMB-231 | Human triple-negative breast cancer |
| MDA-MB-468 | Epithelial, human breast cancer cell line |
| MG-63 | Human osteosarcoma cell line |
| MGC803 | Human gastric cancer cell line |
| MIC | minimum inhibitory concentrations |
| MIP-1α | Macrophage inflammatory protein-1 Alpha |
| MLR | One-way mixed lymphocyte reaction |
| mRNA | Messenger ribonucleic acid |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| MVOCs | Microbial volatile organic compounds |
| NCI-H460 | Human lung carcinoma cell line |
| NCI-H1655/1650 | Non-small-cell lung adenocarcinoma |
| NRTIs | Nucleoside reverse transcriptase inhibitors |
| RP-18 | Rephrased phase-18 |
| PAP-1 | (5-(4-Phenoxybutoxy)psoralen) |
| PC-12 | Human pheochromocytoma neuronal cell line |
| PDGFRb | β-Type platelet-derived growth factor receptor |
| SK-N-SH | Human neuroblastoma cell line |
| SiO2CC | Silica gel column chromatography |
| SMMC-7721 | Human hepatocellular carcinoma cell line |
| SW480 | Human colon cancer cell line |
| U-2OS | Human osteosarcoma cell line |
| TC | Total cholesterol |
| TG | Total glyceride |
| Tie2 | Tyrosine-protein kinase receptor |
| TRKB | Tropomyosin receptor kinase B |
| VSV-G | Vesicular stomatitis virus G protein |
| WT | Cell Division Cycle 37 |
References
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Bright side of Fusarium oxysporum: Secondary metabolites bioactivities and industrial relevance in biotechnology and nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Altyar, A.E.; Mohamed, S.G.A.; Mohamed, G.A. Genus Thielavia: Phytochemicals, industrial importance and biological relevance. Nat. Prod. Res. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Altyar, A.E.; Mohamed, G.A. Natural products of the fungal genus Humicola: Diversity, biological activity, and industrial importance. Curr. Microbiol. 2021, 78, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Bagalagel, A.A.; Diri, R.M.; Noor, A.O.; Bakhsh, H.T.; Muhammad, Y.A.; Mohamed, G.A.; Omar, A.M. Exploring the activity of fungal phenalenone derivatives as potential CK2 inhibitors using computational methods. J. Fungi 2022, 8, 443. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped potential of marine–associated Cladosporium species: An overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 2021, 19, 645. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Qiao, X.G.; Miao, C.P.; Liu, K.; Chen, Y.W.; Xu, L.H.; Zhao, L.X. Diversity, distribution and biotechnological potential of endophytic fungi. Ann. Microbiol. 2016, 66, 529–542. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; Elkhayat, E.S.; Al Musayeib, N.M.; Asfour, H.Z.; Zayed, M.F.; Mohamed, G.A. Fusaripeptide A: New antifungal and anti-malarial cyclodepsipeptide from the endophytic fungus Fusarium sp. J. Asian Nat. Prod. Res. 2018, 20, 75–85. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Fungal depsides–naturally inspiring molecules: Biosynthesis, structural characterization, and biological activities. Metabolites 2021, 11, 683. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Eshmawi, B.A.; Mohamed, S.G.A.; Mohamed, G.A. Fungal naphthalenones; promising metabolites for drug discovery: Structures, biosynthesis, sources, and pharmacological potential. Toxins 2022, 14, 154. [Google Scholar] [CrossRef]
- Noor, A.O.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 2020, 25, 395. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.M.; Mohamed, G.A.; Ibrahim, S.R.M. Chaetomugilins and chaetoviridins–promising natural metabolites: Structures, separation, characterization, biosynthesis, bioactivities, molecular docking, and molecular dynamics. J. Fungi 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El–Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef] [PubMed]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive secondary metabolites from endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Beekman, A.M.; Barrow, R.A. Fungal metabolites as pharmaceuticals. Aust. J. Chem. 2014, 67, 827–843. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 2011, 50, 3–19. [Google Scholar] [CrossRef]
- Hoeksma, J.; Misset, T.; Wever, C.; Kemmink, J.; Kruijtzer, J.; Versluis, K.; Liskamp, R.M.J.; Boons, G.J.; Heck, A.J.R.; Boekhout, T.; et al. A New perspective on fungal metabolites: Identification of bioactive compounds from fungi using Zebrafish Embryogenesis as read–out. Sci. Rep. 2019, 9, 17546. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A Rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti–malarial agents from endophytic fungi: A Review. Mini Rev. Med. Chem. 2018, 18, 1110–1132. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; Zayed, M.F.; El-Kholy, A.A.; Elkhayat, E.S.; Ross, S.A. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium Chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorg. Med. Chem. 2018, 26, 786–790. [Google Scholar] [CrossRef]
- Hodgson, M.J.; Morey, P.; Leung, W.Y.; Morrow, L.; Miller, D.; Jarvis, B.B.; Robbins, H.; Halsey, J.F.; Storey, E. Building-associated pulmonary disease from exposure to stachybotrys Chartarum and Aspergillus versicolor. J. Occup. Environ. Med. 1998, 40, 241–249. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Ghannoum, M.A. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: Infectious disease perspective. Clin. Microbio. Rev. 2003, 16, 144–172. [Google Scholar] [CrossRef] [Green Version]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.; Hyten, A.S.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johanning, E.; Biagini, R.; Hull, D.; Morey, P.; Jarvis, B.; Landsbergis, P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water–damaged office environment. Int. Arch. Occup. Environ. Health 1996, 68, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Dearborn, D.G.; Smith, P.G.; Dahms, B.B.; Allan, T.M.; Sorenson, W.G.; Montana, E.; Etzel, R.A. Clinical profile of 30 infants with acute pulmonary hemorrhage in Cleveland. Pediatrics 2002, 110, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Copeland, L.B.; Doerfler, D.L.; Ward, M.D.W. The relative allergenicity of Stachybotrys chartarum compared to house dust mite extracts in a mouse model. Inhal. Toxicol. 2010, 22, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Köck, J.; Gottschalk, C.; Ulrich, S.; Schwaiger, K.; Gareis, M.; Niessen, L. Rapid and selective detection of macrocyclic trichothecene producing Stachybotrys chartarum strains by loop–mediated isothermal amplification (LAMP). Anal. Bioanal. Chem. 2021, 413, 4801–4813. [Google Scholar] [CrossRef]
- Vesper, S.J.; Magnuson, M.L.; Dearborn, D.G.; Yike, I.; Haugland, R.A. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect. Immun. 2001, 69, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, B.B. Chemistry and toxicology of molds isolated from water damaged buildings. In Mycotoxins and Food Safety; DeVries, J.W., Trucksess, M.W., Jackson, L.S., Eds.; Kluver Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 43–52. [Google Scholar]
- Yike, I.; Rand, T.; Dearborn, D.G. The Role of fungal proteinases in pathophysiology of Stachybotrys chartarum. Mycopathologia 2007, 164, 171–181. [Google Scholar] [CrossRef]
- Pestka, J.J.; Yike, I.; Dearborn, D.G.; Ward, M.D.W.; Harkema, J.R. Stachybotrys chartarum, trichothecene mycotoxins, and damp building–related illness: New insights into a public health enigma. Toxicol. Sci. 2008, 104, 4–26. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.D.; Rand, T.G.; Jarvis, B.B. Stachybotrys chartarum: Cause of human disease or media darling? Med. Mycol. 2003, 41, 271–291. [Google Scholar] [CrossRef] [Green Version]
- Al-Ahmad, M.; Manno, M.; Ng, V.; Ribeiro, M.; Liss, G.M.; Tarlo, S.M. Symptoms after mould exposure including Stachybotrys chartarum, and comparison with darkroom disease. Allergy 2010, 65, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Jain, S.; Pareek, A. Estimation of possible biodegradation of polythene by fungal isolates growing on polythene debris. Pollution 2022, 8, 567–577. [Google Scholar]
- Andersen, B.; Poulsen, R.; Hansen, G.H. Cellulolytic and xylanolytic activities of common indoor fungi. Int. Biodeter. Biodegr. 2016, 107, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Noreen, N.; Ramzan, N.; Perveen, Z.; Shahzad, S. Assessing the enzymatic activities of compost associated mesophilic, thermotolerant and thermophilic bacteria and fungi. Int. J. Biol. Biotech. 2018, 15, 815–825. [Google Scholar]
- Moharram, A.M.; Zohri, A.A.; Hussein, D.A. Cellulase and Xylanase Production by Sugarcane Bagasse Mycobiota. Egypt. Sugar J. 2021, 16, 41–76. [Google Scholar] [CrossRef]
- Kordula, T.; Banbula, A.; Macomson, J.; Travis, J. Isolation and properties of stachyrase A, a chymotrypsin–like serine proteinase from Stachybotrys chartarum. Infect. Immun. 2002, 70, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Sindi, I.A.; Gamal, A.M. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): An Updated review from 2016 to 2021. Mycol. Prog. 2021, 20, 595–639. [Google Scholar] [CrossRef]
- Sousa, A.C.; Martins, L.O.; Robalo, M.P. Laccases: Versatile biocatalysts for the synthesis of heterocyclic cores. Molecules 2021, 26, 3719. [Google Scholar] [CrossRef]
- Crestini, C.; Jurasek, L.; Argyropoulos, D.S. On the mechanism of the laccase–mediator system in the oxidation of lignin. Chemistry 2003, 9, 5371–5378. [Google Scholar] [CrossRef]
- Mander, G.J.; Wang, H.; Bodie, E.; Wagner, J.; Vienken, K.; Vinuesa, C.; Foster, C.; Leeder, A.C.; Allen, G.; Hamill, V. Use of laccase as a novel, versatile reporter system in filamentous fungi. Appl. Environ. Microbiol. 2006, 72, 5020–5026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, G.G.; Baldwin, T.M.; Winetzky, D.S.; Tierney, L.M.; Wang, H.; Murray, C.J. Selective targeting of a laccase from Stachybotrys chartarum covalently linked to a carotenoid–binding peptide. J. Pept. Res. 2004, 64, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, C.; Liu, W.; Zheng, Y.; Chen, C.; Liang, A.; Luo, X.; Li, Z.; Ma, W.; Song, Y.; et al. Functional and structural investigation of a novel Β–mannanase BaMan113A from Bacillus sp. N16-5. Int. J. Biol. Macromol. 2021, 182, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Ganaie, M.A.; Pranaw, K.; Kango, N. Design–of–experiment strategy for the production of mannanase biocatalysts using plam karnel cake and its application to degrade Locust bean and Guar gum. Biocatal. Agric. Biotechnol. 2015, 4, 229–234. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications–A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ye, T.; Zhou, B.; Li, J.; He, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of gastric floating controlled release tablet based on Konjac glucomannan. Food Res. Int. 2015, 72, 47–53. [Google Scholar] [CrossRef]
- Huang, J.; Chen, C.; Huang, C.; Huang, T.; Wu, T.; Cheng, Y.; Ko, T.; Lin, C.; Liu, J.; Guo, R. Improving the specific activity of β–mannanase from aspergillus niger bk01 by structure–based rational design. Biochim. Biophys. Acta. 2014, 1844, 663–669. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Kango, N. Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chem. 2021, 334, 127428. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Lu, F. Expression in Aspergillus niger and characterization of β–mannanases from Stachybotrys chartarum. Wei Sheng Wu Xue Bao 2016, 56, 1242–1255. [Google Scholar]
- Chaari, F.; Chaabouni, S.E. Fungal β–1,3–1,4–glucanases: Production, proprieties and biotechnological applications. J. Sci. Food Agric. 2019, 99, 2657–2664. [Google Scholar] [CrossRef]
- Picart, P.; Goedegebuur, F.; Diaz, P.; Pastor, F.I. Expression of a novel beta–glucanase from Stachybotrys atra in bacterial and fungal hosts. Fungal Biol. 2012, 116, 443–451. [Google Scholar] [PubMed]
- Picart, P.; Pastor, F.I.J.; Orejas, M. Transcriptional analysis of the lichenase–like gene cel12A of the filamentous fungus Stachybotrys Atra BP-A and its relevance for lignocellulose depolymerization. Int. Microbiol. 2021, 24, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Shuanghong, Y.; Yongmei, H.; Bo, Z.; Bo, W.; Dawei, L.; Rihe, P.; Quanhong, Y. Expression, purification, biochemical characterization and structural modeling of an endo-b-1,4-glucanase from Stachybotrys chartarum in Pichia Pastoris. J. Pure Appl. Microbiol. 2016, 10, 1–10. [Google Scholar]
- Hongxia, W.; Huaming, W.; Dalong, Z.; Cheng, L. Heterologous expression and characterization of xylanase XYA6205 from Stachybotrys chartarum. Biotechnol.Bull. 2013, 0, 130. [Google Scholar]
- Dharani, S.R.; Srinivasan, R.; Sarath, R.; Ramya, M. Recent progress on engineering microbial alginate lyases towards their versatile role in biotechnological applications. Folia Microbiol. 2020, 65, 937–954. [Google Scholar]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate–chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; Dos Santos Vasconcelos, M.R.; Passarini, M.R.; Vieira, G.A.; Lopes, V.C.; Mainardi, P.H.; Dos Santos, J.A.; de Azevedo Duarte, L.; Otero, I.V.; da Silva Yoshida, A.M.; et al. Marine–Derived fungi: Diversity of enzymes and biotechnological applications. Front. Microbiol. 2015, 6, 269. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kusaykin, M.I.; Kurilenko, V.V.; Zakharenko, A.M.; Isakov, V.V.; Zaporozhets, T.S.; Gazha, A.K.; Zvyagintseva, T.N. Hydrolysis of fucoidan by fucoidanase isolated from the marine Bacterium, Formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef] [Green Version]
- de Vries, R.P.; Patyshakuliyeva, A.; Garrigues, S.; Agarwal-Jans, S. The Current Biotechnological Status and Potential of Plant and Algal Biomass Degrading/Modifying Enzymes from Ascomycete Fungi; Springer: Cham, Switzerland, 2020; pp. 81–120. [Google Scholar]
- Yang, B.; He, Y.; Lin, S.; Zhang, J.; Li, H.; Wang, J.; Hu, Z.; Zhang, Y. Antimicrobial dolabellanes and atranones from a marine–derived strain of the toxigenic fungus Stachybotrys chartarum. J. Nat. Prod. 2019, 82, 1923–1929. [Google Scholar] [CrossRef]
- Qin, Y.; Fang, F.; Zhou, J.; Wang, R.; Xu, S.; Li, L.; Zhang, H. Atranones from Stachybotrys chartarum and their antitumor activities in MG–63 human osteosarcoma cells. Fitoterapia 2020, 146, 104727. [Google Scholar] [CrossRef]
- Li, C.; Matsuda, Y.; Gao, H.; Hu, D.; Yao, X.S.; Abe, I. Biosynthesis of LL-Z1272β: Discovery of a new member of NRPS–like enzymes for aryl–aldehyde formation. Chembiochem 2016, 17, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Jagels, A.; Hövelmann, Y.; Zielinski, A.; Esselen, M.; Köhler, J.; Hübner, F.; Humpf, H. Stachybotrychromenes A–C: Novel cytotoxic meroterpenoids from Stachybotrys sp. Mycotoxin Res. 2018, 34, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, B.B.; Salemme, J.; Morais, A. Stachybotrys toxins. 1. Nat. Toxins 1995, 3, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with anti-HIV activity from the sponge–derived fungus Stachybotrys chartarum MXH–X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, J.; Tan, Z.; Liu, J.; Zhao, J.; Chen, R.; Xie, K.; Zhang, D.; Li, Y.; Yu, L. Stachybotrysins A–G, phenylspirodrimane derivatives from the fungus Stachybotrys chartarum. J. Nat. Prod. 2017, 80, 1819–1826. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and anti-inflammatory meroterpenoids: Stachybonoids A–F from the crinoid–derived fungus Stachybotrys cchartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef] [Green Version]
- Chunyu, W.; Ding, Z.; Li, M.; Zhao, J.; Gu, S.; Gao, Y.; Wang, F.; Ding, J.; Wen, M. Stachartins A–E, phenylspirodrimanes from the tin mine tailings-associated fungus Stachybotrys chartarum. Helv. Chim. Acta 2016, 99, 583–587. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Zhuo, F.; Gao, N.; Cheng, X.; Wang, X.; Pei, Y.; Kong, L. Seven New Cytotoxic Phenylspirodrimane Derivatives from the Endophytic Fungus Stachybotrys Chartarum. RSC Adv. 2019, 9, 3520–3531. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, M.; Zhao, J.; Jia, X.; Liu, J.; Zhang, M.; Chen, R.; Xie, K.; Chen, D.; Yu, H. Three new phenylspirodrimane derivatives with inhibitory effect towards potassium channel Kv1. 3 from the fungus Stachybotrys chartarum. J. Asian Nat. Prod. Res. 2019, 21, 887–894. [Google Scholar] [CrossRef]
- Bao, Y.R.; Feng, H.L.; Ya, X.S. Stachybotranes A–D, phenylspirodrimanes from the wetland fungus Stachybotrys chartarum with cytotoxic activities. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Yang, B.; Long, J.; Pang, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Li, Y.; Liu, Y. Structurally diverse polyketides and phenylspirodrimanes from the soft coral-associated fungus Stachybotrys chartarum SCSIO41201. J. Antibiot. 2021, 74, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A–P, phenylspirodrimanes from the sponge–associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, S.F.; Jiang, J.; Mazzola, E.P.; Jarvis, B.B. Atranones: Novel diterpenoids from the toxigenic mold Stachybotrys atra. Tetrahedron Lett. 1999, 40, 2725–2728. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Shen, Y.; Tan, Z.; Zhang, M.; Chen, R.; Zhao, J.; Zhang, D.; Yu, L.; Dai, J. Stachybotrysams A–E, prenylated isoindolinone derivatives with anti–hiv activity from the fungus Stachybotrys chartarum. Phytochem. Lett. 2017, 20, 289–294. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Vega, A.; Rivera–Sagredo, A.; Jiménez–Alfaro, M.D.; Díez, E.; Hueso-Rodríguez, J.A. Novel sesquiterpenoids as tyrosine kinase inhibitors produced by Stachybotrys chortarum. Tetrahedron 2004, 60, 2379–2385. [Google Scholar] [CrossRef]
- Ding, Z.; Ding, J.; Zhao, J.; Li, M.; Hu, D.; Jiang, X.; Gu, S.; Wang, F.; Wen, M. Phenylspirodrimane derivatives from cultures of the fungus Stachybotrys chartarum YIM DT 10079. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Feng, J.; Tan, Z.; Liu, J.; Zhang, M.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Chen, X.; et al. Bistachybotrysins A–C, three phenylspirodrimane dimers with cytotoxicity from Stachybotrys chartarum. Bioorg. Med. Chem. Lett. 2018, 28, 355–359. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, J.; Jia, X.; Zhao, J.; Liu, J.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Zhang, D. Bistachybotrysins D and E, one stereoisomeric pair of cytotoxic phenylspirodrimane dimers from Stachybotrys chartarum. Chin. Chem. Lett. 2019, 30, 435–438. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, M.; Jia, X.; Zhao, J.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Liu, J.; Dai, J. Bistachybotrysins F–J, five new phenylspirodrimane dimers with a central cyclopentanone linkage from Stachybotrys chartarum. Fitoterapia 2019, 136, 104158. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, J.; Feng, J.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Liu, J.; Dai, J. Bistachybotrysin K, one new phenylspirodrimane dimer from Stachybotrys chartarum with potent cytotoxic activity. J. Asian Nat. Prod. Res. 2020, 22, 496–502. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Zhao, J.; Feng, J.; Chen, M.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Zhang, D. Bistachybotrysins L–V, bioactive phenylspirodrimane dimers from the fungus Stachybotrys chartarum. Org. Chem. Front. 2020, 7, 531–542. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Jia, X.; Zhao, J.; Chen, R.; Xie, K.; Chen, D.; Li, Y.; Dai, J. Bistachybotrysins W–Y, three new phenylspirodrimane dimers with a 6/7 oxygen heterocycle from Stachybotrys chartarum. Phytochem. Lett. 2020, 35, 73–77. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Li, X.; Cheng, Z.; Huang, J.; Proksch, P.; Lin, W. Chartarolides A–C, novel meroterpenoids with antitumor activities. Tetrahedron Lett. 2017, 58, 1826–1829. [Google Scholar] [CrossRef]
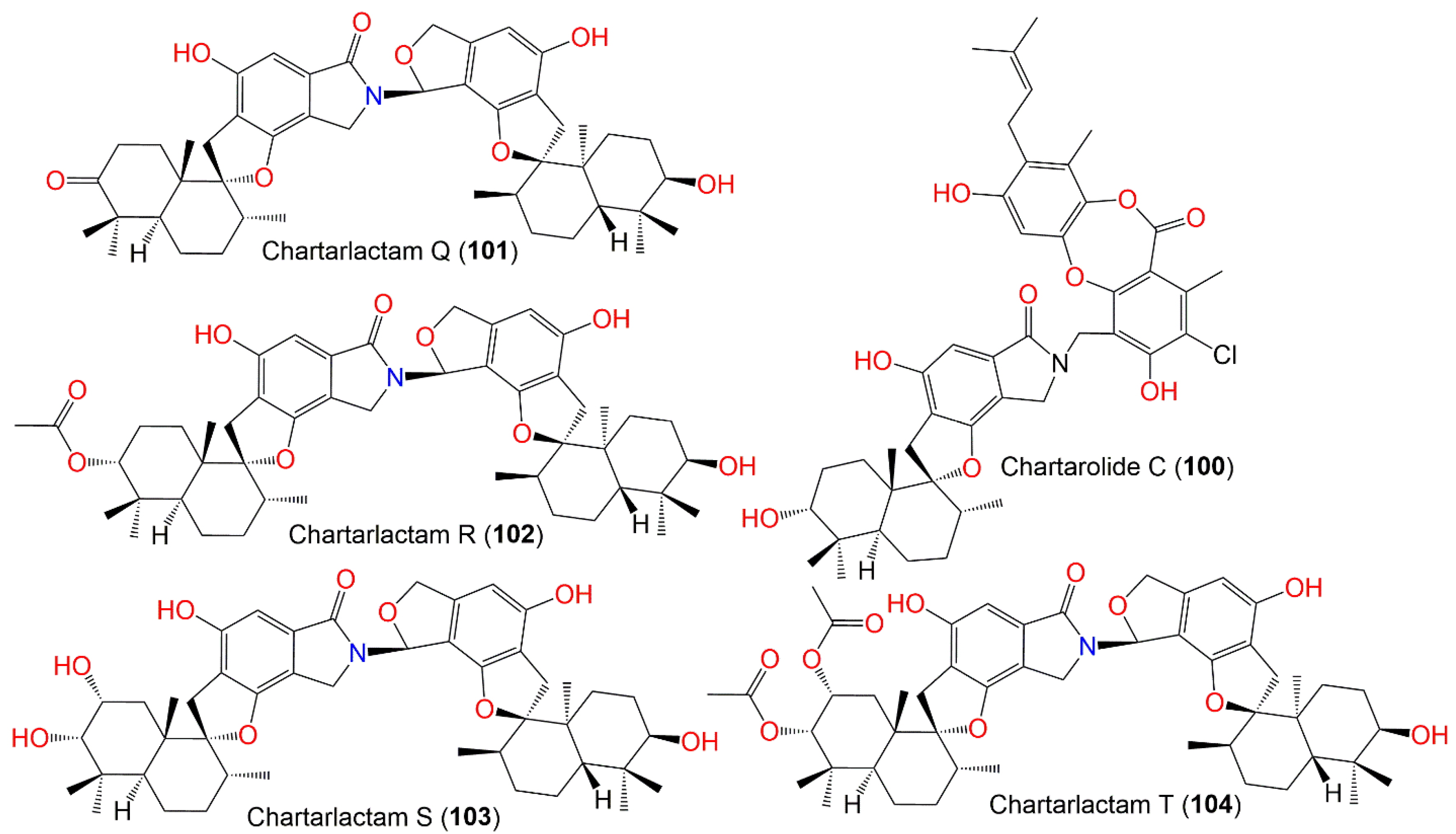
- Liu, D.; Li, Y.; Guo, X.; Ji, W.; Lin, W. Chartarlactams Q−T, dimeric phenylspirodrimanes with antibacterial and antiviral activities. Chem. Biodivers. 2020, 17, e2000170. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhao, J.; Ding, J.; Chunyu, W.; Li, M.; Gu, S.; Wang, F.; Wen, M. A Novel phenylspirodrimane dimer from cultures of the fungus Stachybotrys chartarum. Nat. Prod. Res. 2018, 32, 2370–2374. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ding, J.; Zhao, J.; Chunyu, W.; Li, M.; Gu, S.; Wang, F.; Wen, M. A New phenylspirodrimane dimer from the fungus Stachybotrys chartarum. Fitoterapia 2018, 125, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ding, J.; Zhao, J.; Chunyu, W.; Li, M.; Wang, H.; Gu, S.; Wang, F.; Wen, M. A New phenylspirodrimane dimer derivative from the tin mine tailings-associated fungus Stachybotrys chartarum. Chem. Nat. Compd. 2019, 55, 1050–1052. [Google Scholar] [CrossRef]
- Eppley, R.M.; Bailey, W.J. 12,13-Epoxy-delta 9–trichothecenes as the probable mycotoxins responsible for stachybotryotoxicosis. Science 1973, 181, 758–760. [Google Scholar] [CrossRef]
- Eppley, R.M.; Mazzola, E.P.; Highet, R.J.; Bailey, W.J. Structure of satratoxin H, a metabolite of Stachybotrys atra. Application of proton and carbon–13 nuclear magnetic resonance. J. Org. Chem. 1977, 42, 240–243. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene–type sesquiterpenes from the sponge–derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, B.B.; Sorenson, W.G.; Hintikka, E.L.; Nikulin, M.; Zhou, Y.; Jiang, J.; Wang, S.; Hinkley, S.; Etzel, R.A.; Dearborn, D. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 1998, 64, 3620–3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloem, R.J.; Smitka, T.A.; Bunge, R.H.; French, J.C.; Mazzola, E.P. Roridin L-2, a new trichothecene. Tetrahedron Lett. 1983, 24, 249–252. [Google Scholar] [CrossRef]
- Harrach, B.; Mirocha, C.J.; Pathre, S.V.; Palyusik, M. Macrocyclic trichothecene toxins produced by a strain of Stachybotrys atra from Hungary. Appl. Environ. Microbiol. 1981, 41, 1428–1432. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, B.B.; Lee, Y.W.; Cömezoglu, S.N.; Yatawara, C.S. Trichothecenes produced by Stachybotrys atra from Eastern Europe. Appl. Environ. Microbiol. 1986, 51, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E1–Maghraby, O.M.O.; Bean, G.A.; Jarvis, B.B.; Aboul-Nasr, M.B. Macrocyclic trichothecenes produced by Stachybotrys isolated from Egypt and Eastern Europe. Mycopathologia 1991, 113, 109–115. [Google Scholar] [CrossRef]
- Islam, Z.; Shinozuka, J.; Harkema, J.R.; Pestka, J.J. Purification and comparative neurotoxicity of the trichothecenes satratoxin G and roridin L2 from Stachybotrys chartarum. J. Toxicol. Environ. Health A 2009, 72, 1242–1251. [Google Scholar] [CrossRef] [Green Version]
- Bata, A.; Harrach, B.; Ujszászi, K.; Kis-Tamás, A.; Lásztity, R. Macrocyclic trichothecene toxins produced by Stachybotrys Atra strains isolated in Middle Europe. Appl. Environ. Microbiol. 1985, 49, 678–681. [Google Scholar] [CrossRef] [Green Version]
- Eppley, R.M.; Mazzola, E.P.; Stack, M.E.; Dreifuss, P.A. Structures of satratoxin F and satratoxin G, metabolites of Stachybotrys atra: Application of proton and carbon–13 nuclear magnetic resonance spectroscopy. J. Org. Chem. 1980, 45, 2522–2523. [Google Scholar] [CrossRef]
- Harrach, B.; Bata, A.; Bajmócy, E.; Benko, M. Isolation of satratoxins from the bedding straw of a sheep flock with fatal stachybotryotoxicosis. Appl. Environ. Microbiol. 1983, 45, 1419–1422. [Google Scholar] [CrossRef] [Green Version]
- Harrach, B.; Nummi, M.; Niku-Paavola, M.L.; Mirocha, C.J.; Palyusik, M. Identification of “water-soluble” toxins produced by a Stachybotrys atra strain from Finland. Appl. Environ. Microbiol. 1982, 44, 494–495. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, C.; Bauer, J.; Meyer, K. Detection of satratoxin G and H in indoor air from a water–damaged building. Mycopathologia 2008, 166, 103–107. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cen, S.; Proksch, P.; Lin, W. Isoindolinone–type alkaloids from the sponge–derived fungus Stachybotrys chartarum. Tetrahedron 2014, 70, 7010–7015. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Li, F.; Zhu, T.; Gu, Q.; Li, D. Stachybotrin G, a sulfate meroterpenoid from a sponge derived fungus Stachybotrys chartarum MXH–X73. Tetrahedron Lett. 2015, 56, 7053–7055. [Google Scholar] [CrossRef]
- Hinkley, S.F.; Mazzola, E.P.; Fettinger, J.C.; Lam, Y.F.; Jarvis, B.B. Atranones A–G, from the toxigenic mold Stachybotrys chartarum. Phytochemistry 2000, 55, 663–673. [Google Scholar] [CrossRef]
- Hinkley, S.F.; Moore, J.A.; Squillari, J.; Tak, H.; Oleszewski, R.; Mazzola, E.P.; Jarvis, B.B. New atranones from the fungus Stachybotrys chartarum. Magn. Reson. Chem. 2003, 41, 337–343. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Yan, S.; Xu, J.; Niaz, S.; Chand, R.; Ma, C.H.E.; Lin, Y.; Li, J.; Liu, L. Atranones with enhancement neurite outgrowth capacities from the crinoid–derived fungus Stachybotrys chartarum 952. Tetrahedron 2017, 73, 7260–7266. [Google Scholar] [CrossRef]
- Yoganathan, K.; Yang, L.; Rossant, C.; Huang, Y.; Ng, S.; Butler, M.S.; Buss, A.D. Cochlioquinones and epi–cochlioquinones: Antagonists of the human chemokine receptor CCR5 from Bipolaris Brizae and Stachybotrys chartarum. J. Antibiot. 2004, 57, 59–63. [Google Scholar] [CrossRef]
- Gan, Q.; Lin, C.; Lu, C.; Chang, Y.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Wu, Z.; Li, M. Staprexanthones, xanthone–type stimulators of pancreatic β-cell proliferation from a mangrove endophytic fungus. J. Nat. Prod. 2020, 83, 2996–3003. [Google Scholar] [CrossRef]
- Sakamoto, K.; Tsujii, E.; Miyauchi, M.; Nakanishi, T.; Yamashita, M.; Shigematsu, N.; Tada, T.; Izumi, S.; Okuhara, M. FR901459, a novel immunosuppressant isolated from Stachybotrys chartarum no. 19392. Taxonomy of the producing organism, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 1993, 46, 1788–1798. [Google Scholar] [CrossRef]
- Kadirova, D.B.; Kamalov, L.S.; Bobakulov, K.M.; Aripova, S.F. Chemical constituents of the toxic mold Stachybotrys chartarum. Chem. Nat. Compd. 2013, 49, 583–584. [Google Scholar] [CrossRef]
- Kamalov, L.S.; Tashkhodzhaev, B.; Aripova, S.F. Crystal structure of a cyclopentanone oxime from the toxic fungus Stachybotrys chartarum. Chem. Nat. Compd. 2015, 51, 597–598. [Google Scholar] [CrossRef]
- Lainer-Carr, D.; Brahn, E. Angiogenesis Inhibition as a therapeutic approach for inflammatory synovitis. Nat. Clin. Pract. Rheumatol. 2007, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.L.; Borriello, L.; Karagiannis, G.S.; Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Targeting Tie2 in the tumor microenvironment from angiogenesis to dissemination. Cancers 2021, 13, 5730. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Guo, J.; Zhang, Z.; Zhang, S.; Zhu, Y.; Cheng, J.; Yu, L.; Ji, Y.; Tao, J. Kv1.3 channel as a key therapeutic target for neuroinflammatory diseases: State of the art and beyond. Front. Neurosci. 2019, 13, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamburg, J.R. Biological and biochemical actions of trichothecene mycotoxins. In Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 1983; pp. 41–110. [Google Scholar]
- Grove, J.F. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993, 10, 429–448. [Google Scholar] [CrossRef]
- Grove, J.F. Non-Macrocyclic Trichothecenes. Nat. Prod. Rep. 1988, 5, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cen, Y.; Ye, W.; Li, S.; Zhang, W. Recent advances on macrocyclic trichothecenes, their bioactivities and biosynthetic pathway. Toxins 2020, 12, 417. [Google Scholar] [CrossRef]
- Islam, Z.; Harkema, J.R.; Pestka, J.J. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006, 114, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Islam, Z.; Hegg, C.C.; Bae, H.K.; Pestka, J.J. Satratoxin G–induced Apoptosis in PC–12 Neuronal Cells is Mediated by PKR and Caspase Independent. Toxicol. Sci. 2008, 105, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Kashiwada, Y.; Yamazaki, K.; Ikeshiro, Y.; Yamagishi, T.; Fujioka, T.; Mihashi, K.; Mizuki, K.; Cosentino, L.M.; Fowke, K.; Morris-Natschke, S.L. Isolation of rhododaurichromanic acid B and the anti–HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron Dauricum. Tetrahedron 2001, 57, 1559–1563. [Google Scholar] [CrossRef]
- Muria–Gonzalez, M.J.; Chooi, Y.; Breen, S.; Solomon, P.S. The Past, present and future of secondary metabolite research in the Dothideomycetes. Mol. Plant Pathol. 2015, 16, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- López-Cotarelo, P.; Gómez-Moreira, C.; Criado-García, O.; Sánchez, L.; Rodríguez-Fernández, J.L. Beyond chemoattraction: Multifunctionality of chemokine receptors in leukocytes. Trends Immunol. 2017, 38, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; De Clercq, E.; Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020, 16, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Palestine, A.G.; Nussenblatt, R.B.; Chan, C. Side Effects of systemic cyclosporine in patients not undergoing transplantation. Am. J. Med. 1984, 77, 652–656. [Google Scholar] [CrossRef]
- Wilson, K.E.; Tsou, N.N.; Guan, Z.; Ruby, C.L.; Pelaez, F.; Gorrochategui, J.; Vicente, F.; Onishi, H.R. Isolation and structure elucidation of coleophomones A and B, novel inhibitors of bacterial cell wall transglycosylase. Tetrahedron Lett. 2000, 41, 8705–8709. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.; Viitanen, H. Volatile metabolites of Serpula lacrymans, Coniophora puteana, Poria placenta, Stachybotrys chartarum and Chaetomium globosum. Build. Environ. 1998, 34, 205–211. [Google Scholar] [CrossRef]
- Wilkins, K. Volatile sesquiterpenes from Stachybotrys chartarum: Indicators for trichothecene producing mold species? Environ. Sci. Pollut. Res. Int. 2000, 7, 77–78. [Google Scholar] [CrossRef]
- Gao, P.; Martin, J. volatile metabolites produced by three strains of Stachybotrys chartarum cultivated on rice and gypsum board. Appl. Occup. Environ. Hyg. 2002, 17, 430–436. [Google Scholar] [CrossRef]
- Wang, H.; Yadav, J.S. DNA damage, redox changes, and associated stress–inducible signaling events underlying the apoptosis and cytotoxicity in murine alveolar macrophage cell line MH–S by methanol-extracted Stachybotrys chartarum toxins. Toxicol. Appl. Pharmacol. 2006, 214, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, L.; Xu, W.; Jiang, Z.; Kang, J.; Wang, F.; Xin, F. Identification and characterisation of a novel protein FIP-Sch3 from Stachybotrys chartarum. PLoS ONE 2016, 11, e0168436. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram–positive and gram–negative bacteria. Nanomedicine 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Mohamed, A.G.T. Stachybotrys chartarum: A Novel Biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones. J. Biotechnol. 2013, 18, 75–82. [Google Scholar] [CrossRef] [Green Version]
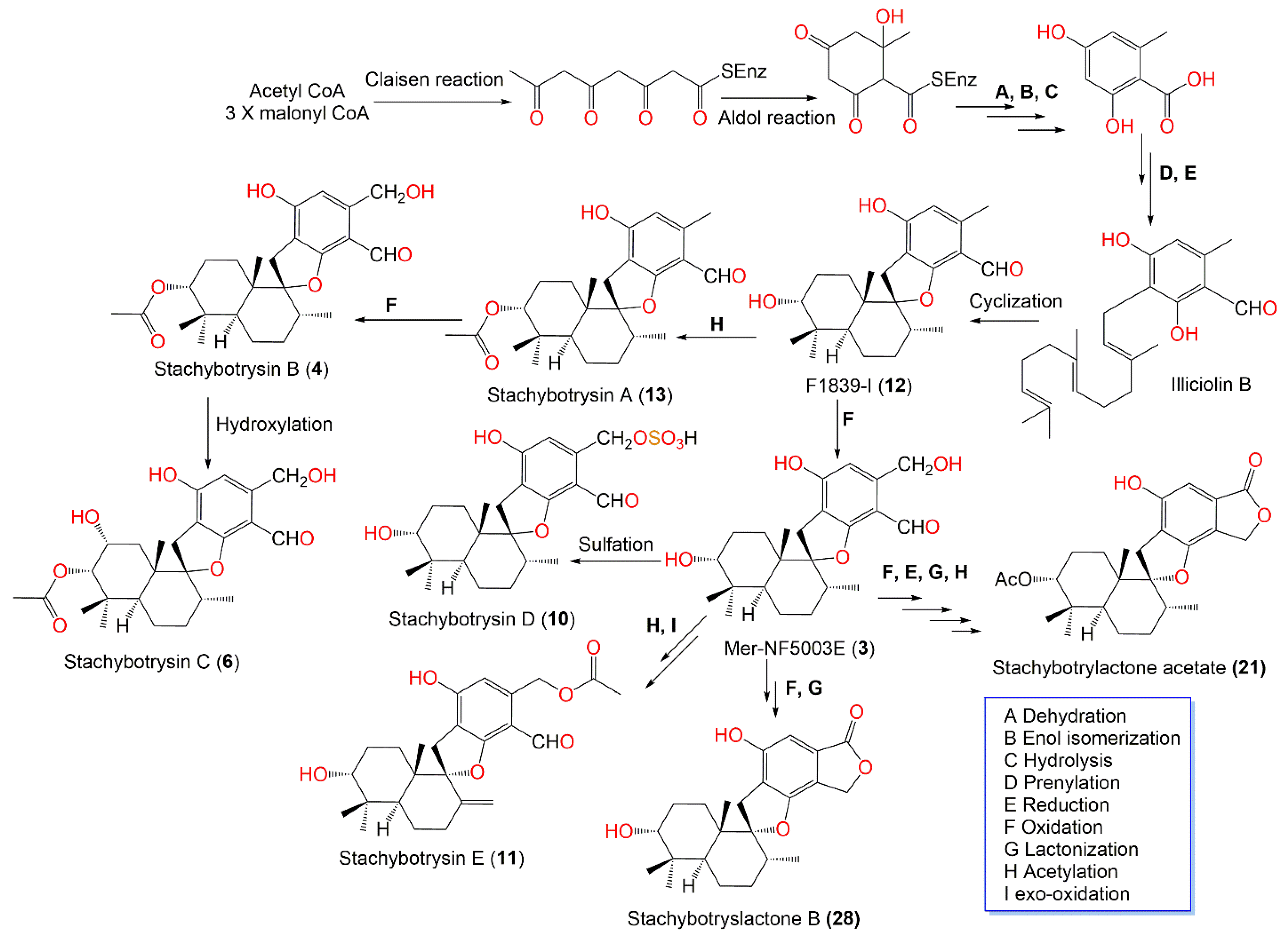
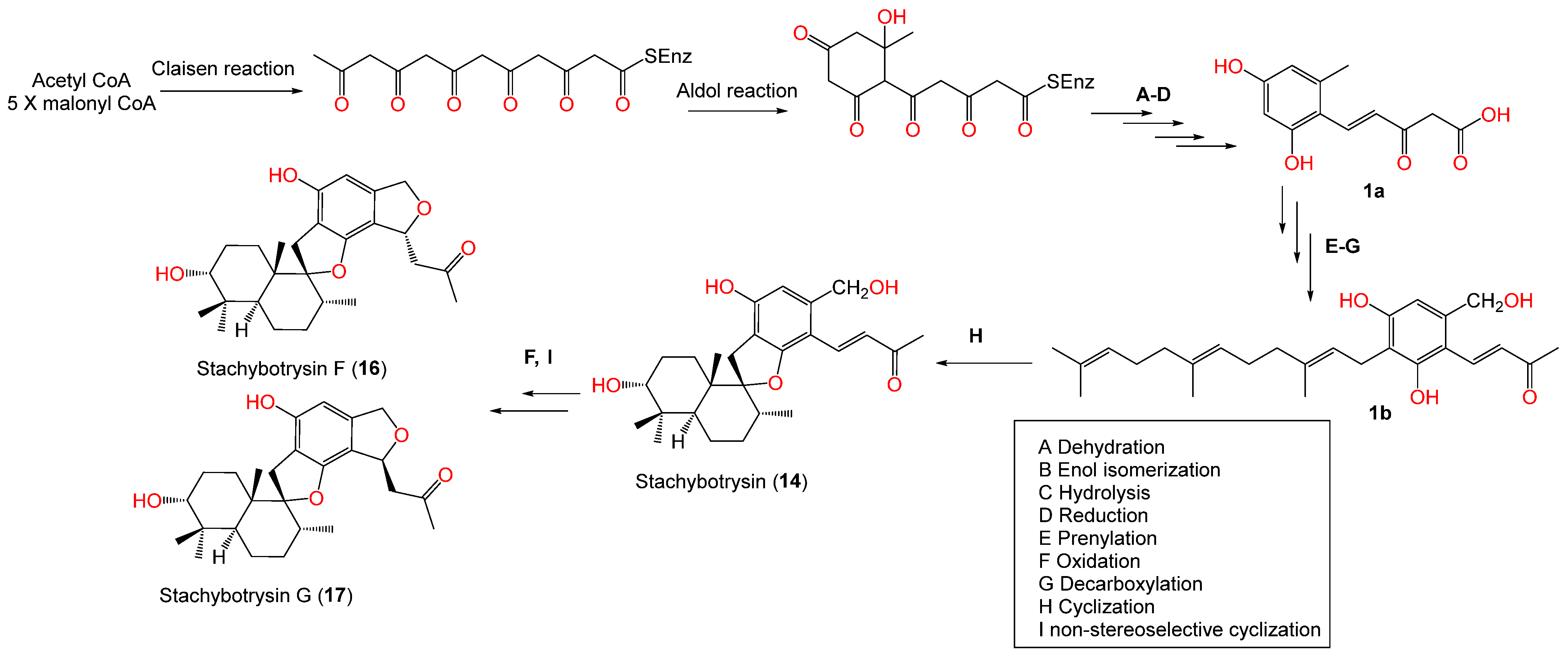

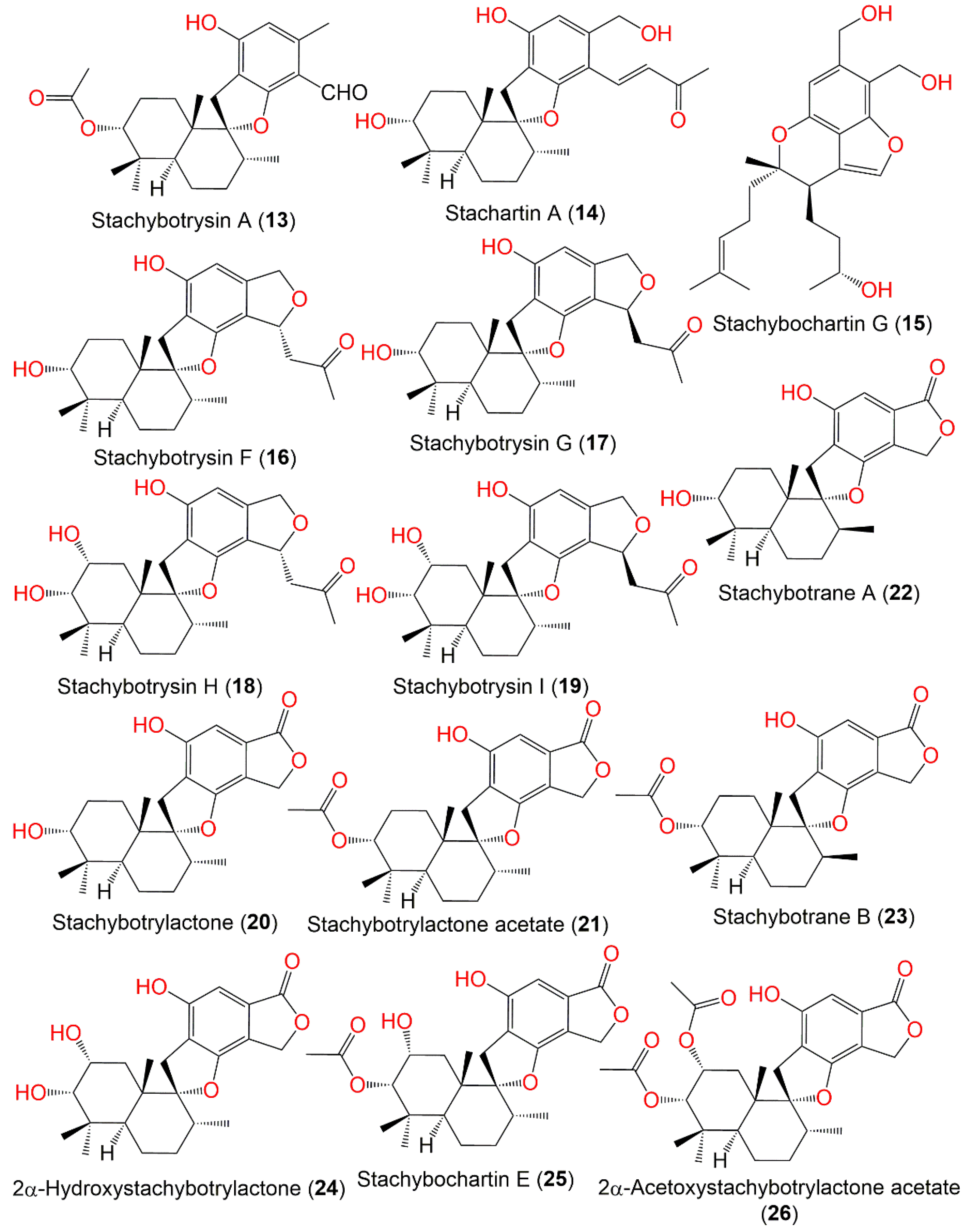
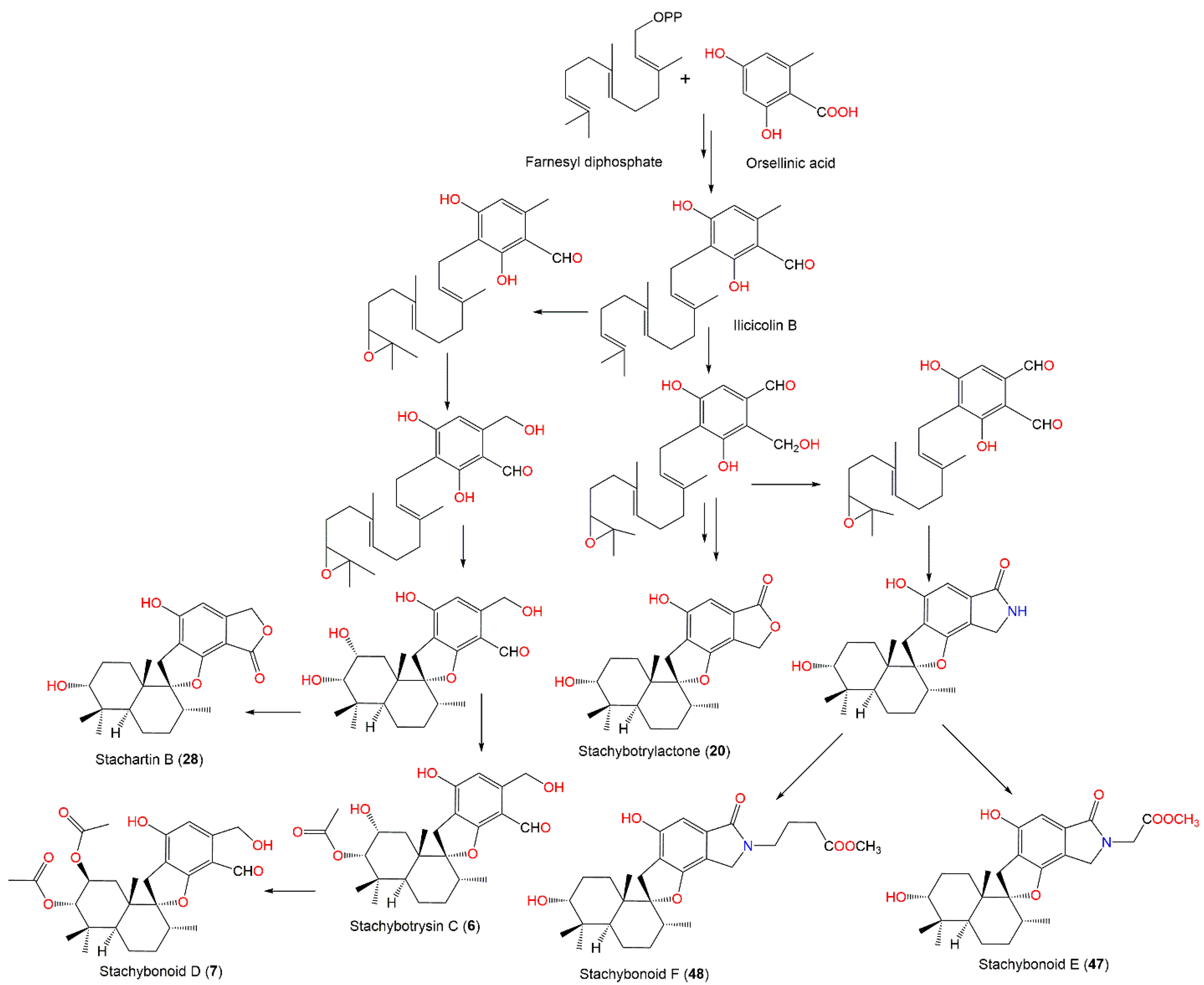

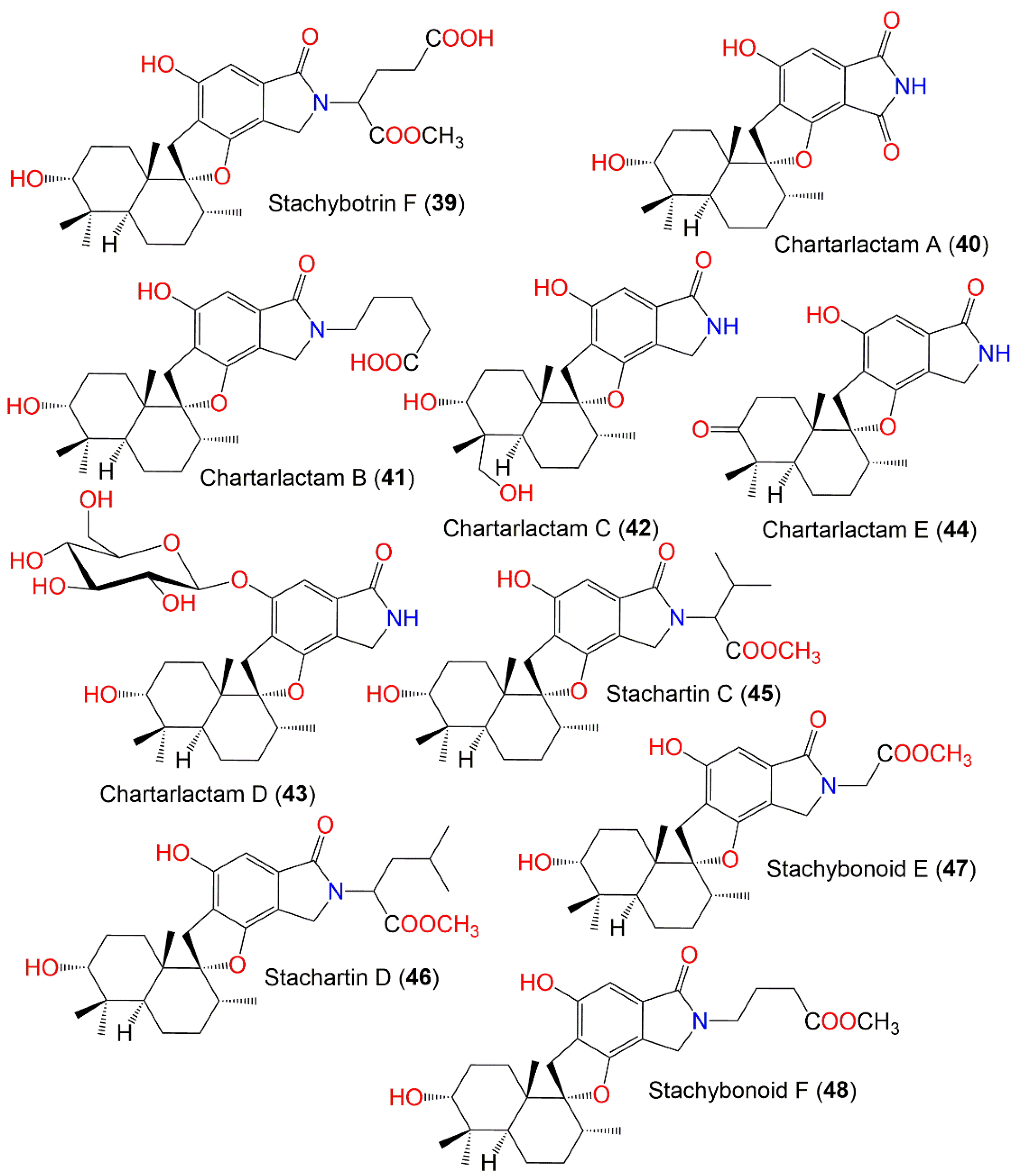

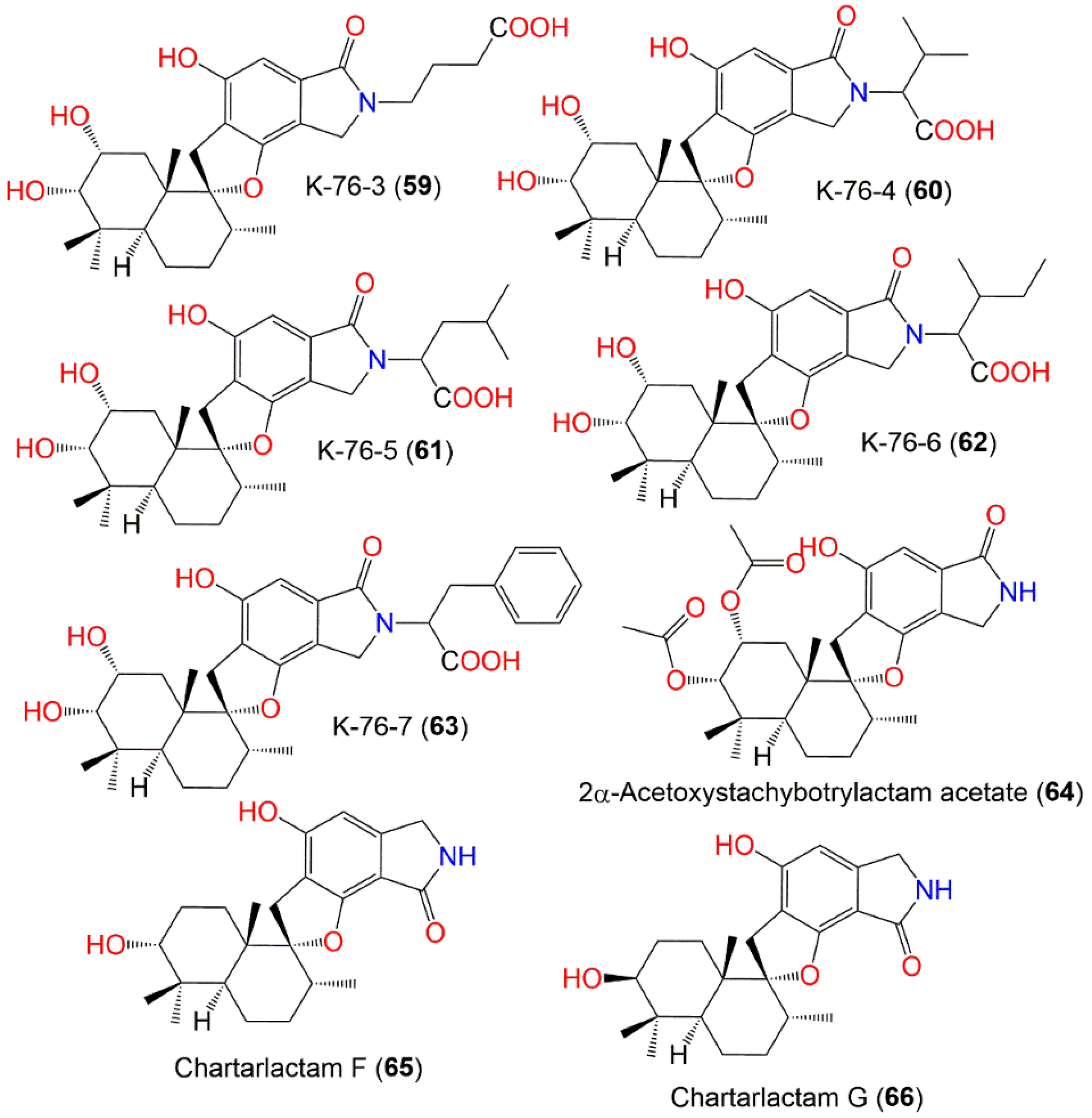

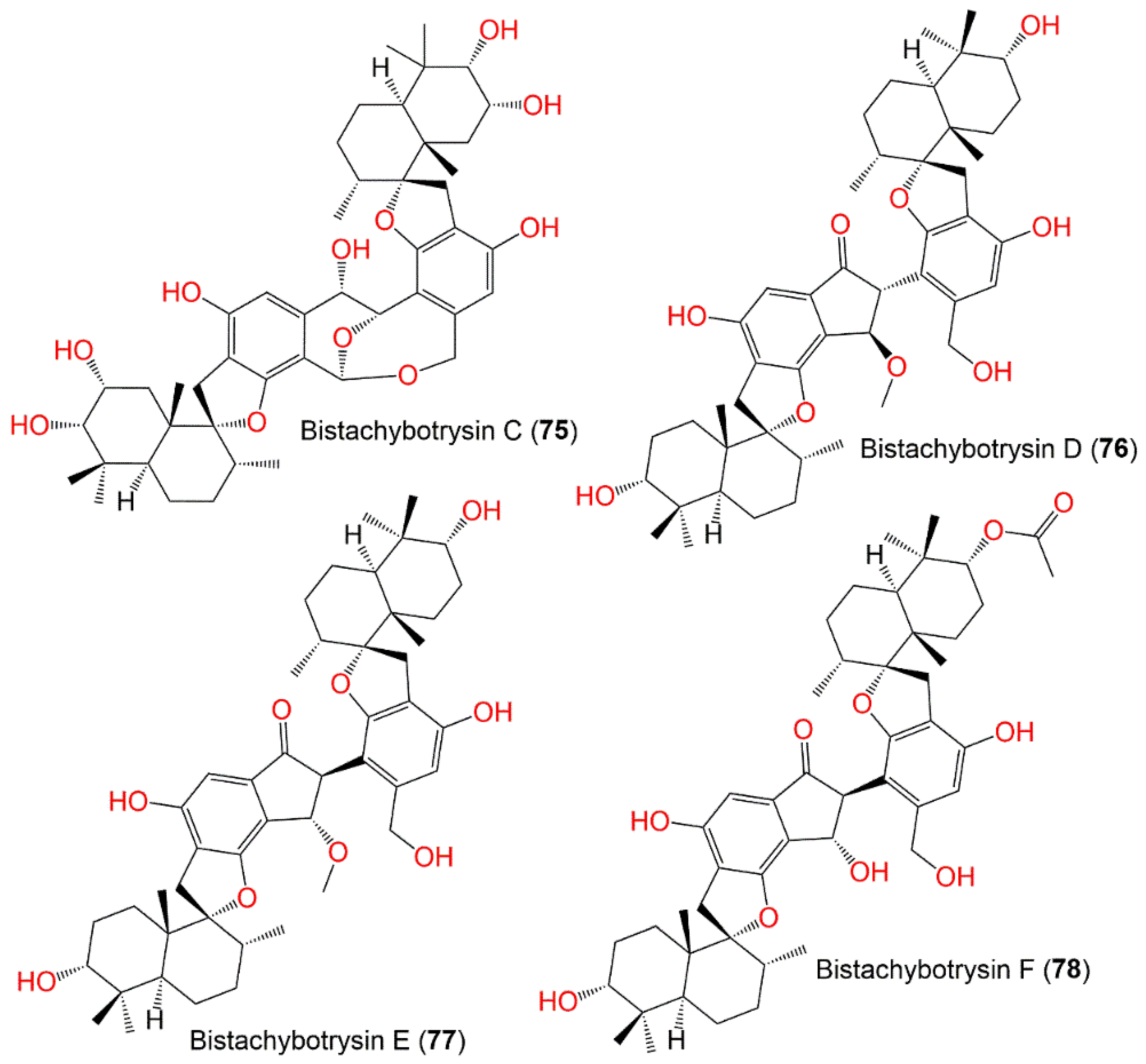








| Compound Name | Mol. Wt. | Mol. Formula | Host (Part, Family) | Place | Ref. |
|---|---|---|---|---|---|
| Stachybotrydial (1) | 386 | C23H30O5 | Cultured | - | [65] |
| K-76 (2) | 402 | C23H30O6 | Cultured | - | [65] |
| Mer-NF5003E (3) | 388 | C23H32O5 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| - | - | Cultured | - | [67] | |
| Stachybotrysin B (4) | 430 | C25H34O6 | Cultured | - | [67] |
| Mer-NF5003B (5) | 404 | C23H32O6 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybotrysin C (6) | 446 | C25H34O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| - | - | Himerometra magnipinna (Crinoids, Himerometridae) | - | [67] | |
| Stachybonoid D (7) | 488 | C27H36O8 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| Stachyboside A (8) | 550 | C29H42O10 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachyboside B (9) | 566 | C29H42O11 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybotrysin D (10) | 468 | C23H32O8S | Cultured | - | [67] |
| Stachybotrysin E (11) | 428 | C25H32O6 | Cultured | - | [67] |
| F1839-I (12) | 372 | C23H32O4 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybotrysin A (13) | 414 | C25H34O5 | Cultured | - | [67] |
| Stachartin A = Stachybotrysin (14) | 428 | C26H36O5 | Cultured | - | [67] |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] | |
| Stachybochartin G (15) | 388 | C23H32O5 | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachybotrysin F (16) | 428 | C26H36O5 | Cultured | - | [67] |
| Stachybotrysin G (17) | 428 | C26H36O5 | Cultured | - | [67] |
| Stachybotrysin H (18) | 444 | C26H36O6 | Cultured | - | [71] |
| Stachybotrysin I (19) | 444 | C26H36O6 | Cultured | - | [71] |
| Stachybotrylactone = Stachybotrolide (20) | 386 | C23H30O5 | Cultured | - | [65] |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] | |
| - | - | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] | |
| Stachybotrylactone acetate (21) | 428 | C25H32O6 | Cultured | - | [65,67] |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| Stachybotrane A (22) | 386 | C23H30O5 | Mud | East Dongting Lake, Hunan, China | [72] |
| - | - | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] | |
| Stachybotrane B (23) | 428 | C25H32O6 | Mud | East Dongting Lake, Hunan, China | [72] |
| - | - | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] | |
| 2α-Hydroxystachybotrylactone (24) | 402 | C23H30O6 | Cultured | - | [65] |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| Stachybochartin E (25) | 444 | C25H32O7 | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| 2α-Acetoxystachybotrylactone acetate (26) | 486 | C27H34O8 | Cultured | - | [65] |
| Stachybotrane C (27) | 402 | C23H30O6 | Mud | East Dongting Lake, Hunan, China | [72] |
| Stachybotryslactone B = Stachartin B (28) | 386 | C23H30O5 | Cultured | - | [67] |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] | |
| - | - | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] | |
| Stachybotrylactam (29) | 385 | C23H31NO4 | Cultured | - | [65] |
| - | - | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] | |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] | |
| - | - | Cultured | - | [71] | |
| - | - | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] | |
| Stachybotrylactam acetate (30) | 427 | C25H33NO5 | Cultured | - | [65] |
| - | - | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] | |
| - | - | Sinularia sp. (Soft coralm Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Arthproliferin E = Stachybotrin E (31) | 399 | C24H33NO4 | Sinularia sp. ( Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| - | - | Cultured | - | [71] | |
| Stachybotrin = Stachybotramide (32) | 429 | C25H35NO5 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| - | - | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] | |
| - | - | Cultured | - | [65,75] | |
| - | - | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] | |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| Stachybochartin F (33) | 471 | C27H37NO6 | Pinellia ternata (rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachybotrin D (34) | 441 | C26H35NO5 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybotrysam E (35) | 471 | C27H37NO6 | Cultured | - | [76] |
| K-76-1 (36) | 515 | C28H37NO8 | Soil | Himalaya, India | [77] |
| - | - | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] | |
| K-76-2 (37) | 487 | C27H37NO7 | Soil | Himalaya, India | [77] |
| Stachybotrin E (38) | 529 | C29H39NO8 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybotrin F (39) | 529 | C29H39NO8 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Chartarlactam A (40) | 399 | C23H29NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] | |
| Chartarlactam B (41) | 485 | C28H39NO6 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam C (42) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam D (43) | 563 | C30H45NO9 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam E (44) | 383 | C23H29NO4 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Stachartin C (45) | 499 | C29H41NO6 | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] |
| Stachartin D (46) | 513 | C30H43NO6 | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] |
| Stachybonoid E (47) | 457 | C26H35NO6 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [78] | |
| Stachybonoid F (48) | 485 | C28H39NO6 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| - | - | Soil sample | Datun tin mine tailings area, Yunnan, China | [78] | |
| Stachartin E (49) | 547 | C33H41NO6 | Soil sample | Datun tin mine tailings area, Yunnan, China | [69] |
| N-(2-Benzenepropanoic acid) stachybotrylactam (50) | 533 | C32H39NO6 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| F1839-A (51) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Chartarlactam I (52) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam J (53) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam K (54) | 443 | C25H33NO6 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam M (55) | 385 | C23H31NO4 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam P (56) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| F1839-E (57) | 445 | C25H35NO6 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| - | - | Mud | East Dongting Lake, Hunan, China | [72] | |
| Stachybotrane D (58) | 445 | C25H35NO6 | Mud | East Dongting Lake, Hunan, China | [72] |
| K-76-3 (59) | 487 | C27H37NO7 | Soil | Himalaya, India | [77] |
| K-76-4 (60) | 501 | C28H39NO7 | Soil | Himalaya, India | [77] |
| K-76-5 (61) | 515 | C29H41NO7 | Soil | Himalaya, India | [77] |
| K-76-6 (62) | 515 | C29H41NO7 | Soil | Himalaya, India | [77] |
| K-76-7 (63) | 549 | C32H39NO7 | Soil | Himalaya, India | [77] |
| 2α-Acetoxystachybotrylactam acetate (64) | 485 | C27H35NO7 | Cultured | - | [65] |
| - | - | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] | |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Chartarlactam F (65) | 385 | C23H31NO4 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Chartarlactam G (66) | 385 | C23H31NO4 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam H (67) | 429 | C25H35NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam N (68) | 429 | C25H35NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam O (69) | 385 | C23H31NO4 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Stachartin F (70) | 485 | C28H39NO6 | Soil sample | Datun tin mine tailings area, Yunnan, China | [78] |
| F1839-D (71) | 401 | C23H31NO5 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
| Chartarlactam L (72) | 766 | C46H58N2O8 | Niphates recondita (Sponge, Niphatidae) | Coral reef near Weizhou Island, Beibuwan Bay, Guangxi, China | [74] |
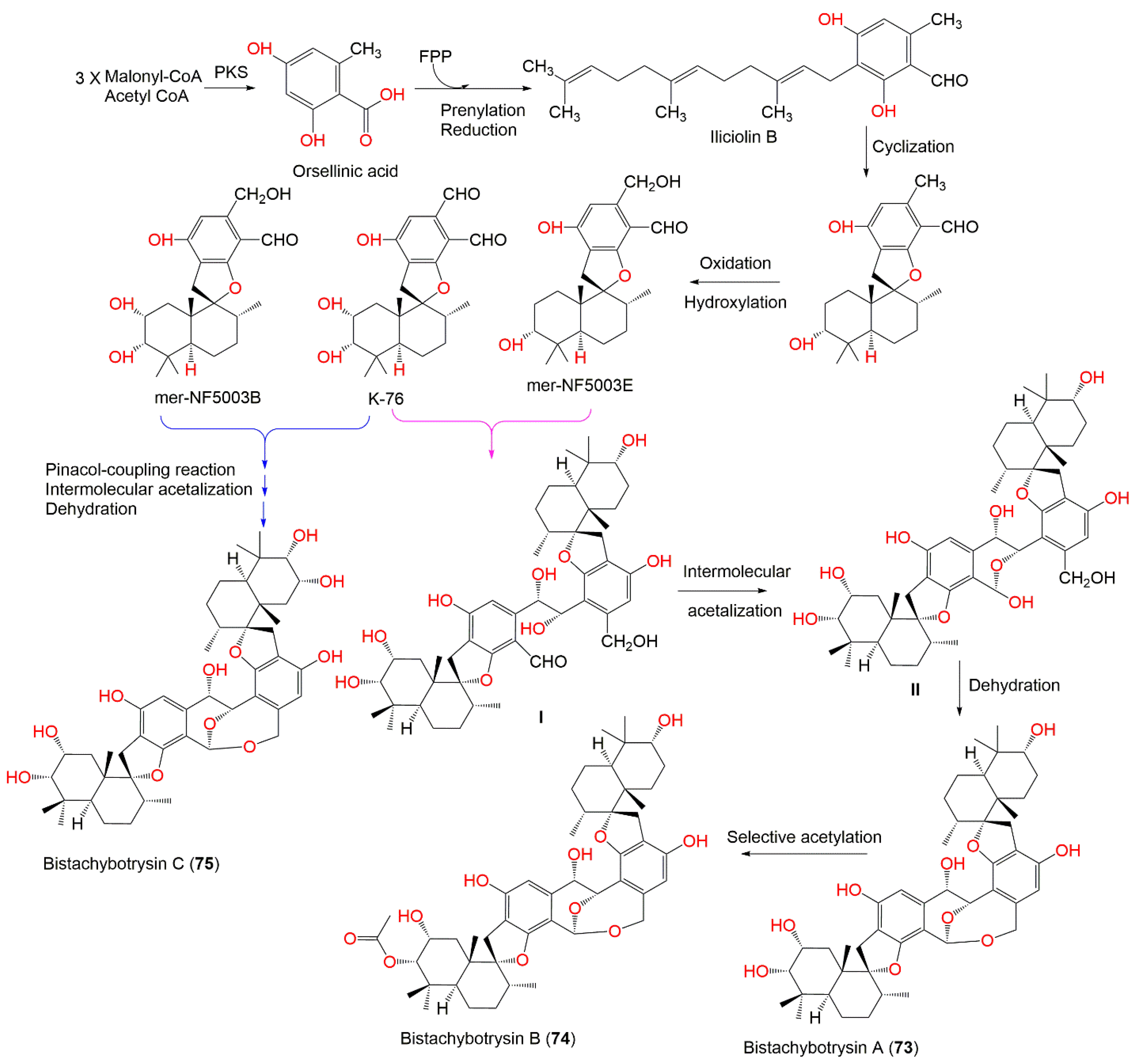
| Bistachybotrysin A (73) | 774 | C46H62O10 | Cultured | - | [79] |
| Bistachybotrysin B (74) | 816 | C48H64O11 | Cultured | - | [79] |
| Bistachybotrysin C (75) | 790 | C46H62O11 | Cultured | - | [79] |
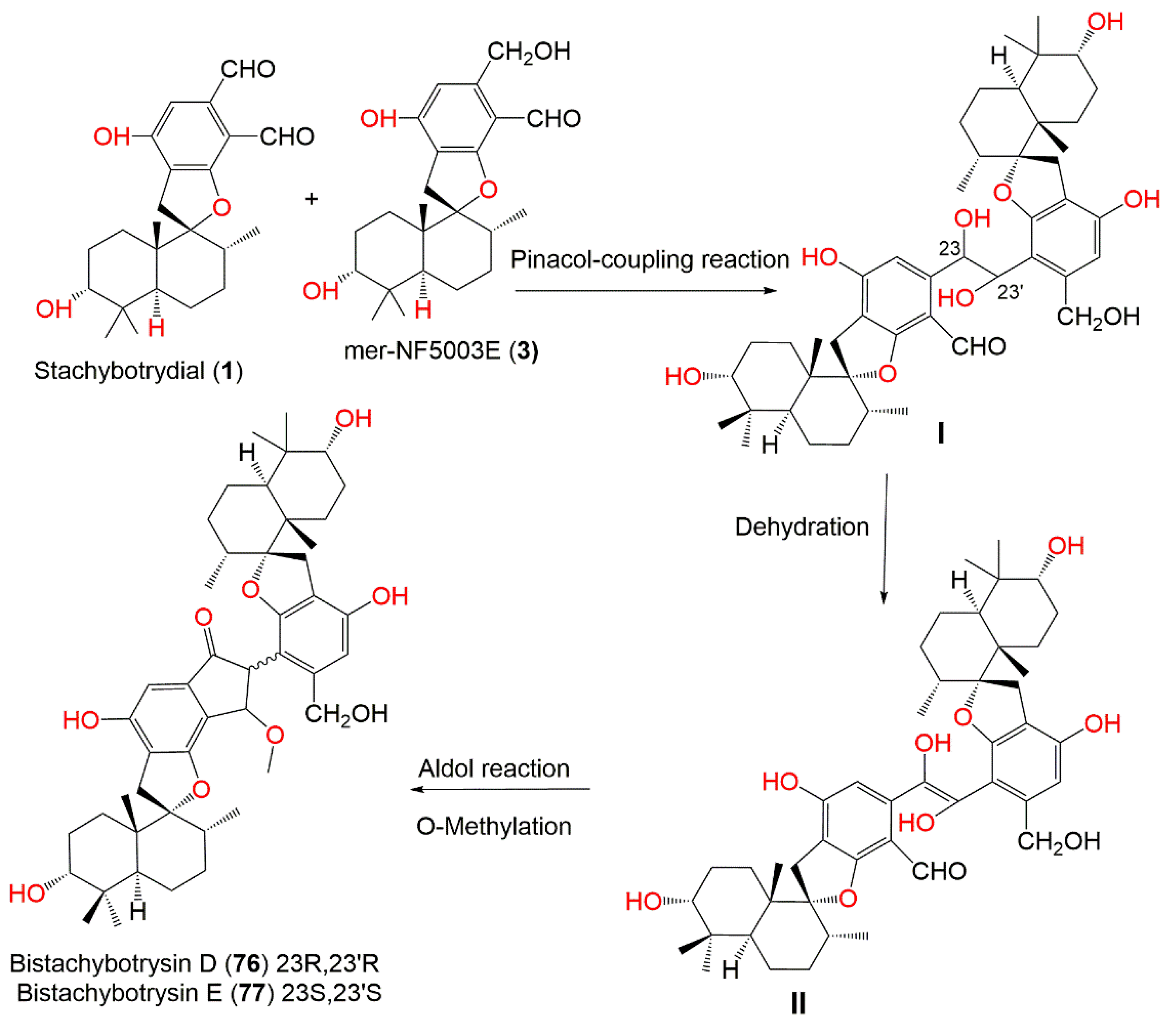
| Bistachybotrysin D (76) | 772 | C47H64O9 | Cultured | - | [80] |
| Bistachybotrysin E (77) | 772 | C47H64O9 | Cultured | - | [80] |
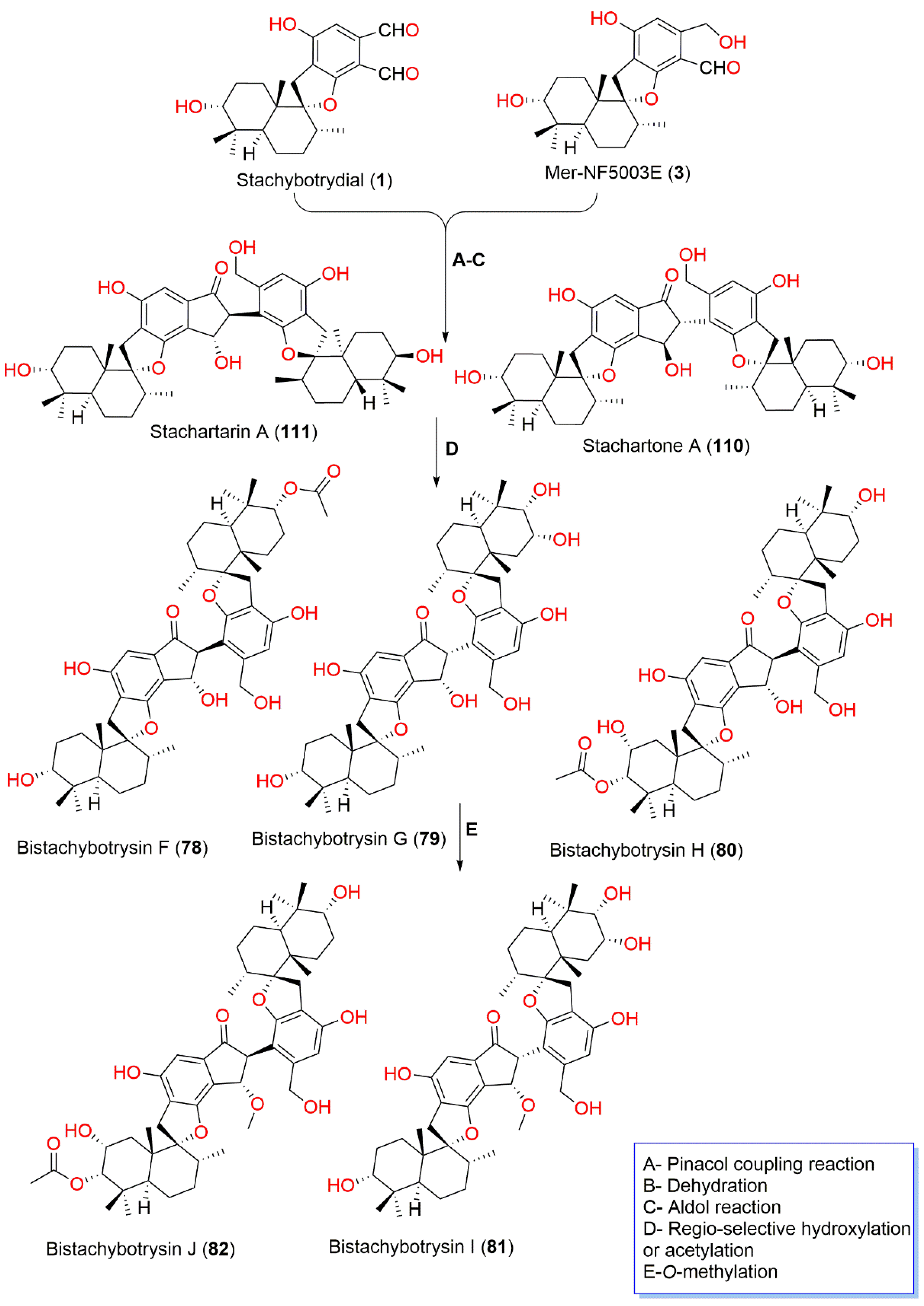
| Bistachybotrysin F (78) | 800 | C48H64O10 | Cultured | - | [81] |
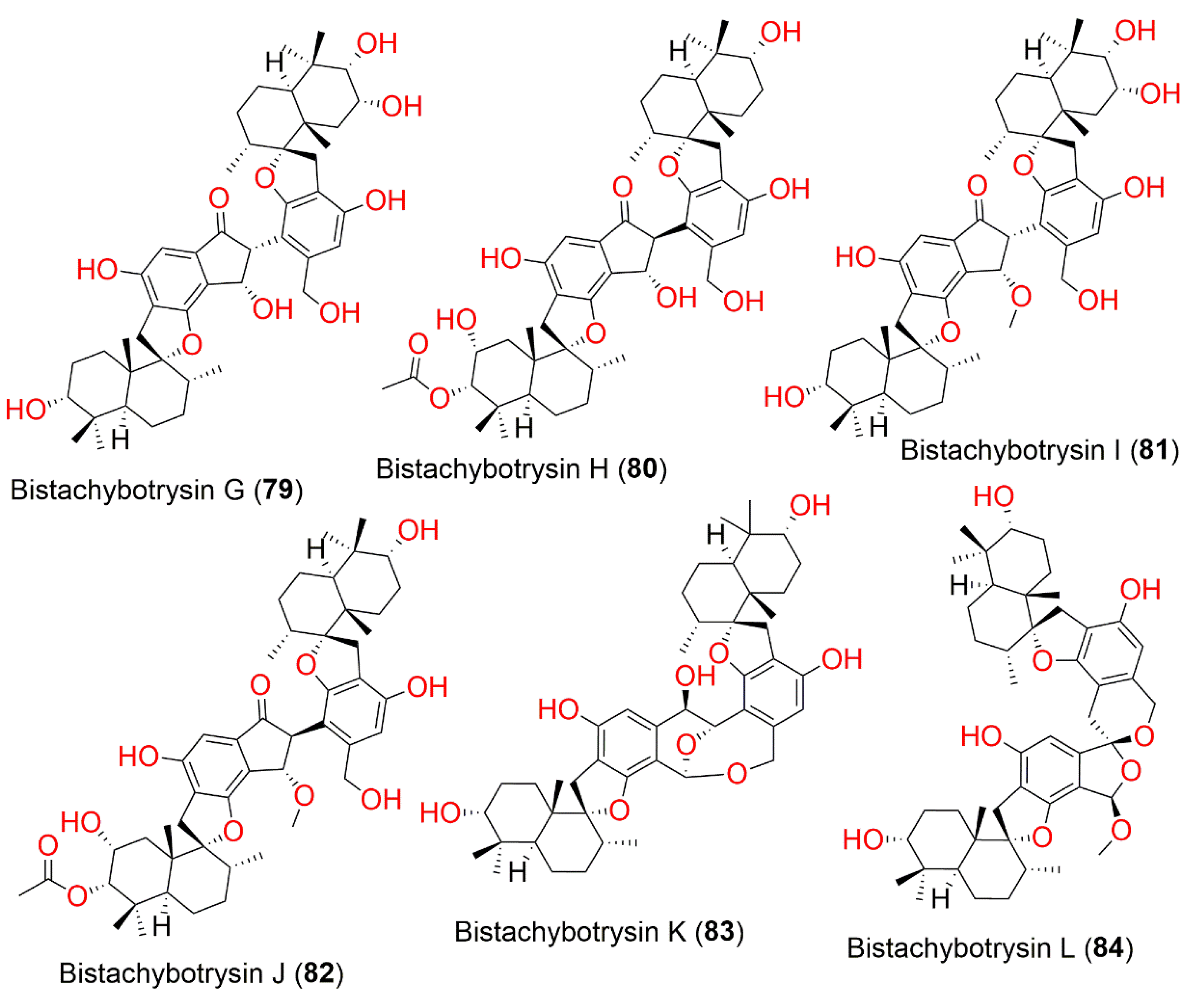
| Bistachybotrysin G (79) | 774 | C46H62O10 | Cultured | - | [81] |
| Bistachybotrysin H (80) | 816 | C48H64O11 | Cultured | - | [81] |
| Bistachybotrysin I (81) | 788 | C47H64O10 | Cultured | - | [81] |
| Bistachybotrysin J (82) | 816 | C49H66O11 | Cultured | - | [81] |
| Bistachybotrysin K (83) | 758 | C46H62O9 | Cultured | - | [82] |
| Bistachybotrysin L (84) | 772 | C47H64O9 | Cultured | - | [83] |
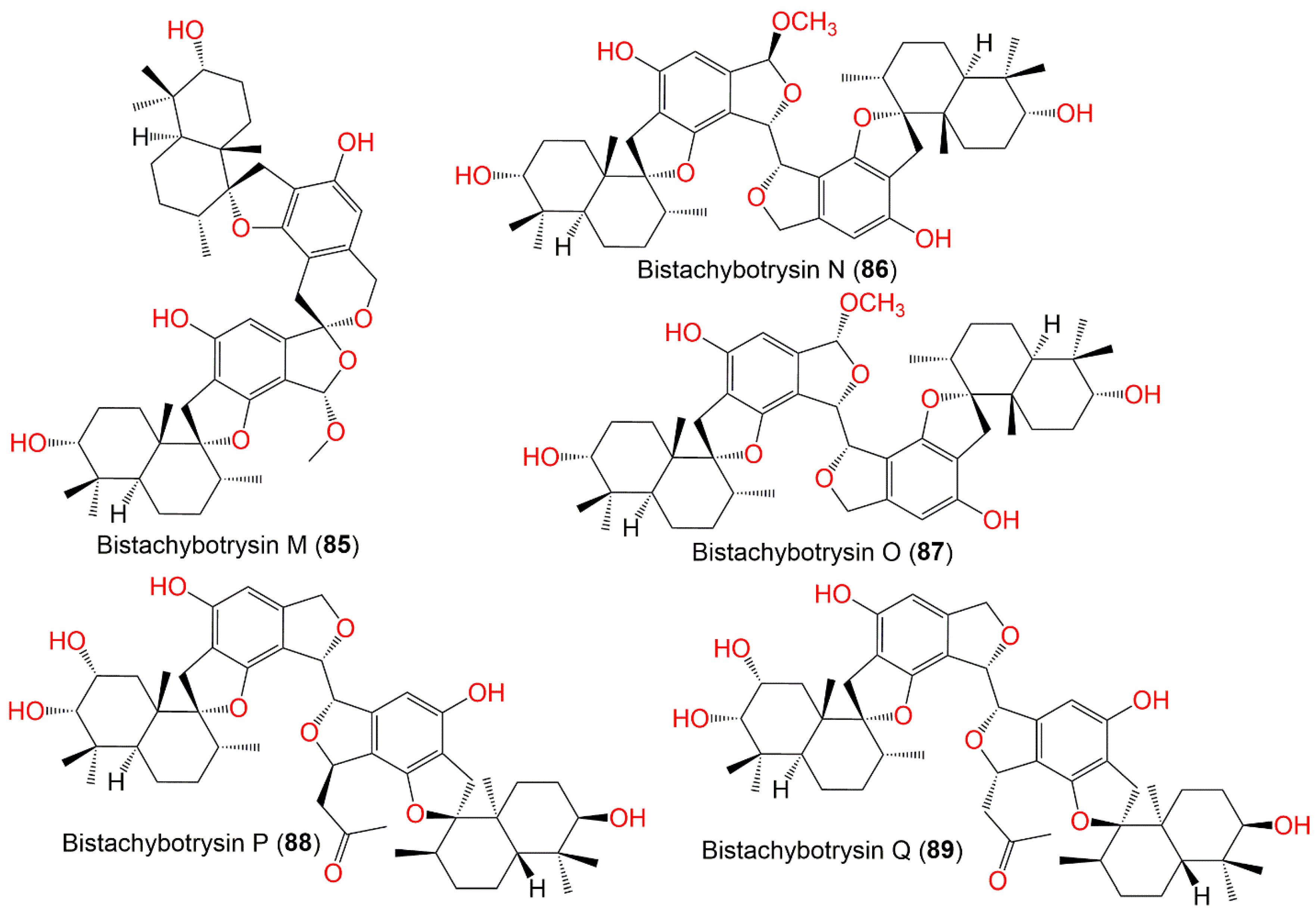
| Bistachybotrysin M (85) | 772 | C47H64O9 | Cultured | - | [83] |
| Bistachybotrysin N (86) | 772 | C47H64O9 | Cultured | - | [83] |
| Bistachybotrysin O (87) | 772 | C47H64O9 | Cultured | - | [83] |
| Bistachybotrysin P (88) | 814 | C49H66O10 | Cultured | - | [83] |
| Bistachybotrysin Q (89) | 814 | C49H66O10 | Cultured | - | [83] |
| Bistachybotrysin R (90) | 854 | C52H70O10 | Cultured | - | [83] |
| Bistachybotrysin S (91) | 854 | C52H70O10 | Cultured | - | [83] |
| Bistachybotrysin T (92) | 798 | C49H66O9 | Cultured | - | [83] |
| Bistachybotrysin U (93) | 756 | C46H60O9 | Cultured | - | [83] |
| Bistachybotrysin V (94) | 758 | C46H62O9 | Cultured | - | [83] |
| Bistachybotrysin W (95) | 832 | C48H64O12 | Cultured | - | [84] |
| Bistachybotrysin X (96) | 832 | C48H64O12 | Cultured | - | [84] |
| Bistachybotrysin Y (97) | 874 | C50H67O13 | Cultured | - | [84] |
| Chartarolide A (98) | 772 | C44H49O10Cl | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [85] |
| Chartarolide B (99) | 772 | C44H49O10Cl | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [85] |
| Chartarolide C (100) | 771 | C44H50NO9Cl | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [85] |
| Chartarlactam Q (101) | 753 | C46H59NO8 | Cultured | - | [86] |
| Chartarlactam R (102) | 797 | C48H63NO9 | Cultured | - | [86] |
| Chartarlactam S (103) | 771 | C46H61NO9 | Cultured | - | [86] |
| Chartarlactam T (104) | 855 | C50H65NO11 | Cultured | - | [86] |
| Stachybochartin A (105) | 774 | C46H62O10 | Pinellia ternata (Rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachybochartin B (106) | 844 | C50H68O11 | Pinellia ternata (Rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachybochartin C (107) | 772 | C47H64O9 | Pinellia ternata (Rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachybochartin D (108) | 858 | C50H66O12 | Pinellia ternata (Rhizomes, Araceae) | Nanjing, Jiangsu, China | [70] |
| Stachyin B (109) | 755 | C46H61NO8 | Cultured | - | [86] |
| Stachartone A (110) | 758 | C46H62O9 | Soil sample | Datun tin mine tailings area, Yunnan, China | [87] |
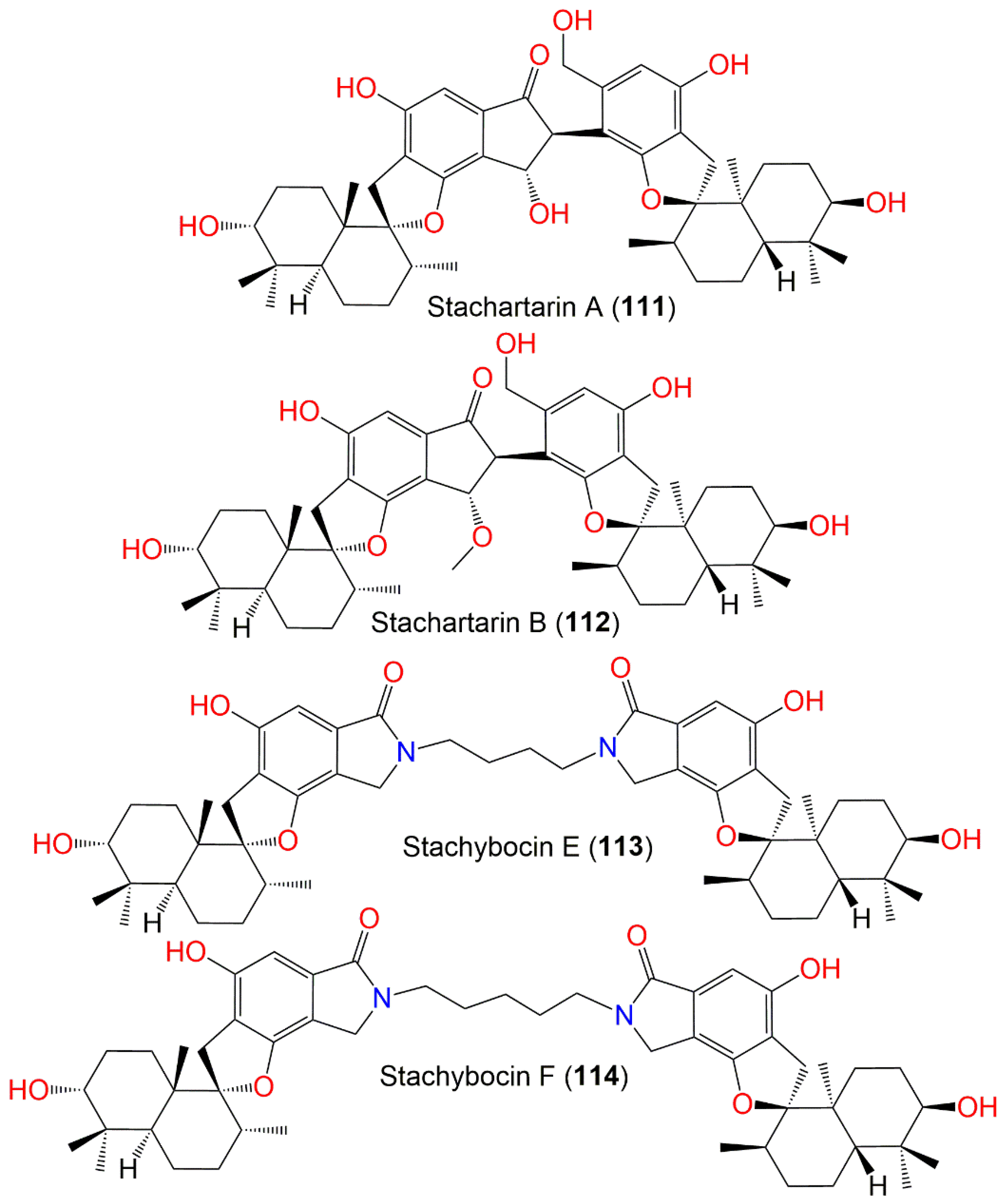
| Stachartarin A (111) | 758 | C46H62O9 | Soil sample | Datun tin mine tailings area, Yunnan, China | [88] |
| Stachartarin B (112) | 772 | C47H64O9 | Soil sample | Datun tin mine tailings area, Yunnan, China | [89] |
| Stachybocin E (113) | 824 | C50H68N2O8 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
| Stachybocin F (114) | 838 | C51H70N2O8 | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [66] |
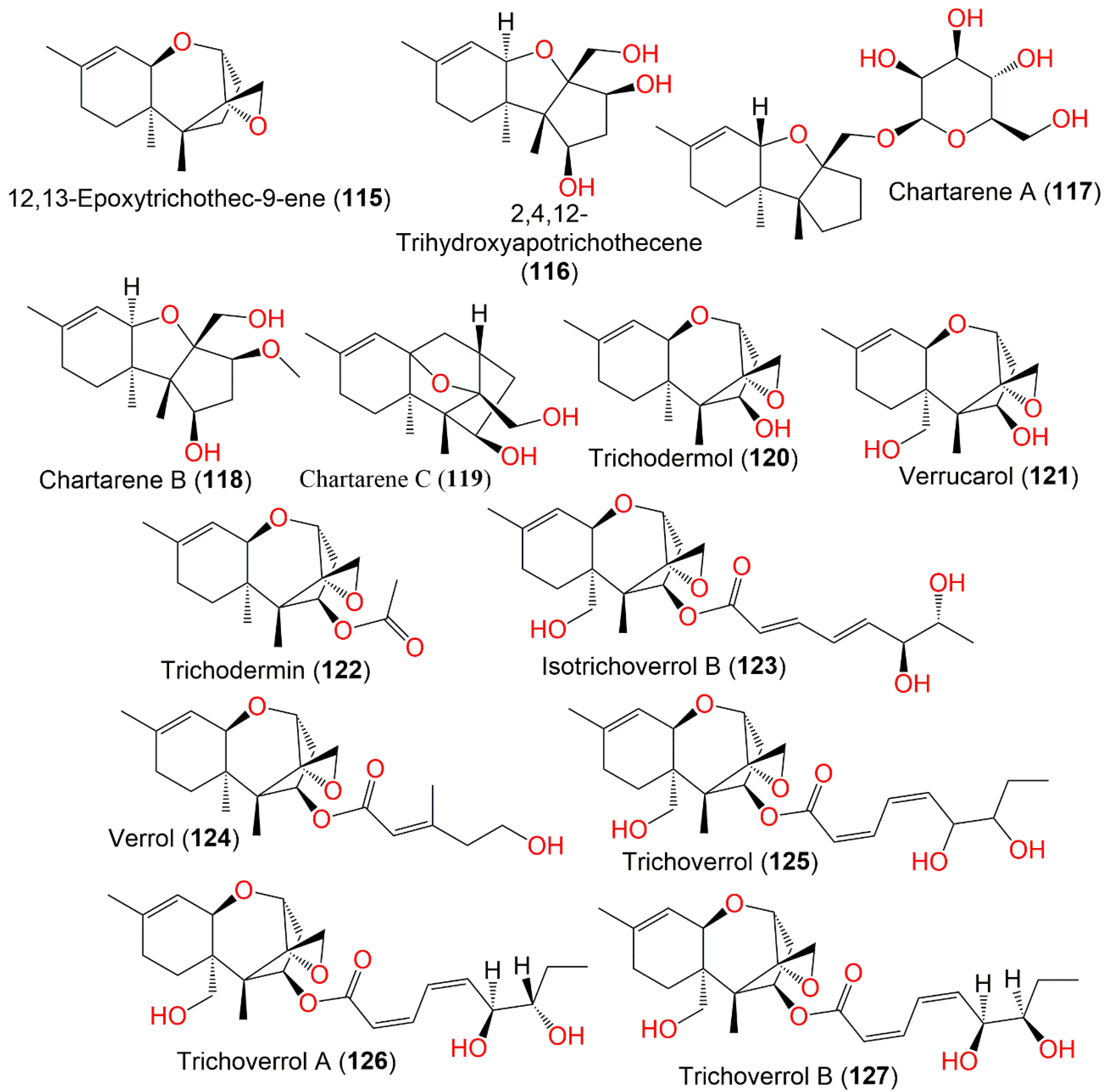
| 12,13-Epoxytrichothec-9-ene (115) | 234 | C15H22O2 | Cultured | - | [90,91] |
| 2,4,12-Trihydroxyapotrichothecene (116) | 268 | C15H24O4 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Chartarene A (117) | 398 | C21H34O7 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Chartarene B (118) | 282 | C16H26O4 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Chartarene C (119) | 264 | C16H24O3 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Trichodermol (120) | 250 | C15H22O3 | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] |
| Verrucarol (121) | 266 | C15H22O4 | Cultured | - | [65,91,94] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [95] | |
| Trichodermin (122) | 292 | C17H24O4 | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] |
| Isotrichoverrol B (123) | 420 | C23H32O7 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Verrol (124) | 362 | C21H30O5 | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] |
| - | - | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] | |
| Trichoverrol (125) | 434 | C24H34O7 | Cultured | - | [65] |
| Trichoverrol A (126) | 434 | C24H34O7 | Dead animals | Various parts of Hungary and Czechoslovakia | [96] |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| Trichoverrol B (127) | 434 | C24H34O7 | Dead animals | Various parts of Hungary and Czechoslovakia | [96] |
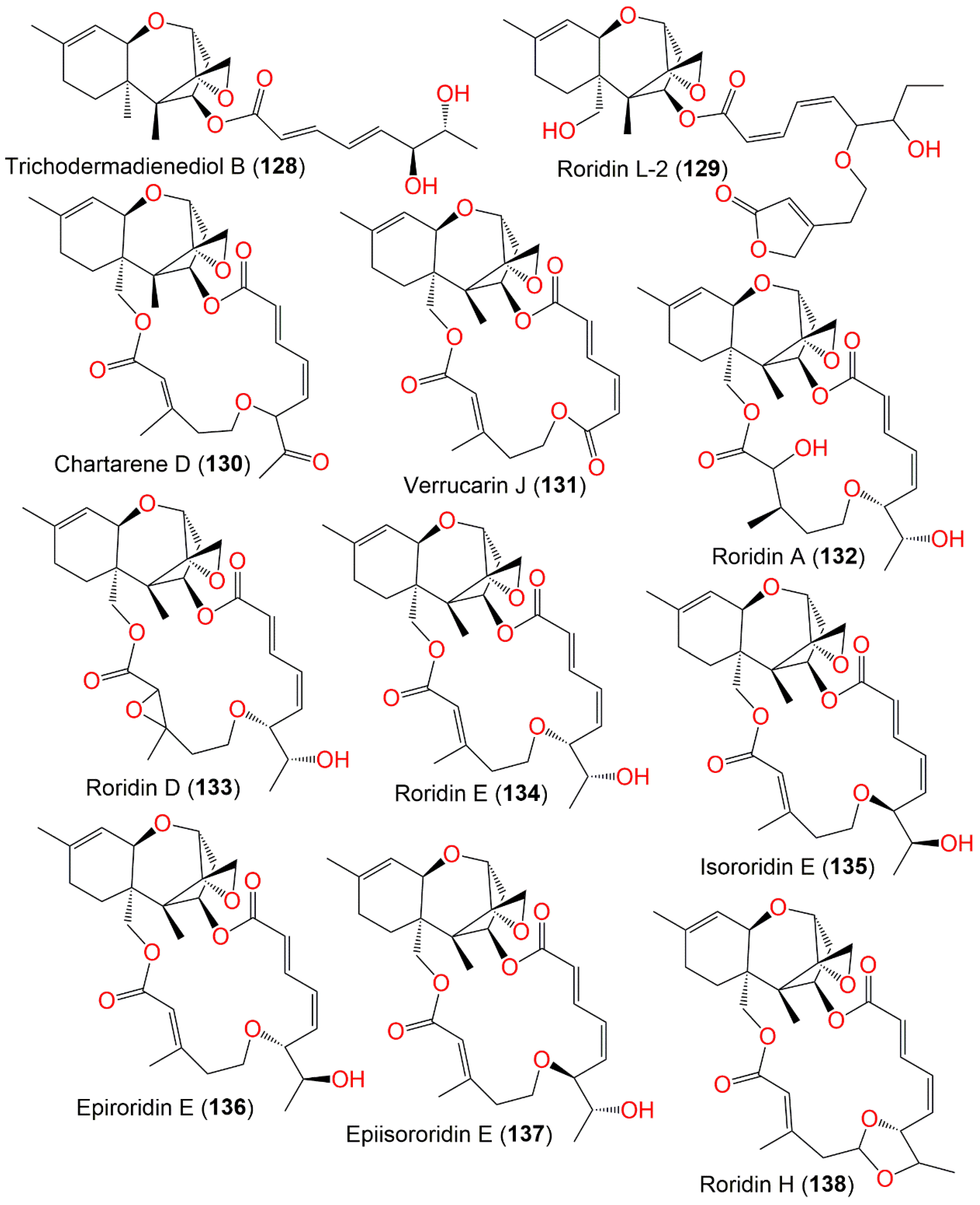
| Trichodermadienediol B (128) | 404 | C23H32O6 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Roridin L-2 (129) | 544 | C30H40O9 | Cultured | - | [65,94,98] |
| - | - | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] | |
| - | - | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] | |
| Chartarene D (130) | 512 | C29H36O8 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Verrucarin J (131) | 484 | C27H32O8 | Cultured | - | [91] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [95] | |
| - | - | Dead animals | Various parts of Hungary and Czechoslovakia | [96,99] | |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| Roridin A (132) | 532 | C29H40O9 | Cultured | - | [91] |
| Roridin D (133) | 530 | C29H38O9 | Cultured | - | [91] |
| Roridin E (134) | 514 | C29H38O8 | Cultured | - | [65,91,94] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [95] | |
| - | - | Dead animals | Various parts of Hungary and Czechoslovakia | [96,99] | |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| - | - | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] | |
| Isororidin E (135) | 514 | C29H38O8 | Cultured | - | [65] |
| Epiroridin E (136) | 514 | C29H38O8 | Cultured | - | [65] |
| Epiisororidin E (137) | 514 | C29H38O8 | Cultured | - | [65] |
| Roridin H (138) | 512 | C29H36O8 | Cultured | - | [91] |
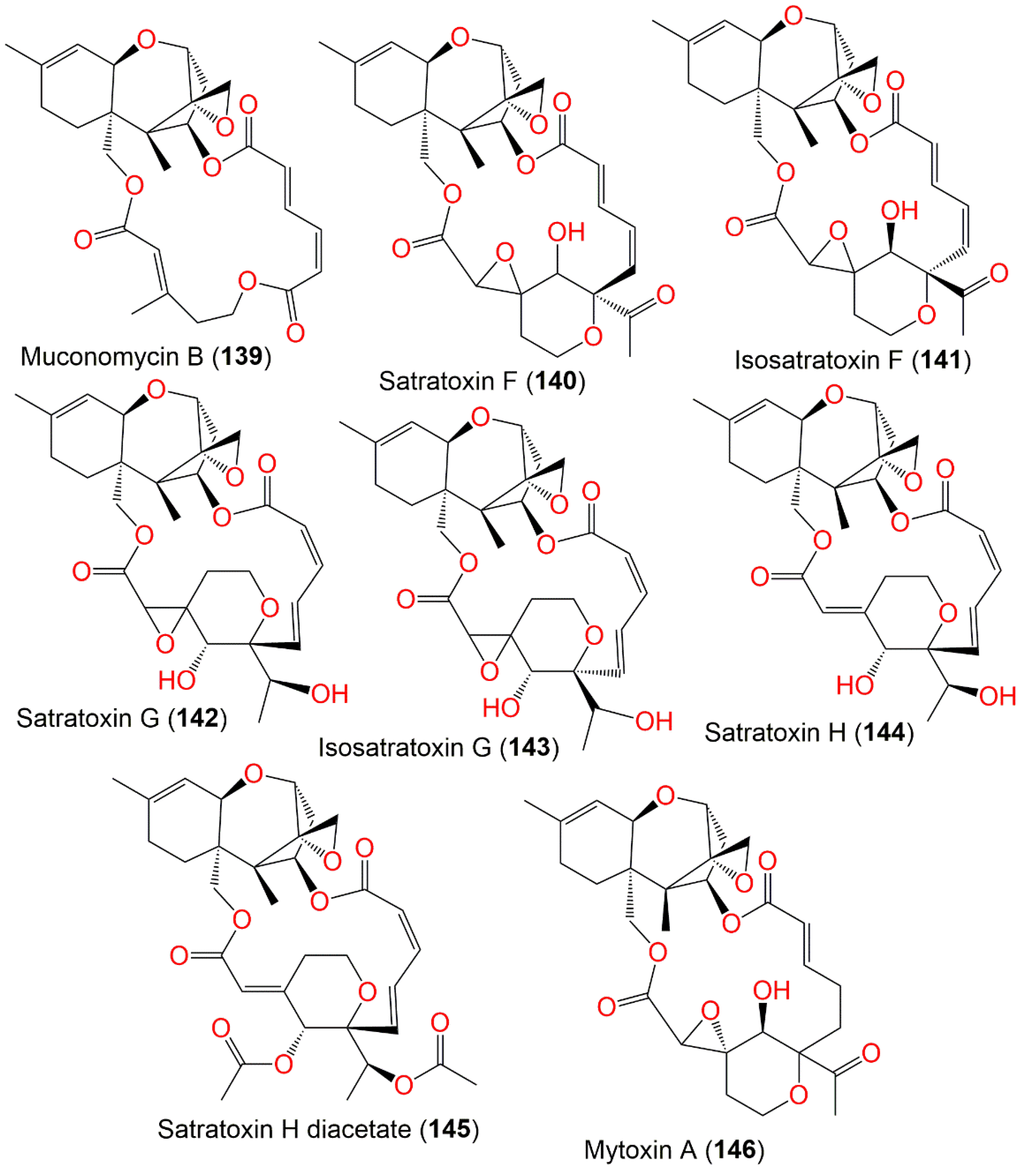
| Muconomycin B (139) | 484 | C27H32O8 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
| Satratoxin F (140) | 542 | C29H34O10 | Cultured | - | [90] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [95,100] | |
| - | - | Dead animals | Various parts of Hungary and Czechoslovakia | [96] | |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| - | - | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] | |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| Isosatratoxin F (141) | 542 | C29H34O10 | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] |
| Satratoxin G (142) | 544 | C29H36O10 | Cultured | - | [65,90,98] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [95,100] | |
| - | - | Dead animals | Various parts of Hungary and Czechoslovakia | [96,99] | |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| - | - | Bedding Straw of a Sheep Flock with Fatal Stachybotryotoxicosis | Hungaria | [101] | |
| - | - | - | Finland | [102] | |
| - | - | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] | |
| - | - | Indoor Air from a Water-Damaged building | Sartorius, Goettingen, Germany | [103] | |
| - | - | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] | |
| Isosatratoxin G (143) | 544 | C29H36O10 | Air and surface samples | Homes enrolled in a case-control study of pulmonary hemosiderosis in infants, Cleveland, Ohio, USA | [93] |
| Satratoxin H (144) | 528 | C29H36O9 | Cultured | - | [65,75,91] |
| - | - | Straw | Hungarian village of Jaszapati from a field case of mycotoxicosis in horses | [75,95] | |
| - | - | Dead animals | Various parts of Hungary and Czechoslovakia | [96,99] | |
| - | - | Egyptian paddy grains | Egypt | [97] | |
| - | - | - | Finland | [102] | |
| - | - | Bedding Straw of a Sheep Flock with Fatal Stachybotryotoxicosis | Hungaria | [101] | |
| - | - | Indoor Air from a Water-Damaged building | Sartorius, Goettingen, Germany | [103] | |
| - | - | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] | |
| - | - | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] | |
| Satratoxin H diacetate (145) | 612 | C33H40O11 | Cultured | - | [91] |
| Mytoxin A (146) | 544 | C29H36O10 | Niphates recondita (Sponge, Niphatidae) | Inner coral reef, Beibuwan Bay, Guangxi, China | [92] |
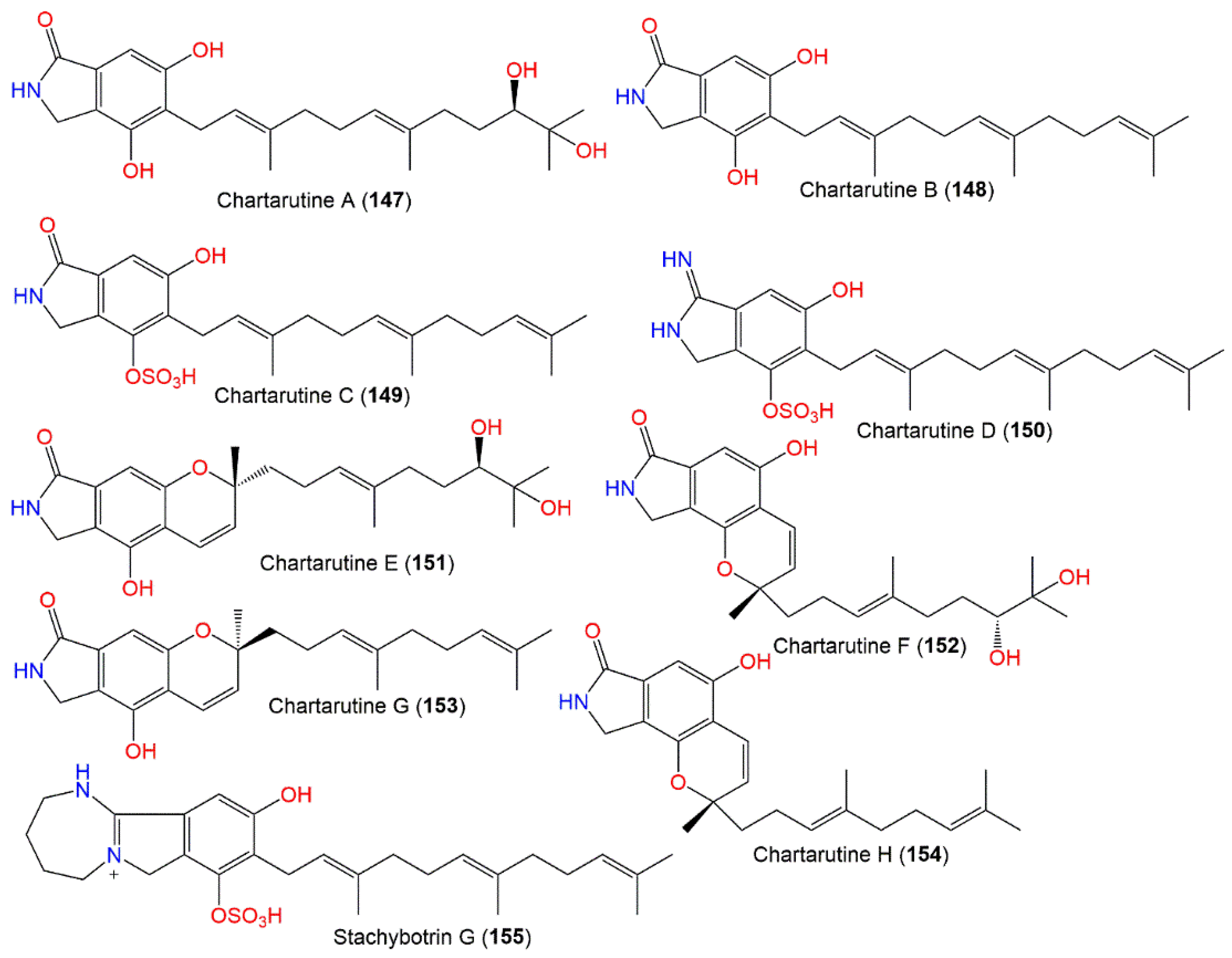
| Chartarutine A (147) | 403 | C23H33NO5 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine B (148) | 369 | C23H31NO3 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| - | - | Cultured | - | [76] | |
| Chartarutine C (149) | 449 | C23H31NO6S | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine D (150) | 448 | C23H32N2O5S | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine E (151) | 401 | C23H31NO5 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine F (152) | 401 | C23H31NO5 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine G (153) | 367 | C23H29NO3 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| Chartarutine H (154) | 367 | C23H29NO3 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
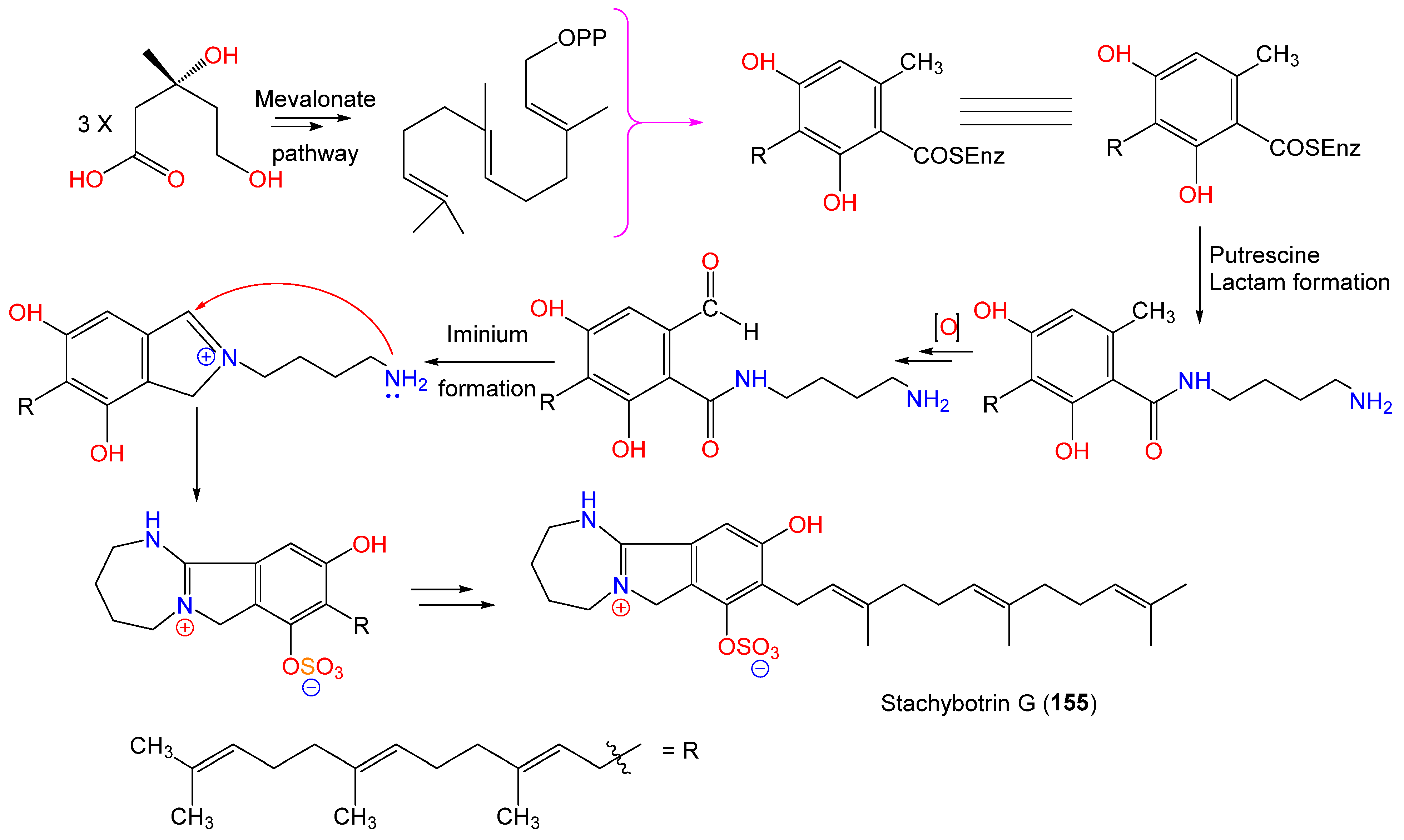
| Stachybotrin G (155) | 503 | C27H39N2O5S | Xestospongia testudinaris (Sponge, Petrosiidae) | Xisha Island, China | [105] |
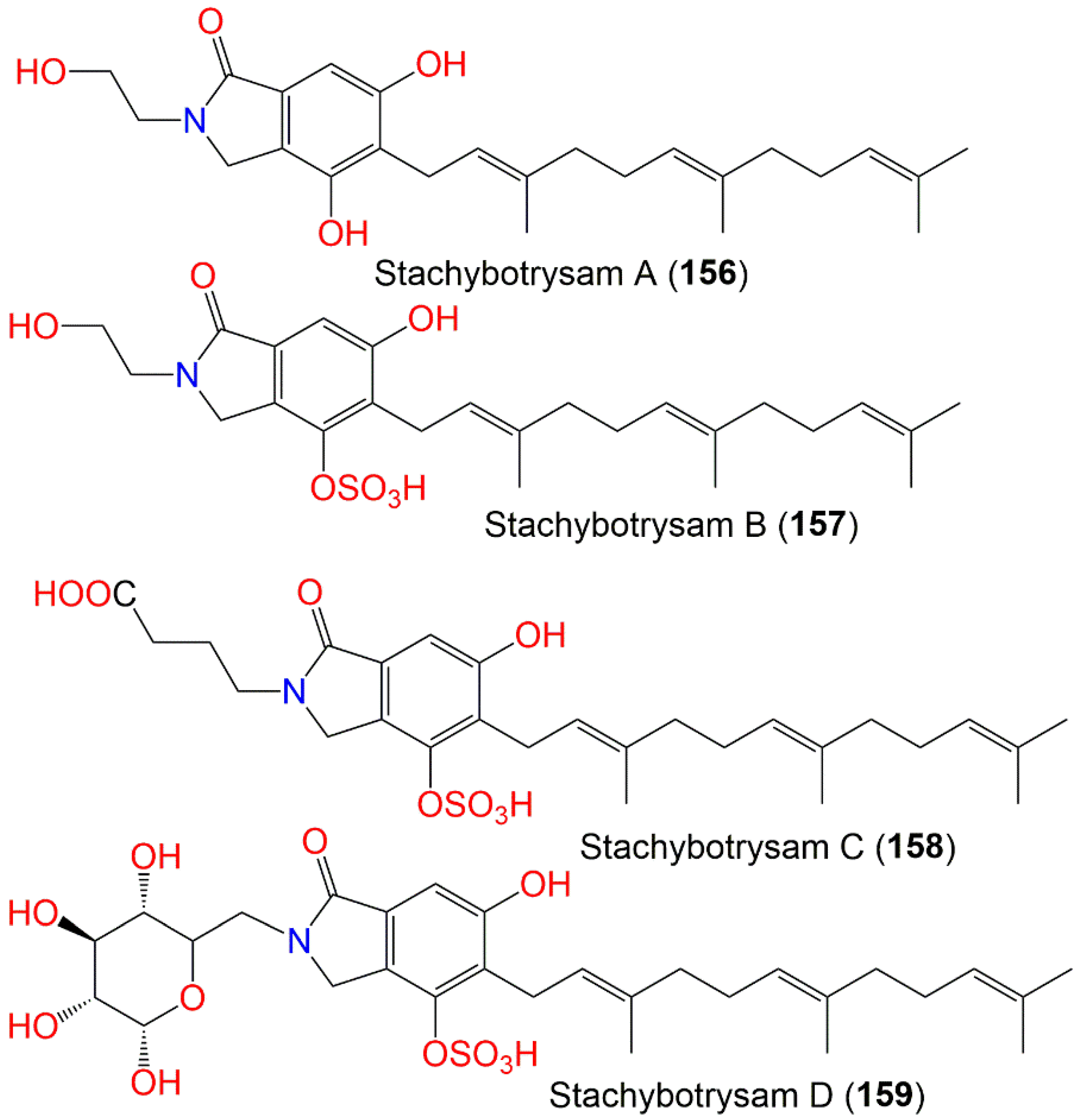
| Stachybotrysam A (156) | 413 | C25H35NO4 | Cultured | - | [76] |
| Stachybotrysam B (157) | 493 | C25H35NO7S | Cultured | - | [76] |
| Stachybotrysam C (158) | 535 | C27H37NO8S | Cultured | - | [76] |
| Stachybotrysam D (159) | 611 | C29H41NO11S | Cultured | - | [76] |
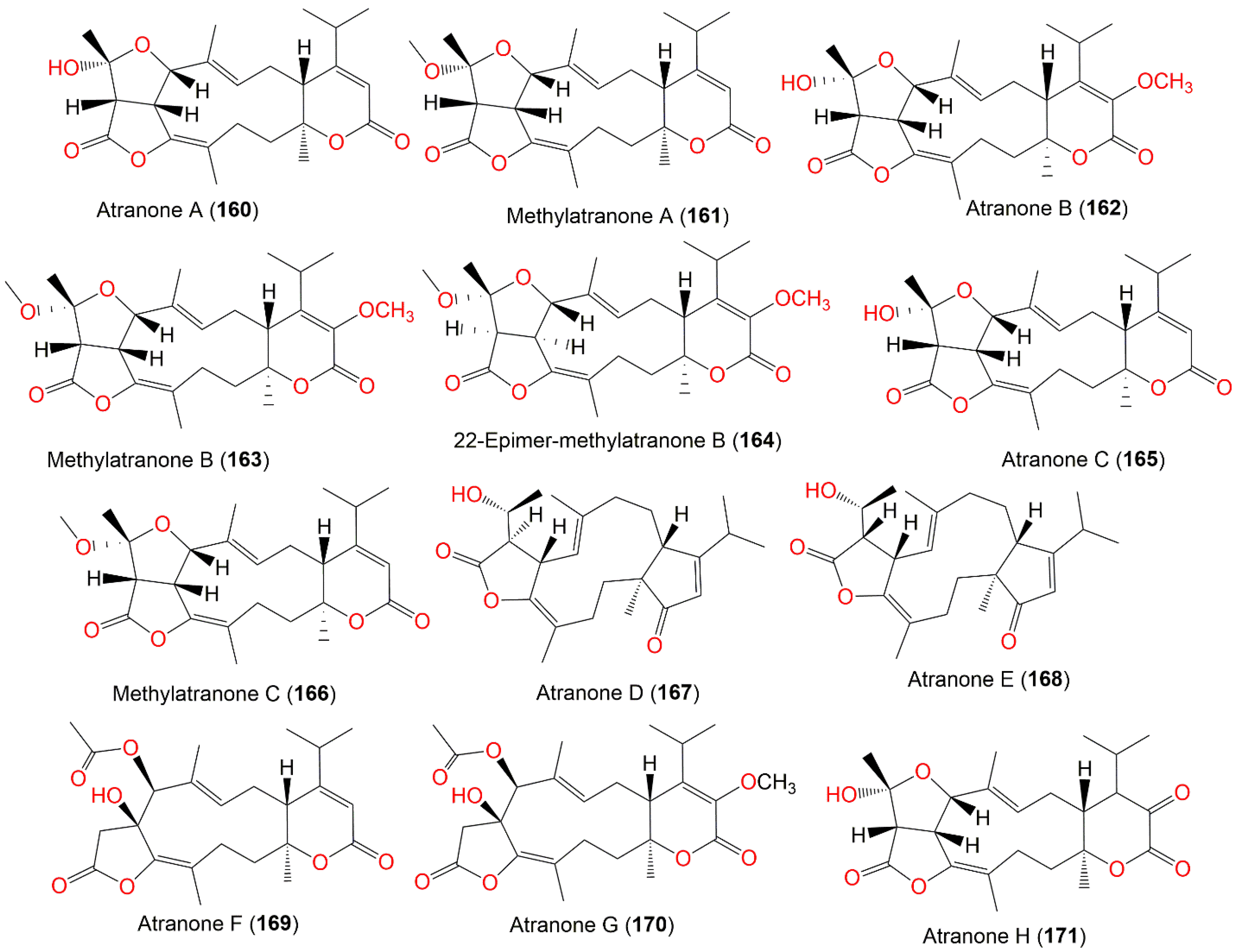
| Atranone A (160) | 416 | C24H32O6 | Cultured | - | [75,106] |
| Methylatranone A (161) | 430 | C25H34O6 | Cultured | - | [107] |
| - | - | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve inGuangdong, China | [108] | |
| Atranone B (162) | 446 | C25H34O7 | Cultured | - | [62,75,106] |
| - | - | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] | |
| Methylatranone B (163) | 460 | C26H36O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| 22-Epimer-methylatranone B (164) | 460 | C26H36O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone C (165) | 416 | C24H32O6 | Cultured | - | [75,106] |
| Methylatranone C (166) | 430 | C25H34O6 | Cultured | - | [107] |
| Atranone D (167) | 386 | C24H34O4 | Cultured | - | [106] |
| Atranone E (168) | 386 | C24H34O4 | Cultured | - | [106] |
| Atranone F (169) | 432 | C24H32O7 | Cultured | - | [106] |
| Atranone G (170) | 462 | C25H34O8 | Cultured | - | [106] |
| Atranone H (171) | 432 | C24H32O7 | Cultured | - | [107] |
| Atranone I (172) | 432 | C24H32O7 | Cultured | - | [107] |
| Atranone J (173) | 404 | C23H32O6 | Cultured | - | [107] |
| Atranone K (174) | 448 | C24H32O8 | Cultured | - | [107] |
| Atranone L (175) | 478 | C26H38O8 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone M (176) | 476 | C26H36O8 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone N (177) | 462 | C26H38O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone O (178) | 480 | C25H36O9 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone P (179) | 404 | C23H32O6 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
| Atranone Q (180) | 390 | C23H34O5 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
| - | - | Cultured | - | [62] | |
| Atranone R (181) | 450 | C25H38O7 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
| Atranone S (182) | 402 | C24H34O5 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
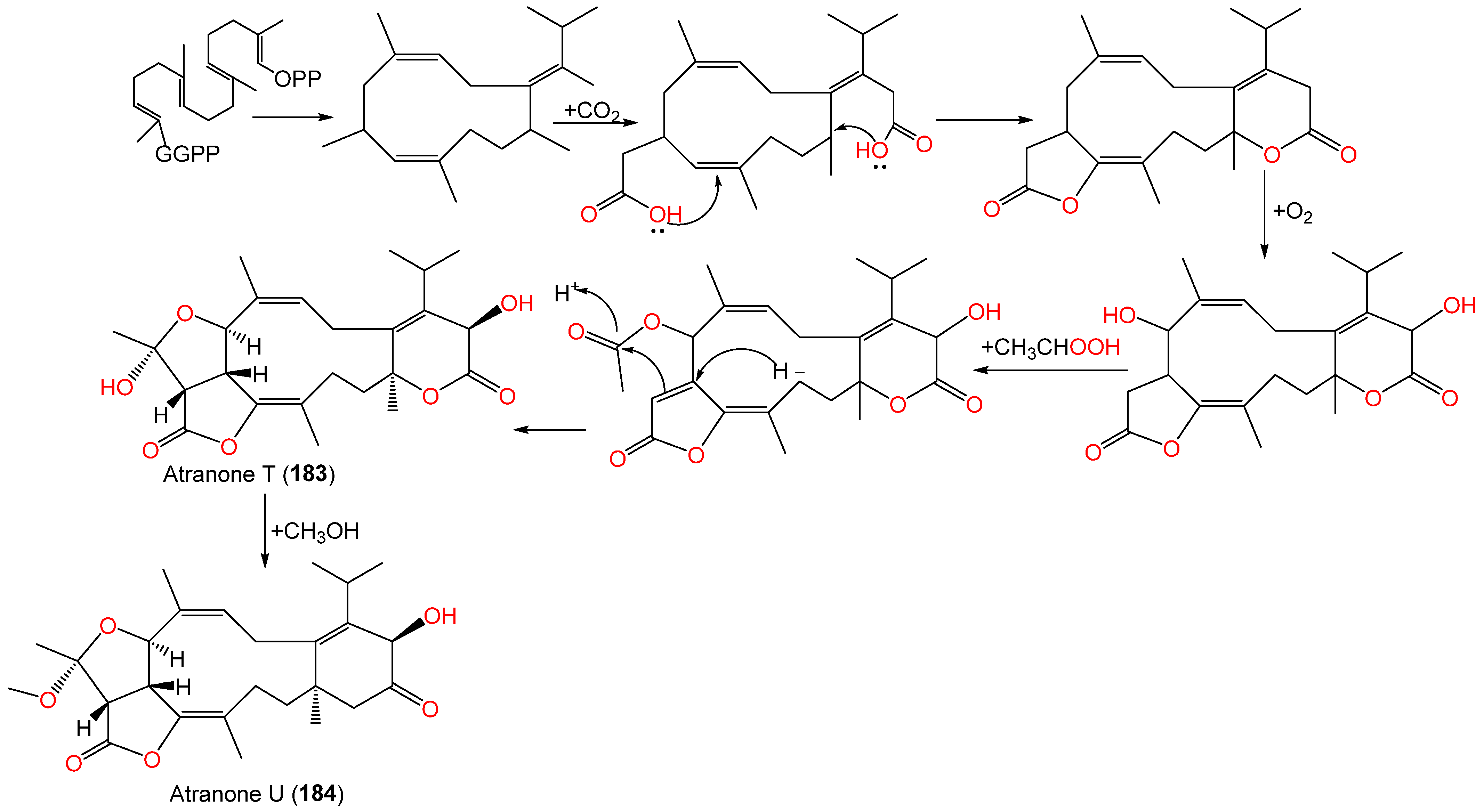
| Atranone T (183) | 432 | C24H32O7 | Cultured | - | [62] |
| Atranone U (184) | 446 | C25H34O7 | Cultured | - | [62] |
| Stachatranone A (185) | 318 | C20H30O3 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
| Stachatranone B (186) | 334 | C20H30O4 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
| Stachatranone C (187) | 334 | C20H30O4 | Sarcophyton subviride (Coral, Alcyoniidae) | Xisha Island, South China Sea, China | [61] |
| - | - | Cultured | - | [62] | |
| 6α-Hydroxydolabella-3E,7E,12-trien-14-one (189) | 302 | C20H30O2 | Cultured | - | [106] |
| (1S*,3R*,4R*,6S*,11S*)-3,4-Epoxy-6-hydroxydolabella-7E,12-dien-14-one (188) | 318 | C20H30O3 | Cultured | - | [106] |
| (1R,6R,11R)-6-Hydroxydolabella-3E,7E, 12-trien-14-one (190) | 302 | C20H30O2 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [108] |
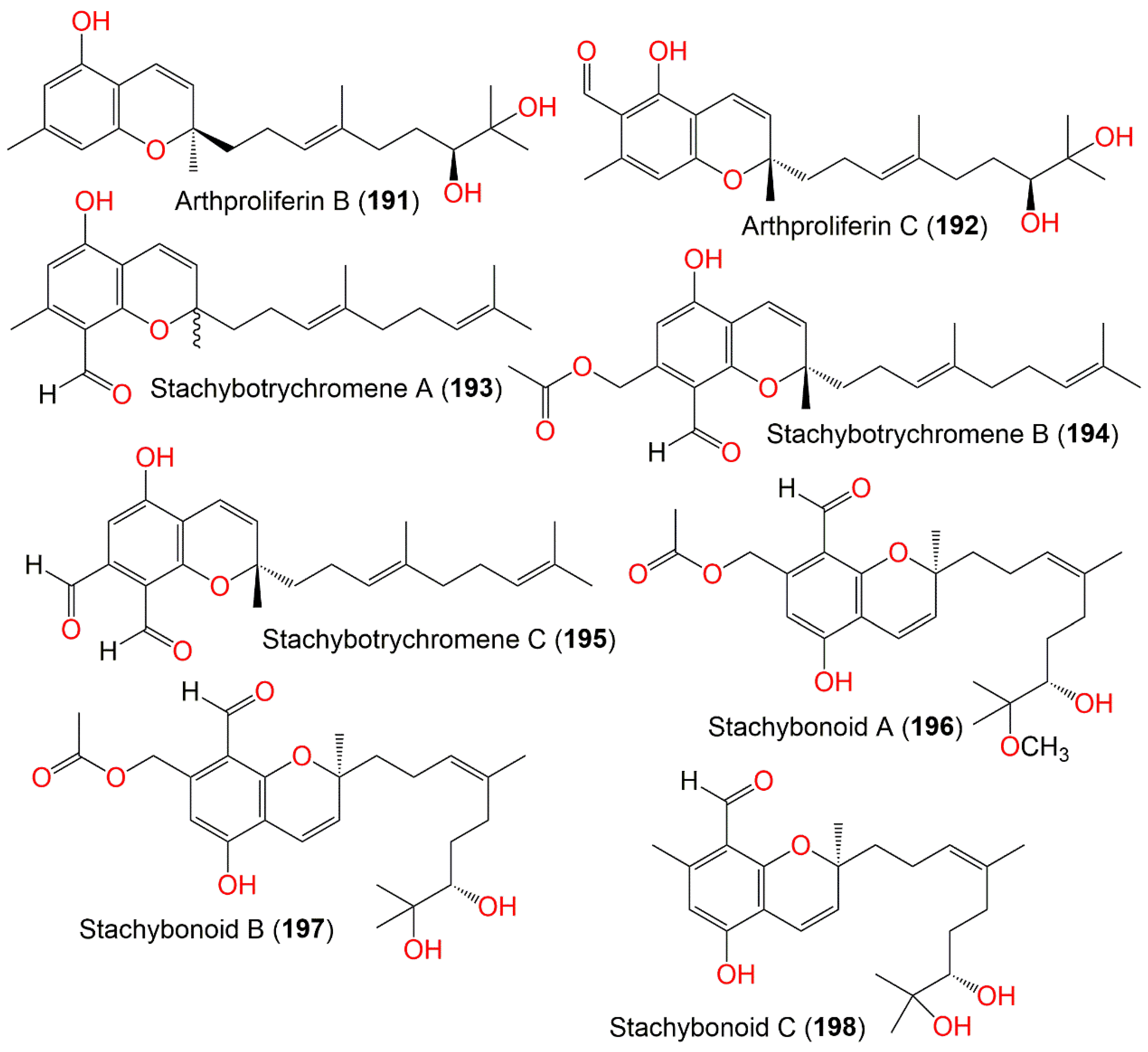
| Arthproliferin B (191) | 360 | C22H32O4 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| Arthproliferin C (192) | 388 | C23H32O5 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| Stachybotrychromene A (193) | 354 | C23H30O3 | Cultured | - | [64] |
| Stachybotrychromene B (194) | 412 | C25H32O5 | Cultured | - | [64] |
| Stachybotrychromene C (195) | 368 | C23H28O4 | Cultured | - | [64] |
| Stachybonoid A (196) | 460 | C26H36O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| Stachybonoid B (197) | 446 | C25H34O7 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
| Stachybonoid C (198) | 388 | C23H32O5 | Himerometra magnipinna (Crinoids, Himerometridae) | Zhanjiang Mangrove National Nature Reserve, Guangdong, China | [68] |
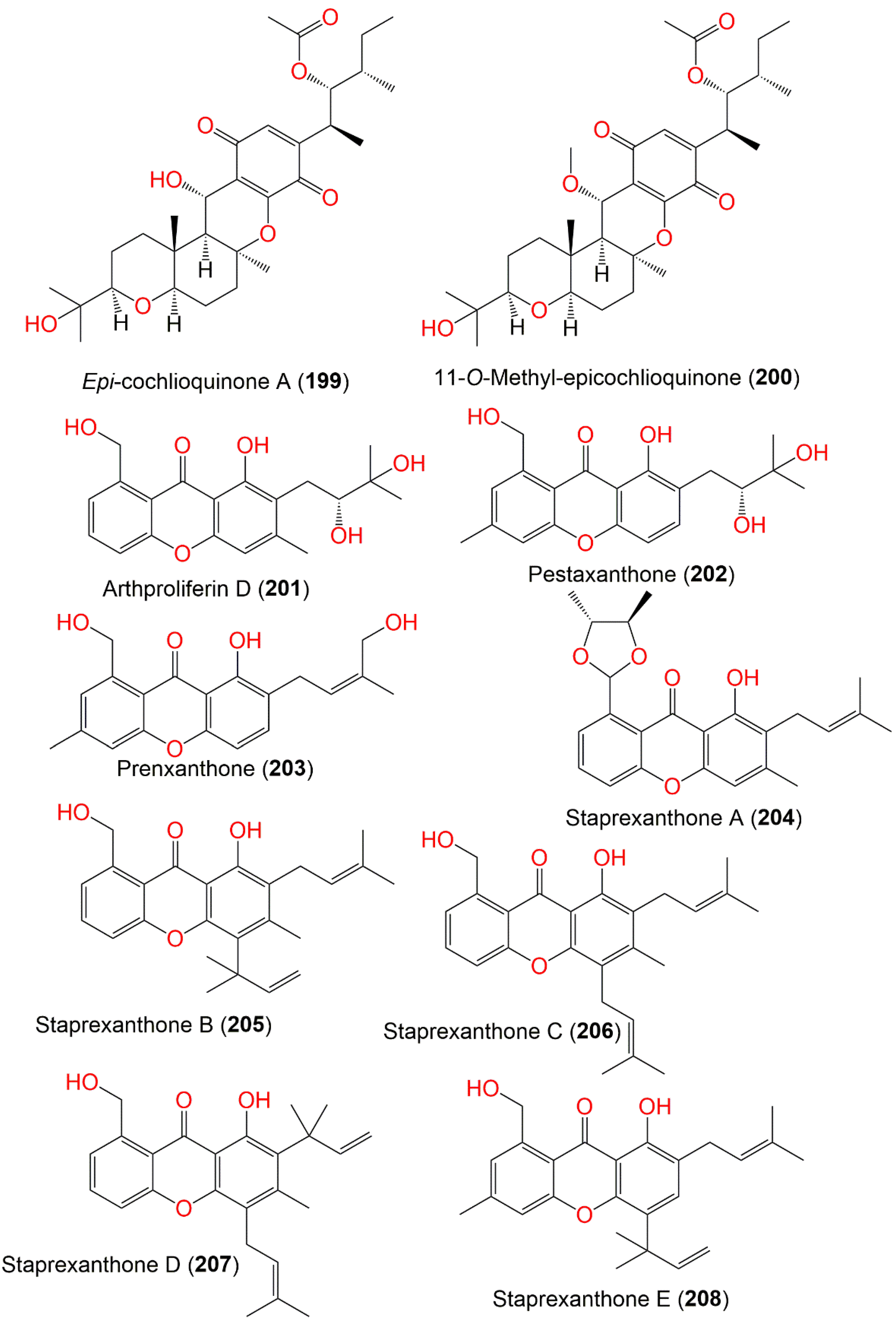
| Epi-cochlioquinone A (199) | 532 | C30H44O8 | Cultured | - | [109] |
| 11-O-Methyl-epicochlioquinone A (200) | 546 | C31H46O8 | Cultured | - | [109] |
| Arthproliferin D (201) | 358 | C20H22O6 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| Pestaxanthone (202) | 358 | C20H22O6 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| Prenxanthone (203) | 340 | C20H20O5 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| Staprexanthone A (204) | 394 | C24H26O5 | Root of an unidentified mangrove plant | Fujian, China | [110] |
| Staprexanthone B (205) | 392 | C25H28O4 | Root of an unidentified mangrove plant | Fujian, China | [110] |
| Staprexanthone C (206) | 392 | C25H28O4 | Root of an unidentified mangrove plant | Fujian, China | [110] |
| Staprexanthone D (207) | 392 | C25H28O4 | Root of an unidentified mangrove plant | Fujian, China | [110] |
| Staprexanthone E (208) | 392 | C25H28O4 | Root of an unidentified mangrove plant | Fujian, China | [110] |
| Cyclosporin A (209) | 1202 | C62H111N11O12 | Soil | Hahajima Island Tokyo, Japan | [111] |
| FR901459 A (210) | 1218 | C62H111N11O13 | Soil | Hahajima Island Tokyo, Japan | [111] |
| Arthproliferin A (211) | 407 | C25H29NO4 | Sinularia sp. (Soft coral, Alcyoniidae) | Yongxing Island, South Chian Sea, China | [73] |
| 5-[(2-Methoxyphenoxy)methyl]-1,3-oxazolidin-2-one (212) | 223 | C11H13NO4 | Black mold | Uzbekistan | [112] |
| Cyclopentanone oxime (213) | 99 | C5H9NO | Black mold | Uzbekistan | [113] |
| BR-011 (214) | 372 | C23H32O4 | Niphates sp. (Sponge, Niphatidae) | Near coral reef, Beibuwan Bay, GuangXi, China | [104] |
| β-Sitosterol (215) | 414 | C29H50O | Black mold | Uzbekistan | [112] |
| Compound Name | Biological Activity | Assay, Organism, or Cell Line | Biological Results | Positive Control | Ref. |
|---|---|---|---|---|---|
| Stachybotrysin B (4) | Anti-HIV-1 virus | Luciferase/VSV-G | 19.2 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Stachybotrysin C (6) | Antiinflammtory | Griess assay/RAW264.7 cells/ LPS-induced NO production inhibition | 27.2 μM (IC50) | Indomethacin 37.5 μM (IC50) | [68] |
| Stachybotrysin E (11) | Anti-HIV-1 virus | Luciferase/VSV-G | 20.5 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Anti-influenza A virus | Luciferase/VSV-G | 45.6 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] | |
| Cytotoxicity | MTT/HepG2 | 36.2 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | |
| F1839-I (12) | Anti-HIV-1 virus | Luciferase/VSV-G | 15.6 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Cytotoxicity | MTT/NCI-H460 | 15.8 μM (IC50) | Paclitaxel 1.0 nM (IC50) | [67] | |
| MTT/BGC823 | 21.9 μM (IC50) | Paclitaxel 0.8 nM (IC50) | [67] | ||
| MTT/Baoy | 41.5 μM (IC50) | Paclitaxel 0.4 nM (IC50) | [67] | ||
| MTT/HepG2 | 18.4 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | ||
| Stachybotrysin A (13) | Anti-HIV-1 virus | Luciferase/VSV-G | 19.6 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Anti-influenza A virus | Luciferase/VSV-G | 12.4 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] | |
| MTT/Baoy | 29.8 μM (IC50) | Paclitaxel 0.4 nM (IC50) | [67] | ||
| MTT/HepG2 | 24.7 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | ||
| Stachartin A = Stachybotrysin (14) | Anti-influenza A virus | Luciferase/VSV-G | 18.7 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] |
| Cytotoxicity | MTT/HepG2 | 34.0 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | |
| Stachybochartin G (15) | Cytotoxicity | MTT/MDA-MB231 | 5.6 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 4.5 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| Stachybotrysin F (16) | Anti-HIV-1 virus | Luciferase/VSV-G | 35.7 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Anti-influenza A virus | Luciferase/VSV-G | 14.6 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] | |
| Cytotoxicity | MTT/HCT116 | 48.5 μM (IC50) | Paclitaxel 1.0 nM (IC50) | [67] | |
| MTT/NCI-H460 | 41.2 μM (IC50) | Paclitaxel 1.0 nM (IC50) | [67] | ||
| MTT/BGC823 | 41.7 μM (IC50) | Paclitaxel 0.8 nM (IC50) | [67] | ||
| MTT/Baoy | 30.6 μM (IC50) | Paclitaxel 0.4 nM (IC50) | [67] | ||
| MTT/HepG2 | 28.4 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | ||
| Stachybotrysin G (17) | Anti-HIV-1 virus | Luciferase/VSV-G | 18.1 μM (IC50) | Efavirenz 4.0 nM (IC50) | [67] |
| Anti-influenza A virus | Luciferase/VSV-G | 23.4 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] | |
| Cytotoxicity | MTT/HCT116 | 44.6 μM (IC50) | Paclitaxel 1.0 nM (IC50) | [67] | |
| MTT/NCI-H460 | 26.9 μM (IC50) | Paclitaxel 1.0 nM (IC50) | [67] | ||
| MTT/BGC823 | 31.6 μM (IC50) | Paclitaxel 0.8 nM (IC50) | [67] | ||
| MTT/Baoy | 56.9 μM (IC50) | Paclitaxel 0.4 nM (IC50) | [67] | ||
| MTT/HepG2 | 24.6 μM (IC50) | Paclitaxel 6.3 nM (IC50) | [67] | ||
| Stachybotrysin H (18) | Potassium channel inhibition | Kv1.3 FLIPR thallium flux /CHO-Kv1.3 | 13.4 μM (IC50) | -Clofazamine 2.01 μM (IC50) -PAP-1 0.17 μM (IC50) | [71] |
| Stachybotrysin I (19) | Potassium channel inhibition | Kv1.3 FLIPR thallium flux /CHO-Kv1.3 | 10.9 μM (IC50) | -Clofazamine 2.01 μM (IC50) -PAP-1 0.17 μM (IC50) | [71] |
| Stachybotrylactone = Stachybotrolide (20) | Antiinflammtory | Griess assay/RAW264.7 cells/ LPS-induced NO production inhibition | 17.9 μM (IC50) | Indomethacin 37.5 μM (IC50) | [68] |
| Stachybotrylactone acetate (21) | Cytotoxicity | MTT/HL-60 | 11.44 μM (IC50) | Cisplatin 1.25 μM (IC50) | [72] |
| MTT/SMMC-7721 | 23.31 μM (IC50) | Cisplatin 7.77 μM (IC50) | [72] | ||
| MTT/A-549 | 22.84 μM (IC50) | Cisplatin 6.12 μM (IC50) | [72] | ||
| MTT/MCF-7 | 18.20 μM (IC50) | Cisplatin 17.63 μM (IC50) | [72] | ||
| MTT/SW480 | 17.38 μM (IC50) | Cisplatin 14.58 μM (IC50) | [72] | ||
| Anti-influenza A virus | Luciferase/VSV-G | 18.9 μM (IC50) | Ribavirion 2.0 nM (IC50) | [67] | |
| Stachybotrane A (22) | Cytotoxicity | MTT/HL-60 | 9.23 μM (IC50) | Cisplatin 1.25 μM (IC50) | [72] |
| MTT/SMMC-7721 | 19.67 μM (IC50) | Cisplatin 7.77 μM (IC50) | [72] | ||
| MTT/A-549 | 31.22 μM (IC50) | Cisplatin 6.12 μM (IC50) | [72] | ||
| MTT/MCF-7 | 18.74 μM (IC50) | Cisplatin 17.63 μM (IC50) | [72] | ||
| Stachybotrane B (23) | Cytotoxicity | MTT/HL-60 | 15.08 μM (IC50) | Cisplatin 1.25 μM (IC50) | [72] |
| MTT/SMMC-7721 | 24.90 μM (IC50) | Cisplatin 7.77 μM (IC50) | [72] | ||
| MTT/MCF-7 | 29.34 μM (IC50) | Cisplatin 17.63 μM (IC50) | [72] | ||
| MTT/SW480 | 31.03 μM (IC50) | Cisplatin 14.58 μM (IC50) | [72] | ||
| K-76-1 (36) | Tyrosine kinase inhibition | Radiometry/33P | >0.2 mM (IC50) | - | [77] |
| K-76-2 (37) | Tyrosine kinase inhibition | Radiometry/33P | >0.031 mM (IC50) | - | [77] |
| Stachybonoid F (48) | Antiinflammtory | Griess assay/RAW264.7 cells/ LPS-induced NO production inhibition | 52.5 μM (IC50) | Indomethacin 37.5 μM (IC50) | [68] |
| Stachybochartin G (51) | Cytotoxicity | MTT/MDA-MB231 | 4.5 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 5.6 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| K-76-3 (59) | Tyrosine kinase inhibition | Radiometry/33P | >0.4 mM (IC50) | - | [77] |
| K-76-4 (60) | Tyrosine kinase inhibition | Radiometry/33P | >0.025 mM (IC50) | - | [77] |
| K-76-5 (61) | Tyrosine kinase inhibition | Radiometry/33P | >0.097 mM (IC50) | - | [77] |
| K-76-6 (62) | Tyrosine kinase inhibition | Radiometry/33P | >0.146 mM (IC50) | - | [77] |
| K-76-7 (63) | Tyrosine kinase inhibition | Radiometry/33P | >0.046 mM (IC50) | - | [77] |
| Bistachybotrysin A (73) | Cytotoxicity | MTT/HCT116 | 6.7 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [79] |
| MTT/NCI-H460 | 7.5 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [79] | ||
| MTT/BGC823 | 14.2 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [79] | ||
| MTT/Daoy | 2.8 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [79] | ||
| MTT/HepG2 | 11.9 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [79] | ||
| Bistachybotrysin B (74) | Cytotoxicity | MTT/HCT116 | 18.0 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [79] |
| MTT/NCI-H460 | 5.5 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [79] | ||
| MTT/BGC823 | 6.6 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [79] | ||
| MTT/Daoy | 4.2 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [79] | ||
| MTT/HepG2 | 19.3 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [79] | ||
| Bistachybotrysin C (75) | Cytotoxicity | MTT/HCT116 | 19.1 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [79] |
| MTT/NCI-H460 | 12.3 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [79] | ||
| MTT/BGC823 | 19.2 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [79] | ||
| MTT/Daoy | 14.6 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [79] | ||
| MTT/HepG2 | 19.3 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [79] | ||
| Bistachybotrysin D (76) | Cytotoxicity | MTT/HCT116 | 6.8 μM (IC50) | Paclitaxel 0.00381 μM (IC50) | [80] |
| MTT/NCI-H460 | 14.7 μM (IC50) | Paclitaxel 0.000384 μM (IC50) | [80] | ||
| MTT/BGC823 | 11.4 μM (IC50) | Paclitaxel 0.00197 μM (IC50) | [80] | ||
| MTT/Daoy | 11.6 μM (IC50) | Paclitaxel 0.000187 μM (IC50) | [80] | ||
| MTT/HepG2 | 7.5 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [80] | ||
| Antiinflammtory | LPS/BV2/NO production inhibition | 61.1% at 10.0 μM | Curcumin 67.6% at 10.0 μM | [80] | |
| Bistachybotrysin E (77) | Cytotoxicity | MTT/HCT116 | 8.9 μM (IC50) | Paclitaxel 0.00381 μM (IC50) | [80] |
| MTT/NCI-H460 | 19.0 μM (IC50) | Paclitaxel 0.000384 μM (IC50) | [80] | ||
| MTT/BGC823 | 6.7 μM (IC50) | Paclitaxel 0.00197 μM (IC50) | [80] | ||
| MTT/Daoy | 59.0 μM (IC50) | Paclitaxel 0.000187 μM (IC50) | [80] | ||
| MTT/HepG2 | 12.4 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [80] | ||
| Bistachybotrysin F (78) | Cytotoxicity | MTT/HCT116 | 22.8 μM (IC50) | Taxol 0.00381 μM (IC50) | [81] |
| MTT/NCI-H460 | 22.5 μM (IC50) | Taxol 0.000384 μM (IC50) | [81] | ||
| MTT/BGC823 | 18.3 μM (IC50) | Taxol 0.00197 μM (IC50) | [81] | ||
| MTT/Daoy | 61.8 μM (IC50) | Taxol 0.000187 μM (IC50) | [81] | ||
| MTT/HepG2 | 18.3 μM (IC50) | Taxol 0.0102 μM (IC50) | [81] | ||
| Bistachybotrysin G (79) | Cytotoxicity | MTT/HepG2 | 22.7 μM (IC50) | Taxol 0.0102 μM (IC50) | [81] |
| Bistachybotrysin H (80) | Cytotoxicity | MTT/HCT116 | 20.7 μM (IC50) | Taxol 0.00381 μM (IC50) | [81] |
| MTT/NCI-H460 | 10.6 μM (IC50) | Taxol 0.000384 μM (IC50) | [81] | ||
| MTT/BGC823 | 21.1 μM (IC50) | Taxol 0.00197 μM (IC50) | [81] | ||
| MTT/Daoy | 20.7 μM (IC50) | Taxol 0.000187 μM (IC50) | [81] | ||
| MTT/HepG2 | 18.4 μM (IC50) | Taxol 0.0102 μM (IC50) | [81] | ||
| Bistachybotrysin I (81) | Cytotoxicity | MTT/HCT116 | 9.1 μM (IC50) | Taxol 0.00381 μM (IC50) | [81] |
| MTT/NCI-H460 | 19.9 μM (IC50) | Taxol 0.000384 μM (IC50) | [81] | ||
| MTT/BGC823 | 17.2 μM (IC50) | Taxol 0.00197 μM (IC50) | [81] | ||
| MTT/Daoy | 18.4 μM (IC50) | Taxol 0.000187 μM (IC50) | [81] | ||
| MTT/HepG2 | 21.4 μM (IC50) | Taxol 0.0102 μM (IC50) | [81] | ||
| Bistachybotrysin J (82) | Cytotoxicity | MTT/HCT116 | 15.8 μM (IC50) | Taxol 0.00381 μM (IC50) | [81] |
| MTT/NCI-H460 | 20.4 μM (IC50) | Taxol 0.000384 μM (IC50) | [81] | ||
| MTT/BGC823 | 16.9 μM (IC50) | Taxol 0.00197 μM (IC50) | [81] | ||
| MTT/Daoy | 25.4 μM (IC50) | Taxol 0.000187 μM (IC50) | [81] | ||
| MTT/HepG2 | 12.2 μM (IC50) | Taxol 0.0102 μM (IC50) | [81] | ||
| Bistachybotrysin K (83) | Cytotoxicity | MTT/HCT116 | 3.4 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [82] |
| MTT/NCI-H460 | 4.7 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [82] | ||
| MTT/BGC823 | 3.3 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [82] | ||
| MTT/Daoy | 1.1 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [82] | ||
| MTT/HepG2 | 4.3 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [82] | ||
| Bistachybotrysin L (84) | Neuroprotective | MTT/SK-N-SH | 0.15 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 10.6 μM (IC50) | Paclitaxel 0.038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 13.5 μM (IC50) | Paclitaxel 0.004 μM (IC50) | [83] | ||
| MTT/BGC823 | 22.3 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/HepG2 | 18.9 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Antiinflammtory | LPS/BV2/NO production inhibition | 5.31% at 10.0 μM | Curcumin 67.6% at 10.0 μM | [83] | |
| Bistachybotrysin M (85) | Neuroprotective | MTT/SK-N-SH | 17.4 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 2.5 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 3.5 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 1.8 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 2.4 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 2.2 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Antiinflammtory | LPS/BV2/NO production inhibition | 26.3% at 10.0 μM | Curcumin 67.6% at 10.0 μM | [83] | |
| Bistachybotrysin N (86) | Neuroprotective | MTT/SK-N-SH | 17.6 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 64.5 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 8.3 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 12.5 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 61.4 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 56.1 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin O (87) | Neuroprotective | MTT/SK-N-SH | 8.4 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 18.8 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 11.5 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 20.5 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 10.7 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 20.1 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin P (88) | Antiinflammtory | LPS/BV2/NO production inhibition | 12.9% at 10.0 μM | Curcumin 67.6% at 10.0 μM | [83] |
| Bistachybotrysin Q (89) | Antiinflammtory | LPS/BV2/NO production inhibition | 10.1% at 10.0 μM | Curcumin 67.6% at 10.0 μM | [83] |
| Cytotoxicity | MTT/HCT116 | 18.5 μM (IC50) | Paclitaxel 0.038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 8.8 μM (IC50) | Paclitaxel 0.004 μM (IC50) | [83] | ||
| MTT/BGC823 | 55.4 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 17.3 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 14.1 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin R (90) | Cytotoxicity | MTT/HCT116 | 8.8 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] |
| MTT/NCI-H460 | 8.2 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 17.8 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 13.1 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 9.4 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin S (91) | Neuroprotective | MTT/SK-N-SH | 6.5 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 8.0 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 11.7 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 8.7 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 11.8 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 6.0 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Antiinflammtory | LPS/BV2/NO production inhibition | 54.2 % at 10.0 μM | Curcumin 67.6% at 10.0 μM | [83] | |
| Bistachybotrysin T (92) | Neuroprotective | MTT/SK-N-SH | 17.4 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Bistachybotrysin U (93) | Neuroprotective | MTT/SK-N-SH | 9.3 % at 10.0 μM, ↑ cell viability | Resveratrol 16.1% at 10.0 μM, ↑ cell viability | [83] |
| Cytotoxicity | MTT/HCT116 | 9.7 μM (IC50) | Paclitaxel 0.038 μM (IC50) | [83] | |
| MTT/NCI-H460 | 10.1 μM (IC50) | Paclitaxel 0.004 μM (IC50) | [83] | ||
| MTT/BGC823 | 9.8 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 8.1 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 9.4 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin V (94) | Cytotoxicity | MTT/HCT116 | 15.0 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [83] |
| MTT/NCI-H460 | 10.9 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [83] | ||
| MTT/BGC823 | 23.9 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [83] | ||
| MTT/Daoy | 27.7 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [83] | ||
| MTT/HepG2 | 12.9 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [83] | ||
| Bistachybotrysin W (95) | Cytotoxicity | MTT/HCT116 | 12.1 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [84] |
| MTT/NCI-H460 | 11.5 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [84] | ||
| MTT/BGC823 | 13.2 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [84] | ||
| MTT/Daoy | 8.8 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [84] | ||
| MTT/HepG2 | 7.0 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [84] | ||
| Bistachybotrysin X (96) | Cytotoxicity | MTT/HCT116 | 22.6 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [84] |
| MTT/NCI-H460 | 10.8 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [84] | ||
| MTT/BGC823 | 15.1 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [84] | ||
| MTT/Daoy | 21.5 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [84] | ||
| MTT/HepG2 | 11.5 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [84] | ||
| Bistachybotrysin Y (97) | Cytotoxicity | MTT/HCT116 | 5.9 μM (IC50) | Paclitaxel 0.0038 μM (IC50) | [84] |
| MTT/NCI-H460 | 13.0 μM (IC50) | Paclitaxel 0.0004 μM (IC50) | [84] | ||
| MTT/BGC823 | 14.1 μM (IC50) | Paclitaxel 0.002 μM (IC50) | [84] | ||
| MTT/Daoy | 6.4 μM (IC50) | Paclitaxel 0.0002 μM (IC50) | [84] | ||
| MTT/HepG2 | 9.8 μM (IC50) | Paclitaxel 0.0102 μM (IC50) | [84] | ||
| Chartarolide A (98) | Cytotoxicity | MTT/HCT-116 | 1.9 μM (IC50) | Taxol 0.03 μM (IC50) | [85] |
| MTT/HepG2 | 1.8 μM (IC50) | Taxol 0.02 μM (IC50) | [85] | ||
| MTT/BGC-823 | 1.3 μM (IC50) | Taxol 0.001 μM (IC50) | [85] | ||
| MTT/NCI-H1650 | 5.5 μM (IC50) | Taxol 0.07 μM (IC50) | [85] | ||
| MTT/A2780 | 1.5 μM (IC50) | Taxol 0.03 μM (IC50) | [85] | ||
| MTT/MCF-7 | 1.4 μM (IC50) | Taxol 0.09 μM (IC50) | [85] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 2.6 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | |
| Spectrophotometric/IGF1R | 6.8 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Spectrophotometric/PDGFRb | 9.1 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Spectrophotometric/TRKB | 8.0 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Chartarolide B (99) | Cytotoxicity | MTT/HCT-116 | 2.3 μM (IC50) | Taxol 0.03 μM (IC50) | [85] |
| MTT/HepG2 | 2.8 μM (IC50) | Taxol 0.02 μM (IC50) | [85] | ||
| MTT/BGC-823 | 1.6 μM (IC50) | Taxol 0.001 μM (IC50) | [85] | ||
| MTT/NCI-H1650 | 4.8 μM (IC50) | Taxol 0.07 μM (IC50) | [85] | ||
| MTT/A2780 | 3.2 μM (IC50) | Taxol 0.03 μM (IC50) | [85] | ||
| MTT/MCF-7 | 3.8 μM (IC50) | Taxol 0.09 μM (IC50) | [85] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 4.9 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | |
| Spectrophotometric/IGF1R | 8.4 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Spectrophotometric/PDGFRb | 20.3 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Spectrophotometric/TRKB | 11.3 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Chartarolide C (100) | Cytotoxicity | MTT/HCT-116 | 7.8 μM (IC50) | Taxol 0.03 μM (IC50) | [85] |
| MTT/HepG2 | 8.9 μM (IC50) | Taxol 0.02 μM (IC50) | [85] | ||
| MTT/BGC-823 | 5.4 μM (IC50) | Taxol 0.001 μM (IC50) | [85] | ||
| MTT/NCI-H1650 | 11.3 μM (IC50) | Taxol 0.07 μM (IC50) | [85] | ||
| MTT/A2780 | 12.5 μM (IC50) | Taxol 0.03 μM (IC50) | [85] | ||
| MTT/MCF-7 | 8.7 μM (IC50) | Taxol 0.09 μM (IC50) | [85] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 21.4 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | |
| Spectrophotometric/TRKB | 18.8 μM (IC50) | Satratoxin H ˂0.5 μM (IC50) | [85] | ||
| Chartarlactam Q (101) | Antibacterial | Broth microdilution/S. aureus ATCC 29213 | 8.0 μg/mL (MIC) | Chloramphenicol 1.0 μg/mL (MIC) | [86] |
| Chartarlactam R (102) | Antibacterial | Broth microdilution/S. aureus ATCC 29213 | 16.0 μg/mL (MIC) | Chloramphenicol 1.0 μg/mL (MIC) | [86] |
| Chartarlactam S (103) | Antibacterial | Broth microdilution/S. aureus ATCC 29213 | 4.0 μg/mL (MIC) | Chloramphenicol 1.0 μg/mL (MIC) | [86] |
| Stachybochartin A (105) | Cytotoxicity | MTT/MDA-MB231 | 21.7 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 19.8 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| Stachybochartin B (106) | Cytotoxicity | MTT/MDA-MB231 | 17.6 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 11.2 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| Stachybochartin C (107) | Cytotoxicity | MTT/MDA-MB231 | 11.6 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 14.5 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| Stachybochartin D (108) | Cytotoxicity | MTT/MDA-MB231 | 10.4 μM (IC50) | -Cisplatin 11.3 μM (IC50) -Doxorubicin 1.0 μM (IC50) | [70] |
| MTT/U2-OS | 9.2 μM (IC50) | -Cisplatin 5.9 μM (IC50) -Doxorubicin 1.2 μM (IC50) | [70] | ||
| Stachyin B (109) | Antibacterial | Broth microdilution/S. aureus ATCC 29213 | 4.0 μg/mL (MIC) | Chloramphenicol 1.0 μg/mL (MIC) | [86] |
| 2,4,12-Trihydroxyapotrichothecene (116) | Cytotoxicity | MTT/HCT-116 | 0.87 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 0.69 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 0.65 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 0.84 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 0.69 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.5 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 1.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 2.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 1.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 2.2 μM (IC50) | - | [92] | ||
| Chartarene A (117) | Cytotoxicity | MTT/HCT-116 | 3.39 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 3.95 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 2.87 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/A2780 | 2.38 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Chartarene B (118) | Cytotoxicity | MTT/HCT-116 | 5.58 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 2.4 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 6.9 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 10.4 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 7.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 12.9 μM (IC50) | - | [92] | ||
| Chartarene C (119) | Cytotoxicity | MTT/HCT-116 | 0.74 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 2.09 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.58 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 2.07 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 1.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 3.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 5.3 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 2.7 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 6.3 μM (IC50) | - | [92] | ||
| Trichodermol (120) | Cytotoxicity | MTT/HCT-116 | 7.22 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 3.69 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 2.55 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.68 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 2.68 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.9 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.8 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 3.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 1.8 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 3.0 μM (IC50) | - | [92] | ||
| Isotrichoverrol B (123) | Cytotoxicity | MTT/HCT-116 | 3.48 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 2.62 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 2.64 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.36 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 2.12 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 24.0 μM (IC50) | - | [92] | |
| Verrol (124) | Cytotoxicity | MTT/HCT-116 | 2.77 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 1.45 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 2.33 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.68 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 2.00 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.2 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 0.7 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 0.4 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 0.9 μM (IC50) | - | [92] | ||
| Trichodermadienediol B (128) | Cytotoxicity | MTT/HCT-116 | 1.65 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 0.86 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 0.81 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 1.31 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 0.68 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.5 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.7 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 1.9 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 1.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 1.9 μM (IC50) | - | [92] | ||
| Roridin L-2 (129) | Cytotoxicity | MTT/HCT-116 | 1.73 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 1.20 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 1.81 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.36 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 1.61 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.4 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.5 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 2.3 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 1.9 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 1.7 μM (IC50) | - | [92] | ||
| Chartarene D (130) | Cytotoxicity | MTT/HCT-116 | 1.48 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 0.90 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 0.68 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 2.23 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 0.69 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 0.8 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 0.7 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 0.7 μM (IC50) | - | [92] | ||
| Roridin E (134) | Cytotoxicity | MTT/HCT-116 | ˂0.01 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | ˂0.01 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.4 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.4 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 1.4 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 1.0 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 2.1 μM (IC50) | - | [92] | ||
| Muconomycin B (139) | Cytotoxicity | MTT/HCT-116 | 1.32 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | 0.81 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | 0.84 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | 0.86 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | 0.68 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.3 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.4 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 1.2 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 0.7 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 1.5 μM (IC50) | - | [92] | ||
| Satratoxin F (140) | Antibacterial | Serial dilution/Methicillin-resistant S. aureus ATCC 29213 | 39.0 μg/mL (MIC) | Ampicillin 10.0 μg/mL (MIC) | [73] |
| Satratoxin G (142) | Cytotoxicity | MTT/HCT-116 | ˂0.01 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | ˂0.01 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | 0.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | 0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | 0.5 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | 0.2 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 0.9 μM (IC50) | - | [92] | ||
| Satratoxin H (144) | Cytotoxicity | MTT/HCT-116 | ˂0.01 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | ˂0.01 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | ˂0.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | 0.1 μM (IC50) | - | [92] | ||
| Mytoxin A (146) | Cytotoxicity | MTT/HCT-116 | ˂0.01 μM (IC50) | Taxol 0.03 μM (IC50) | [92] |
| MTT/HepG2 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/BGC-823 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| MTT/NCI-H1650 | ˂0.01 μM (IC50) | Taxol 0.04 μM (IC50) | [92] | ||
| MTT/A2780 | ˂0.01 μM (IC50) | Taxol 0.01 μM (IC50) | [92] | ||
| Tumor-related kinases inhibition | Spectrophotometric/FGFR3 | ˂0.1 μM (IC50) | - | [92] | |
| Spectrophotometric/IGF1R | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/PDGFRb | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/TRKB | ˂0.1 μM (IC50) | - | [92] | ||
| Spectrophotometric/WT | ˂0.1 μM (IC50) | - | [92] | ||
| Chartarutine A (147) | Anti-HIV virus | Luciferase/VSV-G | 74.00 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine B (148) | Anti-HIV virus | Luciferase/VSV-G | 4.90 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine C (149) | Anti-HIV virus | Luciferase/VSV-G | 24.00 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine D (150) | Anti-HIV virus | Luciferase/VSV-G | 51.76 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine E (151) | Anti-HIV virus | Luciferase/VSV-G | 40.70 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine F (152) | Anti-HIV virus | Luciferase/VSV-G | 18.63 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine G (153) | Anti-HIV virus | Luciferase/VSV-G | 5.57 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Chartarutine H (154) | Anti-HIV | Luciferase/VSV-G | 5.58 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
| Stachybotrysam A (156) | Anti-HIV virus | Luciferase/293T cells | 9.3 μM (IC50) | Efavirenz 2.0 nM (IC50) | [76] |
| Stachybotrysam B (157) | Anti-HIV virus | Luciferase/293T cells | 1.0 μM (IC50) | Efavirenz 2.0 nM (IC50) | [76] |
| Stachybotrysam C (158) | Anti-HIV virus | Luciferase/293T cells | 9.6 μM (IC50) | Efavirenz 2.0 nM (IC50) | [76] |
| Atranone Q (180) | Cytotoxicity | MTT/MG-63 | 8.6 μM (IC50) | 5-FU 10.4 μM (IC50) | [62] |
| Antimicrobial | Broth microdilution/S. aureus ATCC 43300 | 32.0 μg/mL (MIC) | Vancomycin 0.5 μg/mL (MIC) | [61] | |
| Broth microdilution/E. faecalis ATCC 29212 | 16.0 μg/mL (MIC) | Vancomycin 0.5 μg/mL (MIC) | [61] | ||
| Broth microdilution/C. albicans ATCC 10231 | 8.0 μg/mL (MIC) | Fluconazole 1.0 μg/mL (MIC) | [61] | ||
| Stachatranone B (186) | Antimicrobial | Broth microdilution/A. baumannii ATCC 19606 | 16.0 μg/mL (MIC) | Ceftriaxone 8 μg/mL (MIC) | [61] |
| Stachybotrychromene A (193) | Cytotoxicity | Alamar Blue assay/HepG2 | 73.7 μM (IC50) | - | [64] |
| Stachybotrychromene B (194) | Cytotoxicity | Alamar Blue assay/HepG2 | 28.2 μM (IC50) | - | [64] |
| Epi-cochlioquinone A (199) | Human chemokine antagonist | CCR-5 | 4.0 μM (IC50) | - | [109] |
| 11-O-Methyl-epi-cochlioquinone A (200) | Human chemokine antagonist | CCR-5 | 7.0 μM (IC50) | - | [109] |
| Cyclosporin A (209) | Immunosuppressant | Lymphocyte murine inhibition/MLR | 9.9 ng/mL (IC50) | - | [111] |
| Mitogen suppression/ConA | 21.9 ng/mL (IC50) | - | [111] | ||
| Skin grafting, TGF-β1/ radioimmunoprecipitation | 19.0 day (Median serivial time)/100 mg/kg orally | Vehicle treated group (Olive oil) | [111] | ||
| FR901459 A (210) | Immunosuppressant | Lymphocyte murine inhibition/MLR | 26.8 ng/mL (IC50) | - | [111] |
| Mitogen suppression/ConA | 50.1 ng/mL (IC50) | - | [111] | ||
| Skin grafting, TGF-β1/ radioimmunoprecipitation | 10.0 day (Median serivial time)/100 mg/kg orally | Vehicle treated group (Olive oil) | [111] | ||
| Arthproliferin A (211) | Antibacterial | Serial dilution/Methicillin-resistant S. aureus ATCC 29213 | 78.0 μg/mL (MIC) | Ampicillin 10.0 μg/mL (MIC) | [73] |
| BR-011 (214) | Anti-HIV virus | Luciferase/VSV-G | 17.90 μM (IC50) | Efavirenz 0.65 μM (IC50) | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.A.; Mohamed, G.A. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. https://doi.org/10.3390/jof8050504
Ibrahim SRM, Choudhry H, Asseri AH, Elfaky MA, Mohamed SGA, Mohamed GA. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. Journal of Fungi. 2022; 8(5):504. https://doi.org/10.3390/jof8050504
Chicago/Turabian StyleIbrahim, Sabrin R. M., Hani Choudhry, Amer H. Asseri, Mahmoud A. Elfaky, Shaimaa G. A. Mohamed, and Gamal A. Mohamed. 2022. "Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance" Journal of Fungi 8, no. 5: 504. https://doi.org/10.3390/jof8050504









