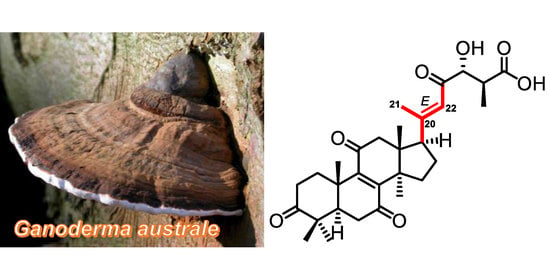
[20(22)E]-Lanostane Triterpenes from the Fungus Ganoderma australe
Abstract
:1. Introduction
2. Experimental Section
2.1. General Experimental Procedures
2.2. Fungal Material
2.3. Extraction and Isolation
2.4. Spectroscopic Data
2.5. Synthesis of the Phenylglycine Methyl Ester (PGME) Derivatives
2.6. Biological Activity Assays
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins-New antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J. Antibiot. 1977, 30, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, X.F.; Fan, Z.J.; Zhang, N.L.; Zhu, Y.J.; Zhang, Z.M.; Khazhieva, I.; Yurievich, M.Y.; Belskaya, N.P.; Bakulev, V.A. Synthesis and fungicidal activity of 3,4-dichloroisothiazole based strobilurins as potent fungicide candidates. RSC Adv. 2017, 7, 3145–3151. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.; Wittstein, K.; Kirk, P.M.; Stadler, M. An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fungal Divers. 2014, 71, 1–15. [Google Scholar] [CrossRef]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia 2010, 81, 1033–1036. [Google Scholar] [CrossRef]
- Mizushinaa, Y.; Hanashimaa, L.; Yamaguchib, T.; Takemurac, M.; Sugawaraa, F.; Saneyoshib, M.; Matsukaged, A.; Yoshidac, S.; Sakaguchi, K. A Mushroom Fruiting Body-Inducing Substance Inhibits Activities of Replicative DNA Polymerases. Biochem. Biophys. Res. Commun. 1998, 249, 17–22. [Google Scholar] [CrossRef]
- Nishitoba, T.; Oda, K.; Sato, H.; Sakamura, S. Novel triterpenoids from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1988, 52, 367–372. [Google Scholar] [CrossRef]
- Wasser, S.P. Reishi or ling zhi (Ganoderma lucidum). Encycl. Diet. Suppl. 2005, 1, 603–622. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [CrossRef]
- Peng, X.-R.; Liu, J.-Q.; Wang, C.-F.; Li, X.-Y.; Shu, Y.; Zhou, L.; Qiu, M.-H. Hepatoprotective effects of triterpenoids from Ganoderma cochlear. J. Nat. Prod. 2014, 77, 737–743. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.E.; Carbonero, E.R.; Simão, R.D.C.G.; Kadowaki, M.K.; Sassaki, G.L.; Osaku, C.A.; Gorin, P.A.; Iacomini, M. An unusual water-soluble β-glucan from the basidiocarp of the fungus Ganoderma resinaceum. Carbohydr. Polym. 2008, 72, 473–478. [Google Scholar] [CrossRef]
- Peng, X.-R.; Liu, J.-Q.; Han, Z.-H.; Yuan, X.-X.; Luo, H.-R.; Qiu, M.-H. Protective effects of triterpenoids from Ganoderma resinaceum on H2O2-induced toxicity in HepG2 cells. Food Chem. 2013, 141, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Ganoderma–A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-C.; Yang, L.; Ma, Q.-Y.; Ge, Y.-Z.; Kong, F.-D.; Zhou, L.-M.; Zhang, F.; Xie, Q.-Y.; Yu, Z.-F.; Dai, H.-F.; et al. Triterpenoids and meroterpenoids with α-glucosidase inhibitory activities from the fruiting bodies of Ganoderma australe. Bioorganic Chem. 2021, 117, 105448. [Google Scholar] [CrossRef]
- León, F.; Valencia, M.; Rivera, A.; Nieto, I.; Quintana, J.; Estévez, F.; Bermejo, J. Novel Cytostatic Lanostanoid Triterpenes from Ganoderma australe. Helvetica Chim. Acta 2003, 86, 3088–3095. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Mayteeworakoon, S.; Laoteng, K.; Choowong, W.; Choeyklin, R. Lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete Ganoderma australe. Nat. Prod. Res. 2017, 32, 1044–1049. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, S.K. The isolation of lanosta-7,9(11),24-trien-3β,21-diol from the fungus Ganoderma australe. Phytochemistry 1984, 23, 686–687. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, Y.; Qin, F.-Y.; Yan, Y.-M.; Cheng, Y.-X. Meroterpenoids and alkaloids from Ganoderma australe. Nat. Prod. Res. 2019, 35, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Dong, Y.; Qin, F.-Y.; Cheng, Y.-X. Australeols A−F, neuroprotective meroterpenoids from Ganoderma australe. Fitoterapia 2019, 134, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Dong, Z.-J.; Wei, K.; Bai, X.; Zhang, L.; Wen, C.-N.; Feng, T.; Liu, J.-K. Novel Natural Oximes and Oxime Esters with a Vibralactone Backbone from the Basidiomycete Boreostereum vibrans. ChemistryOpen 2016, 5, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, Z.-H.; Yao, J.-N.; Peng, Y.-L.; Huang, R.; Feng, T.; Liu, J.-K. Isoindolinone-containing meroterpenoids with α-glucosidase inhibitory activity from mushroom Hericium caput-medusae. Fitoterapia 2017, 122, 107–114. [Google Scholar] [CrossRef]
- Ren, Y.-M.; Zhang, R.; Feng, Z.; Ke, C.-Q.; Yao, S.; Tang, C.; Lin, L.; Ye, Y. Macrocephatriolides A and B: Two Guaianolide Trimers from Ainsliaea macrocephala as PTP1B Inhibitors and Insulin Sensitizers. J. Org. Chem. 2021, 86, 17782–17789. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, P.; Yu, H.; Li, J.; Wang, M.W.; Zhao, W. Anti-Helicobacter pylori and Thrombin Inhibitory Components from Chinese Dragon’s Blood, Dracaena cochinchinensis. J. Nat. Prod. 2007, 70, 1570–1577. [Google Scholar] [CrossRef]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Zhao, J.; Chen, L.-X.; Wang, S.-F.; Wang, Y.; Li, S.-P. Lanostane triterpenes from the mushroom Ganoderma resinaceum and their inhibitory activities against α-glucosidase. Phytochemistry 2018, 149, 103–115. [Google Scholar] [CrossRef]
- Peng, X.-R.; Wang, Q.; Su, H.-G.; Zhou, L.; Xiong, W.-Y.; Qiu, M.-H. Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum. J. Fungi 2022, 8, 331. [Google Scholar] [CrossRef]
- Pu, D.; Li, X.; Lin, J.; Zhang, R.; Luo, T.; Wang, Y.; Gao, J.; Zeb, M.A.; Zhang, X.; Li, X.; et al. Triterpenoids from Ganoderma gibbosum: A Class of Sensitizers of FLC-Resistant Candida albicans to Fluconazole. J. Nat. Prod. 2019, 82, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Ryu, J.; Kim, J.S.; Kang, S.S.; Xu, Y.; Jung, S.H.; Lee, Y.S.; Lee, S.; Shin, K.H. New Lanostane-Type Triterpenoids from Ganoderma applanatum. J. Nat. Prod. 2004, 67, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-G.; Peng, X.-R.; Shi, Q.-Q.; Huang, Y.-J.; Zhou, L.; Qiu, M.-H. Lanostane triterpenoids with anti-inflammatory activities from Ganoderma lucidum. Phytochemistry 2020, 173, 112256. [Google Scholar] [CrossRef] [PubMed]






| No. | 1 ad | 2 ac | 3 bc | 4 ae | 5 ac |
|---|---|---|---|---|---|
| 1 | 2.87, ddd (14.3, 8.5, 5.8) | 2.85, ddd (14.2, 9.8, 7.6) | 2.93, overlapped | 2.75, ddd (13.8, 9.7, 7.8) | 2.95, ddd (14.2, 8.4, 6.2) |
| 1.82, ddd (14.3, 9.7, 6.2) | 1.84, ddd (14.2, 11.9, 2.1) | 1.70, overlapped | 1.94, ddd (13.8, 12.0, 2.5) | 1.73, ddd (14.2, 9.2, 6.7) | |
| 2 | 2.71, ddd (15.2, 9.7, 5.8) | 2.74, ddd (14.4, 11.9, 7.6) | 2.60, ddd (15.5, 9.8, 6.2) | 2.86, ddd (14.5, 12.0, 7.8) | 2.60, ddd (15.5, 9.2, 6.2) |
| 2.45, ddd (15.2, 8.5, 6.2) | 2.33, ddd (14.4, 9.8, 2.1) | 2.44, ddd (15.0, 8.3, 6.2) | 2.16, ddd (14.5, 9.7, 2.5) | 2.50, ddd (15.5, 8.4, 6.7) | |
| 5 | 2.38, dd (14.7, 2.4) | 2.31, d (13.7) | 2.11, dd (9.9, 5.5) | 2.46, d (13.6) | 2.25, dd (15.3, 2.6) |
| 6 | 2.68, t (14.7) | 4.44, d (13.7) | 1.70, overlapped, 2H | 4.57, d (13.6) | 2.53, dd (15.3, 14.7) |
| 2.36, dd (14.7, 2.4) | 2.40, dd (14.7, 2.6) | ||||
| 7 | 4.47, t (2.3) | ||||
| 12 | 2.96, d (16.3) | 2.83, d (17.0) | 2.77, d (17.3) | 3.02, d (16.9) | 2.81, d (16.2) |
| 2.41, d (16.3) | 2.51, d (17.0) | 2.38. d (17.3) | 2.45, d (16.9) | 2.47, d (16.2) | |
| 15 | 2.24, m | 2.29, overlapped | 1.86, m | 2.22, overlapped | 2.30, ddd (12.4, 8.3, 2.7) |
| 1.87, overlapped | 1.74, m | 2.05, m | 1.81, td (12.0, 7.0) | 1.83, m | |
| 16 | 2.04, m | 1.95, overlapped, 2H | 1.98, m, 2H | 2.07, overlapped | 1.92, overlapped, 2H |
| 1.91, dt (11.3, 2.6) | 1.93, overlapped | ||||
| 17 | 2.99, overlapped | 2.91, t (9.2) | 2.95, overlapped | 3.06, overlapped | 2.87, t (9.1) |
| 18 | 0.75, s | 0.69, s | 0.67, s | 0.77, s | 0.70, s |
| 19 | 1.28, s | 1.23, s | 1.00, s | 1.23, s | 1.27, s |
| 21 | 2.16, s | 2.18, s | 2.17, s | 2.18, s | 2.19, s |
| 22 | 6.51, s | 6.23, br.s | 6.25, s | 6.56, s | 6.22, br.s |
| 24 | 4.21, d (4.9) | 4.29, d (2.9) | 4.33, d (3.1) | 4.22, d (4.0) | 4.23, dd (5.7, 3.2) |
| 25 | 2.93, dd (7.3, 4.9) | 3.01, dd (7.2, 2.9) | 3.00, dd (7.2, 3.1) | 3.04, dd (7.1, 4.0) | 2.99, dd (7.2, 3.2) |
| 27 | 1.18, d (7.3) | 1.28, d (7.2) | 1.24, d (7.2) | 1.18, d (7.1) | 1.28, d (7.2) |
| 28 | 1.13, s | 1.34, s | 1.13, s | 1.30, s | 1.13, s |
| 29 | 1.10, s | 1.42, s | 1.06, s | 1.38, s | 1.11, s |
| 30 | 1.35, s | 1.38, s | 1.35, s | 1.42, s | 1.31, s |
| OCH3 | 3.57, s | 3.63, s | |||
| 6-OH | 4.17, br.s | ||||
| 24-OH | 3.89, d (5.7) |
| No. | 1 be | 2 bd | 3 ad | 4 bf | 5 bd | 7 cd |
|---|---|---|---|---|---|---|
| 1 | 36.1, CH2 | 35.3, CH2 | 34.9, CH2 | 35.8, CH2 | 35.0, CH2 | 36.0, CH2 |
| 2 | 35.0, CH2 | 33.4, CH2 | 34.2, CH2 | 33.7, CH2 | 34.1, CH2 | 34.5, CH2 |
| 3 | 218.6, C | 216.8, C | 218.6, C | 216.3, C | 215.6, C | 214.7, C |
| 4 | 47.8, C | 47.7, C | 46.5, C | 48.1, C | 46.8, C | 47.9, C |
| 5 | 50.8, CH | 54.9, CH | 45.0, CH | 54.8, CH | 49.8, CH | 49.9, CH |
| 6 | 37.7, CH2 | 72.2, CH | 29.3, CH2 | 72.8, CH | 37.1, CH2 | 24.3, CH2 |
| 7 | 202.7, C | 202.6, C | 67.1, CH | 203.4, C | 201.1, C | 131.2, CH |
| 8 | 151.3, C | 148.3, C | 160.3, C | 149.2, C | 150.9, C | 138.3, C |
| 9 | 152.4, C | 150.0, C | 140.1, C | 150.1, C | 150.2, C | 160.4, C |
| 10 | 40.1, C | 39.9, C | 37.9, C | 40.5, C | 39.0, C | 38.2, C |
| 11 | 202.8, C | 200.1, C | 200.1, C | 200.7, C | 200.8, C | 119.1, CH |
| 12 | 50.9, CH2 | 49.8, CH2 | 50.1, CH2 | 50.5, CH2 | 50.0, CH2 | 203.3, C |
| 13 | 49.7, C | 48.2, C | 48.9, C | 48.9, C | 48.5, C | 57.8, C |
| 14 | 49.7, C | 48.1, C | 50.8, C | 48.9, C | 48.4, C | 56.4, C |
| 15 | 33.5, CH2 | 31.8, CH2 | 30.4, CH2 | 32.6, CH2 | 32.4, CH2 | 77.0, CH |
| 16 | 24.2, CH2 | 23.4, CH2 | 23.3, CH2 | 23.8, CH2 | 23.4, CH2 | 36.9, CH2 |
| 17 | 54.6, CH | 53.5, CH | 54.4, CH | 53.9, CH | 53.6, CH | 47.8, CH |
| 18 | 19.1, CH3 | 18.7, CH3 | 18.8, CH3 | 18.9, CH3 | 18.6, CH3 | 17.3, CH3 |
| 19 | 18.4, CH3 | 19.3, CH3 | 17.7, CH3 | 19.7, CH3 | 18.0, CH3 | 21.3, CH3 |
| 20 | 161.3, C | 161.7, C | 162.6, C | 160.1, C | 161.5, C | 157.9, C |
| 21 | 22.2, CH3 | 22.2, CH3 | 21.9, CH3 | 22.0, CH3 | 22.3, CH3 | 21.0, CH3 |
| 22 | 122.0, CH | 120.0, CH | 119.6, CH | 121.4, CH | 120.1, CH | 126.2, CH |
| 23 | 202.0, C | 198.7, C | 199.0, C | 200.3, C | 198.8, C | 199.0, C |
| 24 | 80.0, CH | 78.2, CH | 78.2, CH | 79.4, CH | 78.5, CH | 47.6, CH2 |
| 25 | 44.6, CH | 43.1, CH | 43.3, CH | 44.0, CH | 43.3, CH | 35.2, CH |
| 26 | 176.9, C | 177.1, C | 177.1, C | 173.6, C | 173.5, C | 176.3, C |
| 27 | 13.8, CH3 | 13.3, CH3 | 13.0, CH3 | 13.9, CH3 | 13.6, CH3 | 20.0, CH3 |
| 28 | 27.7, CH3 | 31.2, CH3 | 27.7, CH3 | 31.2, CH3 | 27.6, CH3 | 25.2, CH3 |
| 29 | 20.7, CH3 | 19.5, CH3 | 20.6, CH3 | 19.6, CH3 | 20.5, CH3 | 22.6, CH3 |
| 30 | 26.4, CH3 | 26.4, CH3 | 27.6, CH3 | 26.4, CH3 | 26.3, CH3 | 26.5, CH3 |
| -OCH3 | 51.8, CH3 | 52.1, CH3 | ||||
| -OCH2CH3 | 60.6, CH2 | |||||
| -OCH2CH3 | 14.3, CH3 |
| No. | 7 ac | 8 ac | 9 bc | 10 bc |
|---|---|---|---|---|
| 1 | 2.28, overlapped | 2.10, ddd (13.9, 10.9, 6.2) | 2.61, ddd (15.5, 9.7, 6.0) | 2.94, ddd (14.4, 8.3, 6.1) |
| 1.86, m | 1.95, ddd (13.9, 9.2, 5.0) | 2.46, ddd (15.5, 8.5, 6.5) | 1.77, ddd (14.4, 9.1, 7.0) | |
| 2 | 2.81, td (14.6, 5.6) | 2.69, overlapped | 2.97, ddd (14.3, 8.5, 6.0) | 2.62, ddd (15.3, 9.1, 6.1) |
| 2.43, overlapped | 2.48, overlapped | 1.70, ddd (14.3, 9.7, 6.5) | 2.52, ddd (15.3, 8.3, 7.0) | |
| 5 | 1.70, dd (12.1, 3.6) | 2.75, dd (13.0, 3.0) | 2.09, dd (11.8, 3.9) | 2.26, dd (15.3, 2.8) |
| 6 | 2.44, overlapped | 2.17, dt (15.0, 3.0) | 1.70, overlapped | 2.62, t (15.3) |
| 2.28, overlapped | 1.75, dd (15.0, 13.0) | 2.49, dd (15.3, 2.8) | ||
| 7 | 6.27, d (6.7) | 4.68, d (3.0) | 4.47, m | |
| 11 | 5.69, s | 6.05, s | ||
| 12 | 2.76, d (17.3) | 2.87, d (16.9) | ||
| 2.38, d (17.3) | 2.44, d (16.9) | |||
| 15 | 4.31, d (6.2) | 2.04, overlapped | 4.40, ddd (10.2, 5.6, 1.8) | |
| 1.84, m | ||||
| 16 | 2.52, ddd (15.4, 8.8, 6.2) | 2.66, dd (12.7, 9.3) | 2.03, overlapped | 2.49, overlapped |
| 2.04, dd (15.4, 8.8) | 2.46, dd (12.7, 9.3) | 1.94, overlapped | 1.82, ddd (15.0, 10.2, 5.6) | |
| 17 | 3.23, t (8.8) | 3.51, t (9.3) | 2.88, t (8.7) | 2.92, overlapped |
| 18 | 1.17, s | 1.13, s | 0.67, s | 0.73, s |
| 19 | 1.36, s | 1.09, s | 1.02, s | 1.28, s |
| 21 | 2.28, s | 2.26, s | 2.11, s | 2.10, s |
| 22 | 6.44, s | 6.34, s | 6.12, s | 6.09, s |
| 24 | 2.94, overlapped | 2.95, overlapped | 2.95, ddd (20.5, 14.2) | 2.94, overlapped |
| 2.55, dd (20.4, 8.6) | 2.54, dd (20.5, 8.5) | 2.52, dd (20.5, 8.6) | 2.51, overlapped | |
| 25 | 2.94, overlapped | 2.94, overlapped | 2.96, ddd (14.2, 8.6, 6.8) | 2.94, overlapped |
| 27 | 1.18, s | 1.19, d (6.9) | 1.19, d (6.8) | 1.19, d (6.8) |
| 28 | 1.12, s | 1.12, s | 1.07, s | 1.15, s |
| 29 | 1.17, s | 1.08, s | 1.14, s | 1.12, s |
| 30 | 1.00, s | 1.29, s | 1.35, s | 1.26, s |
| -OCH3 | 3.68, s | |||
| -OCH2CH3 | 4.13, overlapped, 2H | 4.13, overlapped, 2H | 4.13, m, 2H | |
| -OCH2CH3 | 1.25, overlapped | 1.25, overlapped | 1.25, t (7.1) | |
| 15-OH | 4.48, d (1.8) |
| No. | 8 cd | 9 bd | 10 bd | 11 bd | 12 bd | 13 ad |
|---|---|---|---|---|---|---|
| 1 | 36.1, CH2 | 34.2, CH2 | 35.1, CH2 | 35.2, CH2 | 34.9, CH2 | 35.1, CH2 |
| 2 | 33.7, CH2 | 34.9, CH2 | 34.0, CH2 | 37.0, CH2 | 34.2, CH2 | 34.1, CH2 |
| 3 | 216.4, C | 218.0, C | 214.9, C | 215.1, C | 218.0, C | 215.6, C |
| 4 | 45.9, C | 46.5, C | 46.6, C | 46.7, C | 46.5, C | 46.8, C |
| 5 | 40.7, CH | 45.2, CH | 49.2, CH | 49.3, CH | 45.1, CH | 49.8, CH |
| 6 | 22.8, CH2 | 29.4, CH2 | 36.9, CH2 | 34.1, CH2 | 29.4, CH2 | 37.1, CH2 |
| 7 | 58.9, CH | 67.3, CH | 204.4, C | 204.5, C | 67.3, CH | 201.2, C |
| 8 | 62.7, C | 159.5, C | 150.4, C | 150.5, C | 159.5, C | 150.9, C |
| 9 | 164.3, C | 140.2, C | 152.8, C | 153, C | 140.2, C | 150.2, C |
| 10 | 40.4, C | 38.0, C | 39.2, C | 39.3, C | 38.0, C | 39.0, C |
| 11 | 129.9, CH | 199.5, C | 200.3, C | 200.4, C | 199.3, C | 200.7, C |
| 12 | 200.7, C | 50.3, CH2 | 50.5, CH2 | 50.6, CH2 | 50.3, CH2 | 50.0, CH2 |
| 13 | 57.7, C | 48.6, C | 48.9, C | 49.0, C | 48.9, C | 48.6, C |
| 14 | 55.2, C | 50.6, C | 52.3, C | 52.4, C | 50.7, C | 48.5, C |
| 15 | 209.9, C | 30.4, CH2 | 72.4, CH | 72.5, CH | 30.4, CH2 | 32.4, CH2 |
| 16 | 38.5, CH2 | 23.2, CH2 | 32.0, CH2 | 32.1, CH2 | 23.3, CH2 | 23.4, CH2 |
| 17 | 42.9, CH | 54.1, CH | 51.9, CH | 52.0, CH | 54.5, CH | 53.9, CH |
| 18 | 18.3, CH3 | 18.6, CH3 | 18.9, CH3 | 19.0, CH3 | 18.8, CH3 | 18.6, CH3 |
| 19 | 25.0, CH3 | 17.7, CH3 | 17.7, CH3 | 17.8, CH3 | 17.7, CH3 | 18.1, CH3 |
| 20 | 154.0, C | 158.0, C | 155.8, C | 156.1, C | 162.0, C | 162.9, C |
| 21 | 20.6, CH3 | 21.4, CH3 | 21.1, CH3 | 21.2, CH3 | 22.2, CH3 | 22.5, CH3 |
| 22 | 127.0, CH | 123.8, CH | 124.5, CH | 124.5, CH | 119.6, CH | 119.6, CH |
| 23 | 198.8, C | 198.4, C | 198.2, C | 198.3, C | 198.8, C | 198.7, C |
| 24 | 47.9, CH2 | 47.9, CH2 | 47.7, CH2 | 47.9, CH2 | 78.6, CH | 77.3, CH |
| 25 | 35.1, CH | 35.0, CH | 35.0, CH | 34.9, CH | 43.2, CH | 42.8, CH |
| 26 | 176.1, C | 176.7, C | 176.0, C | 176.6, C | 173.0, C | 173.4, C |
| 27 | 17.3, CH3 | 17.3, CH3 | 17.2, CH3 | 17.3, CH3 | 13.8, CH3 | 9.5, CH3 |
| 28 | 28.8, CH3 | 27.8, CH3 | 27.4, CH3 | 27.5, CH3 | 27.7, CH3 | 27.6, CH3 |
| 29 | 21.7, CH3 | 20.6, CH3 | 20.4, CH3 | 20.5, CH3 | 20.6, CH3 | 20.5, CH3 |
| 30 | 17.8, CH3 | 27.8, CH3 | 20.7, CH3 | 20.9, CH3 | 27.7, CH3 | 26.4, CH3 |
| -OCH3 | 52.0, CH3 | 52.1, CH3 | ||||
| -OCH2CH3 | 60.7, CH2 | 60.6, CH2 | 61.0, CH2 | 61.3, CH2 | ||
| -OCH2CH3 | 29.8, CH3 | 14.2, CH3 | 14.3, CH3 | 14.3, CH3 |
| No. | 11 bc | 12 bc | 13 ac |
|---|---|---|---|
| 1 | 2.96, overlapped | 2.98, overlapped | 2.87, overlapped |
| 1.76, ddd (17.3, 9.9, 3.2) | 1.70, overlapped | 1.68, overlapped | |
| 2 | 2.60, overlapped | 2.61, ddd (14.9, 9.0, 5.4) | 2.55, ddd (15.5, 9.6, 6.0) |
| 2.48, overlapped | 2.45, ddd (14.9, 8.6, 6.5) | 2.45, overlapped | |
| 5 | 2.26, dd (15.1, 2.6) | 2.09, dd (9.9, 5.4) | 2.19, dd (15.1, 2.7) |
| 6 | 2.60, overlapped | 1.70, overlapped | 2.48, dd (15.1, 14.4) |
| 2.52, overlapped | 1.24, m | 2.34, dd (14.4, 2.7) | |
| 7 | 4.47, br.s | ||
| 12 | 2.87, d (16.8) | 2.78, d (17.4) | 2.76, d (16.3) |
| 2.44, d (16.8) | 2.37, d (17.4) | 2.40, d (16.3) | |
| 15 | 4.39, dd (9.7, 5.7) | 2.05, m | 2.27, overlapped |
| 1.87, m | 1.77, overlapped | ||
| 16 | 1.82, ddd (15.1, 10.2, 5.5) | 2.00, overlapped | 1.89, overlapped |
| 2.49, overlapped | 1.96, overlapped | 1.68, overlapped | |
| 17 | 2.92, overlapped | 2.96, overlapped | 2.80, overlapped |
| 18 | 0.73, s | 0.68, s | 0.63, s |
| 19 | 1.27, s | 1.02, s | 1.21, s |
| 21 | 2.10, s | 2.22, s | 2.15, s |
| 22 | 6.08, m | 6.25, s | 6.16, s |
| 24 | 2.52, overlapped | 4.21, br.s | 4.62, dd (5.2, 2.9) |
| 2.95, overlapped | |||
| 25 | 2.94, overlapped | 2.96, overlapped | 2.78, overlapped |
| 27 | 1.18, d (6.7) | 1.30, d (7.3) | 0.92, d (7.1) |
| 28 | 1.14, s | 1.14, s | 1.07, s |
| 29 | 1.12, s | 1.07, s | 1.04, s |
| 30 | 1.26, s | 1.36, s | 1.25, s |
| -OCH3 | 3.68, s | ||
| -OCH2CH3 | 4.08, m, 2H | 4.14, q (7.1), 2H | |
| -OCH2CH3 | 1.19, t (7.3) | 1.24, t (7.1) | |
| 24-OH | 3.93, br.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Guo, L.-L.; Isaka, M.; Li, Z.-H.; Chen, H.-P. [20(22)E]-Lanostane Triterpenes from the Fungus Ganoderma australe. J. Fungi 2022, 8, 503. https://doi.org/10.3390/jof8050503
Zhou L, Guo L-L, Isaka M, Li Z-H, Chen H-P. [20(22)E]-Lanostane Triterpenes from the Fungus Ganoderma australe. Journal of Fungi. 2022; 8(5):503. https://doi.org/10.3390/jof8050503
Chicago/Turabian StyleZhou, Lin, Li-Li Guo, Masahiko Isaka, Zheng-Hui Li, and He-Ping Chen. 2022. "[20(22)E]-Lanostane Triterpenes from the Fungus Ganoderma australe" Journal of Fungi 8, no. 5: 503. https://doi.org/10.3390/jof8050503