Mercury in Macrolepiota procera (Scop.) Singer and Its Underlying Substrate—Environmental and Health Risks Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis and Data Processing
2.2. Study Areas, Sampling, and Sample Preparation
2.3. Sample Analysis
2.4. Risk Assessment
2.5. Health Risk Assessment
Provisional Tolerable Weekly Intake—PTWI
2.6. Statistical Analysis
3. Results and Discussion
3.1. Mercury in Soil/Substrate Samples
3.2. Soil Pollution
3.3. Mercury in M. procera Fruiting Bodies
3.4. Bioaccumulation Factor (BAF) and Cap/Stem Quotient (Qc/s)
3.5. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haro, A.; Trescastro, A.; Lara, L.; Fernández-Fígares, I.; Nieto, R.; Seiquer, I. Mineral elements contents of wild growing edible mushrooms from the southeast of Spain. J. Food Compos. Anal. 2020, 91, 103504. [Google Scholar] [CrossRef]
- Kunca, V.; Pavlík, M. Fruiting Body Production of, and Suitable Environmental Ranges for, Growing the Umbrella Polypore Medicinal Mushroom, Polyporus umbellatus (Agaricomycetes) in Natural Conditions in Central Europe. Int. J. Med. Mushrooms 2019, 2, 121–129. [Google Scholar] [CrossRef]
- Kunca, V.; Čiliak., M. Habitat preferences of Hericium erinaceus in Slovakia. Fungal Ecol. 2017, 27, 189–192. [Google Scholar] [CrossRef]
- Árvay, J.; Demková, L.; Hauptvogl, M.; Michalko, M.; Bajčan, D.; Stanovič, R.; Tomáš, J.; Hrstková, M.; Trebichalský, P. Assessment of Environmental and Health Risks in Former Polymetallic Ore Mining and Smelting Area, Slovakia: Spatial Distribution and Accumulation of Mercury in Four Different Ecosystems. Ecotoxicol. Environ. Saf. 2017, 144, 236–244. [Google Scholar] [CrossRef]
- Melgar, M.J.; Alonso, J.; García, M.A. Cadmium in edible mushrooms from NW Spain: Bioconcentration factors and consumer health implications. Food Chem. Toxicol. 2016, 88, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Sarikurkcu, C.; Yalcin, O.U.; Cengiz, M.; Gungor, H. Metal concentration, phenolics profiling, and antioxidant activity of two wild edible Melanoleuca mushrooms (M. cognata and M. stridula). Microchem. J. 2019, 150, 104172. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species: Part I. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health 2013, 48, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Melgar, M.J.; Alonso, J.; García, M.A. Mercury in edible mushrooms and underlying soil: Bioconcentration factors and toxicological risk. Sci. Total Environ. 2009, 407, 5328–5334. [Google Scholar] [CrossRef]
- Falandysz, J.; Chudzińska, M.; Barałkiewicz, D.A.N.U.T.A.; Saba, M.; Wang, Y.; Zhang, J. Occurrence, variability and associations of trace metallic elements and arsenic in sclerotia of medicinal Wolfiporia extensa from polymetallic soils in Yunnan, China. Acta Pol. Pharm. Drug Res 2017, 74, 1379–1387. [Google Scholar]
- Falandysz, J.; Hanć, A.; Barałkiewicz, D.; Zhang, J.; Treu, R. Metallic and metalloid elements in various developmental stages of Amanita muscaria (L.) Lam. Fungal Biol. 2020, 124, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Sapkota, A.; Dryżałowska, A.; Mędyk, M.; Feng, X. Analysis of some metallic elements and metalloids composition and relationships in parasol mushroom Macrolepiota procera. Environ. Sci. Pollut. Res. 2017, 24, 15528–15537. [Google Scholar] [CrossRef] [Green Version]
- Gucia, M.; Jarzyńska, G.; Rafał, E.; Roszak, M.; Kojta, A.K.; Osiej, I.; Falandysz, J. Multivariate analysis of mineral constituents of edible Parasol Mushroom (Macrolepiota procera) and soils beneath fruiting bodies collected from Northern Poland. Environ. Sci. Pollut. Res. 2012, 19, 416–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukojević, V.; Đurđić, S.; Stefanović, V.; Trifković, J.; Čakmak, D.; Perović, V.; Mutić, J. Scandium, yttrium, and lanthanide contents in soil from Serbia and their accumulation in the mushroom Macrolepiota procera (Scop.) Singer. Environ. Sci. Pollut. Res. 2019, 26, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Eckley, C.S.; Achá, D.; Feng, X.; Gilmour, C.C.; Jonsson, S.; Mitchell, C.P. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio 2018, 47, 141–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhang, J.; Lv, Y.; Xu, K.; Lu, S.; Liu, X.; Yang, Y. Effect of soil mercury pollution on ginger (Zingiber officinale Roscoe): Growth, product quality, health risks and silicon mitigation. Ecotoxicol. Environ. Saf. 2020, 195, 110472. [Google Scholar] [CrossRef]
- Tang, W.L.; Liu, Y.R.; Guan, W.Y.; Zhong, H.; Qu, X.M.; Zhang, T. Understanding mercury methylation in the changing environment: Recent advances in assessing microbial methylators and mercury bioavailability. Sci. Total Environ. 2020, 714, 136827. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. Mineral Composition and Radioactivity of Edible Mushrooms; Academic Press: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-817565-1. [Google Scholar]
- Nayab, G.U.L.; Sardar, K.H.A.N.; Abbas, K.H.A.N.; Nawab, J.; Sarwar, A.; Nida, G.U.L. Organic and Inorganic Mercury in Biological Samples of Flouresecent Lamp Industries Workers and Health Risks. Biomed. Environ. Sci. 2020, 33, 89–102. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Šefčík, P.; Pramuka, S.; Gluch, A. Assessment of soil contamination in Slovakia according index of geoaccumulation. Agriculture 2008, 54, 119–130. [Google Scholar]
- Islam, S.; Ahmed, K.; Masunaga, S. Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Sci. Total Environ. 2015, 512, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, S.; Zhang, X.; Wu, D.; Luo, W.; Zhang, T.; Wu, L. Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J. Geochem. Explor. 2015, 148, 71–78. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Dryźalowska, A.; Falandysz, J. Bioconcentration of mercury by mushroom Xerocomus chrysenteron from the spatially distinct locations: Levels, possible intake and safety. Ecotoxicol. Environ. Saf. 2014, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Joint, F.A.O.; World Health Organization; WHO Expert Committee on Food Additives. Safety evaluation of certain food additives/prepared by the by the Seventy Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2012. Available online: https://books.google.ro/books?hl=ro&lr=&id=YFAMU9qYD_YC&oi=fnd&pg=PP7&ots=e5skGh31Ln&sig=W99eNu8GUWHlWsG-ooJzM1dlkws&redir_esc=y#v=onepage&q&f=false (accessed on 2 July 2021).
- Statistical Organization of Slovak Republic 2019. Food Consumption in the Slovak Republic 2018. Available online: www.statistics.sk (accessed on 17 December 2020).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 2 July 2021).
- Addinsoft. XLSTAT, Analyse de Données et Statistique avec MS Excel; Addinsoft: New York, NY, USA, 2014. [Google Scholar]
- Árvay, J.; Tomáš, J.; Hauptvogl, M.; Massányi, P.; Harangozo, Ľ. Human Exposure to Heavy Metals and Possible Public Health Risks Via Consumption of Wild Edible Mushrooms from Slovak Paradise National Park, Slovakia. J. Environ. Sci. Health Part B 2015, 50, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Árvay, J.; Záhorcová, Z.; Tomáš, J.; Hauptvogl, M.; Stanovič, R.; Harangozo, Ľ. Mercury in edible wild-grown mushrooms from historical mining area–Slovakia: Bioaccumulation and risk assessment. J. Microbiol. Biotechnol. Food Sci. 2021, 1–4. [Google Scholar] [CrossRef]
- Falandysz, J.; Bielawski, L.; Kawano, M.; Brzostowski, A.; Chudzyński, K. Mercury in mushrooms and soil from the Wieluńska Upland in south-central Poland. J. Environ. Sci. Health Part A 2002, 37, 1409–1420. [Google Scholar] [CrossRef]
- Falandysz, J.; Gucia, M. Bioconcentration factors of mercury by Parasol Mushroom (Macrolepiota procera). Environ. Geochem. Health 2008, 30, 121–125. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzyńska, S.; Szostek, M.; Goliński, P. Toxicological risks and nutritional value of wild edible mushroom species-a half-century monitoring study. Chemosphere 2021, 263, 128095. [Google Scholar] [CrossRef] [PubMed]
- Environmental Regionalization of the Slovak Republic. 2016. Available online: https://www.minzp.sk/files/environmentalna-regionalizacia-sr.pdf (accessed on 17 December 2017).
- Mleczek, M.; Budka, A.; Kalač, P.; Siwulski, M.; Niedzielski, P. Family and species as determinants modulating mineral composition of selected wild-growing mushroom species. Environ. Sci. Pollut. Res. 2020, 1–16. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zocher, A.L.; Kraemer, D.; Merschel, G.; Bau, M. Distribution of major and trace elements in the bolete mushroom Suillus luteus and the bioavailability of rare earth elements. Chem. Geol. 2018, 483, 491–500. [Google Scholar] [CrossRef]
- Falandysz, J.; Gucia, M.; Mazur, A. Content and bioconcentration factors of mercury by Parasol Mushroom Macrolepiota procera. J. Environ. Sci. Health Part B 2007, 42, 735–740. [Google Scholar] [CrossRef]
- Giannaccini, G.; Betti, L.; Palego, L.; Mascia, G.; Schmid, L.; Lanza, M.; Lucacchini, A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ. Monit. Assess. 2012, 184, 7579–7595. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants. Twenty-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; No. 631; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- EPA, US. Toxicological Review of Zinc and Compounds; US Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
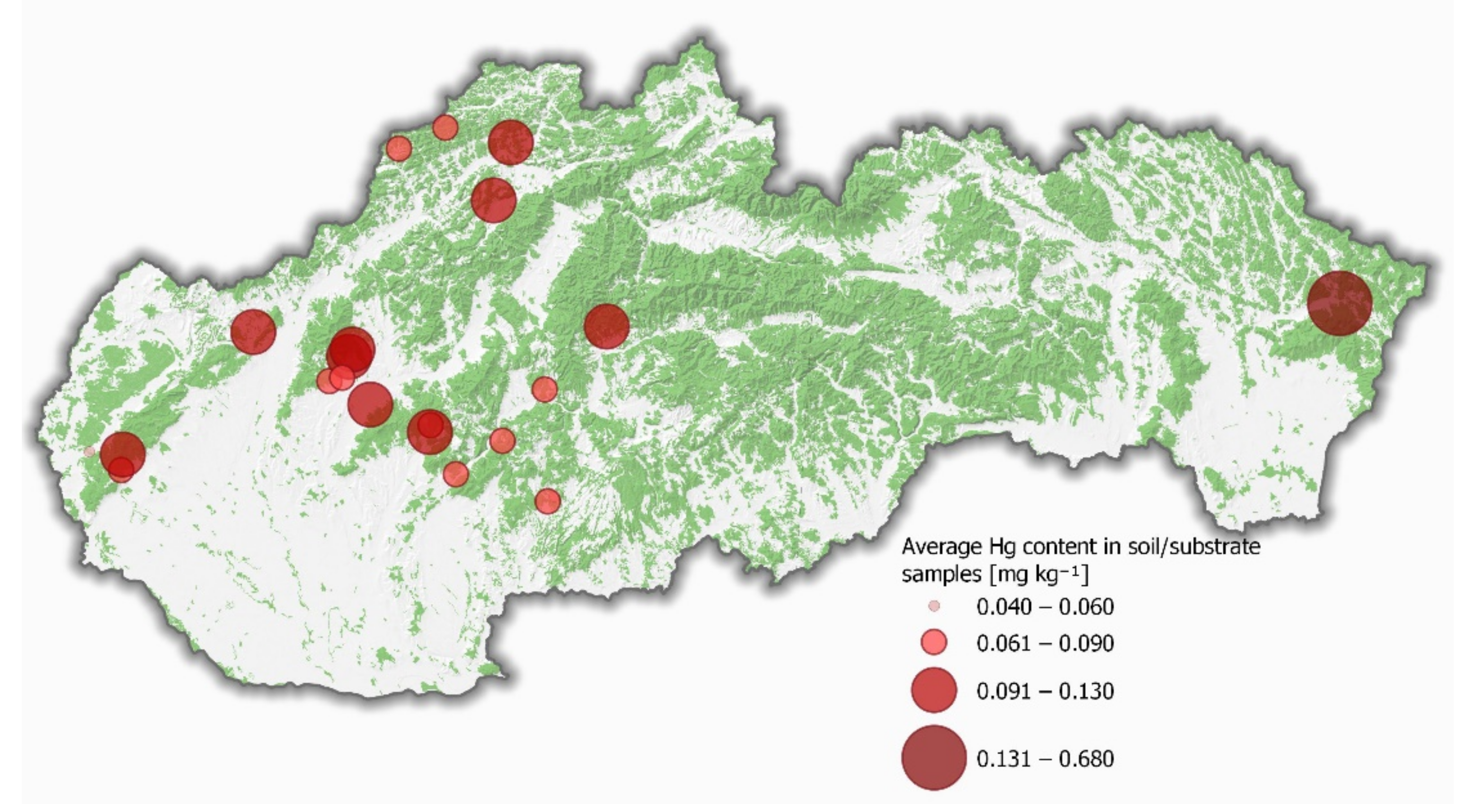
| Locality | n | Coordinates (VGS 84) | Altitude (m) | |
|---|---|---|---|---|
| Bojná | 15 | 48.591916 | 18.021600 | 390 |
| Divina | 6 | 49.286883 | 18.691366 | 423 |
| Duchonka | 9 | 48.663350 | 18.094166 | 275 |
| Hodruša-Hámre | 21 | 48.468566 | 18.755716 | 409 |
| Jedľové Kostoľany | 9 | 48.495283 | 18.454450 | 420 |
| Krajné | 15 | 48.707103 | 17.688867 | 289 |
| Kráľovce | 20 | 48.326200 | 18.986500 | 229 |
| Lazy pod Makytou | 12 | 49.244400 | 18.225650 | 542 |
| Limbach | 7 | 48.294658 | 17.200095 | 420 |
| Lozorno | 8 | 48.335600 | 17.063083 | 220 |
| Nemečky | 6 | 48.683833 | 18.105333 | 298 |
| Orovnica | 8 | 48.366516 | 18.575166 | 254 |
| Pezinská Baba | 8 | 48.337583 | 17.200451 | 537 |
| Pitelová | 5 | 48.618097 | 18.913429 | 480 |
| Sitnianska Lehôtka | 20 | 48.312333 | 18.961416 | 232 |
| Skýcov | 10 | 48.476183 | 18.454166 | 473 |
| Snina | 7 | 48.976100 | 22.194766 | 260 |
| Solčany | 8 | 48.537475 | 18.198736 | 385 |
| Šachtička | 6 | 48.803309 | 19.150328 | 1009 |
| Štiavnik | 6 | 49.312900 | 18.412250 | 655 |
| Tesáre | 15 | 48.604333 | 18.073000 | 251 |
| Zbyňov | 9 | 49.124650 | 18.639466 | 378 |
| Sampling Locality | Soil/Substrate | Mushroom | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hg (mg kg−1 DW) AVG ± S.D. Min–Max | Igeo * | Cf * | PER * | Hgcap (mg kg−1 DW) AVG ± S.D. Min–Max | Hgstem (mg kg−1 DW) AVG ± S.D. Min–Max | BAFcap * | BAFstem * | Qc/s * | %PTWIcap * | %PTWIstem * | |
| Bojná | 0.09 ± 0.03 0.03–0.15 | 1.41 | −0.20 | 56.5 | 2.09 ± 1.17 0.06–4.57 | 1.00 ± 0.46 0.05–1.55 | 24.7 | 11.7 | 2.10 | 61.3 | 29.2 |
| Divina | 0.13 ± 0.02 0.11–0.16 | 2.23 | 0.56 | 89.3 | 3.57 ± 1.66 1.84–6.29 | 1.75 ± 0.66 0.84–2.70 | 26.6 | 13.0 | 2.04 | 105 | 51.2 |
| Duchonka | 0.11 ± 0.05 0.05–0.18 | 1.79 | 0.12 | 71.6 | 1.89 ± 0.67 0.75–3.01 | 1.18 ± 0.29 0.65–1.61 | 17.6 | 10.9 | 1.61 | 55.3 | 34.5 |
| Hodruša-Hámre | 0.07 ± 0.03 0.03–0.14 | 1.10 | −0.54 | 44.1 | 1.07 ± 0.60 0.07–2.86 | 0.69 ± 0.36 0.04–1.59 | 16.2 | 10.5 | 1.55 | 31.5 | 20.3 |
| Jedľové Kostoľany | 0.08 ± 0.03 0.04–0.11 | 1.31 | −0.30 | 52.6 | 1.64 ± 1.49 0.77–5.75 | 0.91 ± 0.61 0.49–2.51 | 20.8 | 11.5 | 1.81 | 48.2 | 26.6 |
| Krajné | 0.10 ± 0.06 0.05–0.27 | 1.71 | 0.00 | 68.2 | 1.94 ± 0.49 1.16–2.95 | 1.31 ± 0.42 0.45–1.82 | 19.0 | 12.8 | 1.49 | 57.0 | 38.3 |
| Kráľovce | 0.06 ± 0.02 0.04–0.11 | 0.94 | −0.73 | 37.5 | 0.89 ± 0.30 0.44–1.72 | 0.73 ± 0.23 0.44–1.59 | 15.7 | 13.0 | 1.21 | 26.0 | 21.5 |
| Lazy pod Makytou | 0.09 ± 0.02 0.06–0.13 | 1.53 | −0.02 | 61.1 | 2.28 ± 0.87 1.22–4.00 | 1.46 ± 0.64 0.67–2.83 | 24.9 | 16.0 | 1.56 | 66.9 | 42.9 |
| Limbach | 0.07 ± 0.04 0.02–0.12 | 1.19 | −0.61 | 47.5 | 2.73 ± 1.28 1.58–5.16 | 1.64 ± 0.60 0.92–2.70 | 38.3 | 23.1 | 1.66 | 80.0 | 48.2 |
| Lozorno | 0.04 ± 0.02 0.02–0.07 | 1.10 | −0.74 | 43.8 | 1.59 ± 0.88 0.04–2.86 | 1.19 ± 0.69 0.03–2.17 | 43.9 | 33.0 | 1.33 | 46.5 | 34.9 |
| Nemečky | 0.11 ± 0.03 0.07–0.17 | 1.15 | −0.38 | 46.1 | 1.03 ± 0.26 0.49–1.37 | 0.73 ± 0.16 0.58–1.04 | 9.76 | 6.95 | 1.40 | 30.1 | 21.5 |
| Orovnica | 0.07 ± 0.00 0.06–0.08 | 1.82 | 0.16 | 72.9 | 1.43 ± 0.41 0.95–2.15 | 0.79 ± 0.22 0.51–1.15 | 20.8 | 11.5 | 1.81 | 42.1 | 23.3 |
| Pezinská Baba | 0.12 ± 0.04 0.07–0.19 | 1.52 | −0.12 | 60.9 | 2.81 ± 1.38 0.29–5.14 | 1.81 ± 0.71 0.24–2.64 | 22.9 | 14.7 | 1.56 | 82.5 | 53.0 |
| Pitelová | 0.09 ± 0.05 0.05–0.18 | 11.4 | 2.86 | 455 | 4.14 ± 1.12 2.32–5.74 | 2.21 ± 0.26 1.86–2.66 | 46.9 | 25.1 | 1.87 | 121 | 64.9 |
| Sitnianska lehôtka | 0.07 ± 0.03 0.04–0.14 | 2.23 | 0.46 | 89.2 | 1.08 ± 0.34 0.47–1.65 | 0.78 ± 0.27 0.31–1.28 | 15.4 | 11.0 | 1.40 | 31.8 | 22.7 |
| Skýcov | 0.13 ± 0.02 0.10–0.17 | 2.04 | 0.43 | 81.5 | 1.77 ± 0.74 0.86–3.40 | 0.91 ± 0.30 0.34–1.45 | 13.3 | 6.81 | 1.95 | 52.0 | 26.7 |
| Snina | 0.68 ± 0.19 0.37–0.89 | 1.56 | −0.01 | 62.3 | 2.32 ± 0.37 1.73–2.89 | 1.70 ± 0.39 1.25–2.53 | 3.40 | 2.50 | 1.36 | 68.1 | 50.0 |
| Solčany | 0.13 ± 0.05 0.04–0.12 | 1.41 | −0.20 | 56.5 | 2.34 ± 0.77 1.44–3.75 | 1.21 ± 0.38 0.78–1.88 | 17.5 | 9.04 | 1.94 | 68.7 | 35.5 |
| Šachtička | 0.12 ± 0.01 0.11–0.14 | 2.23 | 0.56 | 89.3 | 2.45 ± 0.13 2.23–2.65 | 1.74 ± 0.17 1.47–1.99 | 20.0 | 14.2 | 1.41 | 71.8 | 51.0 |
| Štiavnik | 0.08 ± 0.02 0.06–0.10 | 1.79 | 0.12 | 71.6 | 1.75 ± 0.75 0.98–3.05 | 0.97 ± 0.43 0.44–1.50 | 22.5 | 12.5 | 1.80 | 51.3 | 28.4 |
| Tesáre | 0.08 ± 0.02 0.05–0.11 | 1.10 | −0.54 | 44.1 | 1.21 ± 0.28 0.81–1.66 | 0.88 ± 0.22 0.61–1.35 | 15.2 | 11.0 | 1.38 | 35.5 | 25.7 |
| Zbyňov | 0.13 ± 0.01 0.11–0.15 | 1.31 | −0.30 | 52.6 | 1.93 ± 0.66 1.06–3.26 | 1.06 ± 0.47 0.07–1.76 | 15.5 | 8.52 | 1.81 | 56.6 | 31.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jančo, I.; Šnirc, M.; Hauptvogl, M.; Demková, L.; Franková, H.; Kunca, V.; Lošák, T.; Árvay, J. Mercury in Macrolepiota procera (Scop.) Singer and Its Underlying Substrate—Environmental and Health Risks Assessment. J. Fungi 2021, 7, 772. https://doi.org/10.3390/jof7090772
Jančo I, Šnirc M, Hauptvogl M, Demková L, Franková H, Kunca V, Lošák T, Árvay J. Mercury in Macrolepiota procera (Scop.) Singer and Its Underlying Substrate—Environmental and Health Risks Assessment. Journal of Fungi. 2021; 7(9):772. https://doi.org/10.3390/jof7090772
Chicago/Turabian StyleJančo, Ivona, Marek Šnirc, Martin Hauptvogl, Lenka Demková, Hana Franková, Vladimír Kunca, Tomáš Lošák, and Július Árvay. 2021. "Mercury in Macrolepiota procera (Scop.) Singer and Its Underlying Substrate—Environmental and Health Risks Assessment" Journal of Fungi 7, no. 9: 772. https://doi.org/10.3390/jof7090772
APA StyleJančo, I., Šnirc, M., Hauptvogl, M., Demková, L., Franková, H., Kunca, V., Lošák, T., & Árvay, J. (2021). Mercury in Macrolepiota procera (Scop.) Singer and Its Underlying Substrate—Environmental and Health Risks Assessment. Journal of Fungi, 7(9), 772. https://doi.org/10.3390/jof7090772







