Steryl Glycosides in Fungal Pathogenesis: An Understudied Immunomodulatory Adjuvant
Abstract
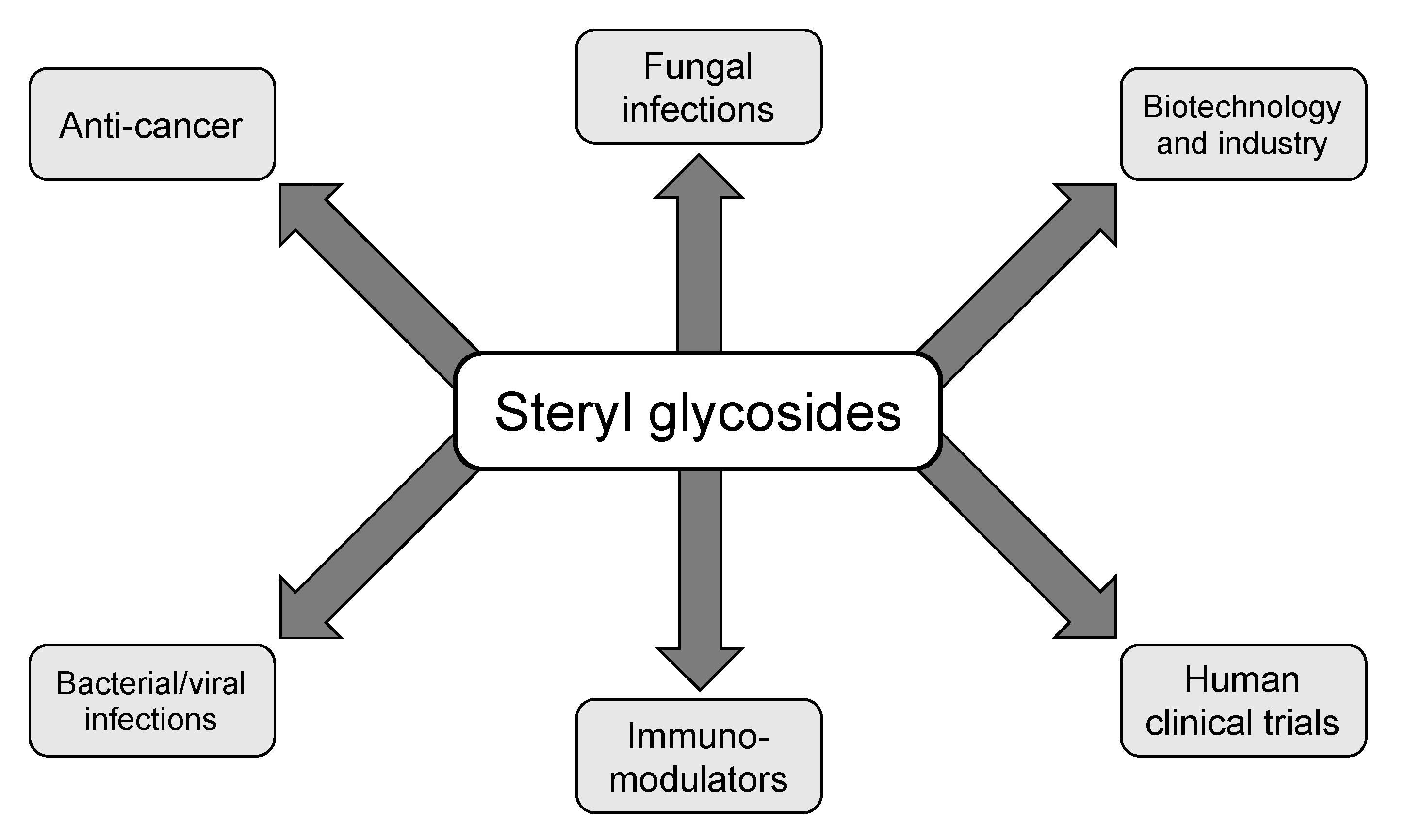
:1. Introduction
2. Lipids as An Emerging Topic of Study in Fungal Infections
3. Sterols and Steryl Glycosides
3.1. Sterols
3.2. Steryl Glycosides and Other Conjugated Sterols
4. Immunomodulatory Roles of Steryl Glycosides
4.1. Fungi
4.2. Plants
4.3. Bacteria
5. Other Known Application Uses Involving Steryl Glycosides
5.1. Anti-Cancer Treatments
5.2. Biotechnology and Industrial Uses
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Watanabe, T.; Ito, T.; Goda, H.M.; Ishibashi, Y.; Miyamoto, T.; Ikeda, K.; Taguchi, R.; Okino, N.; Ito, M. Sterylglucoside Catabolism in Cryptococcus neoformans with Endoglycoceramidase-related Protein 2 (EGCrP2), the First Steryl-β-glucosidase Identified in Fungi. J. Biol. Chem. 2015, 290, 1005–1019. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.C.; Rella, A.; Normile, T.; Joffe, L.S.; Tavares, P.M.; de SAraújo, G.R.; Frases, S.; Orner, E.P.; Farnoud, A.M.; Fries, B.C.; et al. Cryptococcus neoformans Glucuronoxylomannan and Sterylglucoside Are Required for Host Protection in an Animal Vaccination Model. Available online: https://mbio.asm.org/content/10/2/e02909-18 (accessed on 22 February 2020).
- Lee, J.-H.; Han, Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int. Immunopharmacol. 2006, 6, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.-P.; Tsai, C.-T.; Chiou, Y.-S.; Chen, Y.-J.; Li, M.-E.; Guo, T.-W.; Lyu, J.W.; Chou, S.-H.; Wu, T.-K. An enzymatic approach to configurationally rare trans-androsteronyl-α-glucoside and Its potential anticancer application. Chem. Biol. Drug Des. 2017, 89, 61–66. [Google Scholar] [CrossRef]
- Mansour, S.; Tocheva, A.S.; Cave-Ayland, C.; Machelett, M.M.; Sander, B.; Lissin, N.M.; Molloy, P.E.; Baird, M.S.; Stübs, G.; Schröder, N.W.J.; et al. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E1266–E1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Exploiting Lipids to Develop Anticryptococcal Vaccines. Curr. Trop. Med. Rep. 2019, 6, 55–63. [Google Scholar] [CrossRef]
- Singh, G.; Dhar, Y.V.; Asif, M.H.; Misra, P. Exploring the functional significance of sterol glycosyltransferase enzymes. Prog. Lipid Res. 2018, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Mutlu, N.; Minard, A.Y.; Kumar, A.; Krysan, D.J.; Wellington, M. A Genome-Wide Screen of Deletion Mutants in the Filamentous Saccharomyces cerevisiae Background Identifies Ergosterol as a Direct Trigger of Macrophage Pyroptosis. Available online: https://mbio.asm.org/content/9/4/e01204-18 (accessed on 22 February 2020).
- Singh, A.; MacKenzie, A.; Girnun, G.; Del Poeta, M. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. J. Lipid Res. 2017, 58, 2017–2036. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Nakajima, K.; Itoh, Y.; Sayano, T.; Ohashi, Y.; Yamaguchi, Y.; Greimel, P.; Hirabayashi, Y. Aglycon diversity of brain sterylglucosides: Structure determination of cholesteryl- and sitosterylglucoside. J. Lipid Res. 2016, 57, 2061–2072. [Google Scholar] [CrossRef] [Green Version]
- Weete, J.D.; Abril, M.; Blackwell, M. Phylogenetic Distribution of Fungal Sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef] [Green Version]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.; Peiru, S.; Eberhardt, F.; Vetcher, L.; Cabrera, R.; Menzella, H.G. Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl. Microbiol. Biotechnol. 2014, 98, 4033–4040. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.; Zaslawski, A.; Thiele, S.; Plat, J.; Warnecke, D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog. Lipid Res. 2010, 49, 262–288. [Google Scholar] [CrossRef] [PubMed]
- Münger, L.H.; Nyström, L. Enzymatic hydrolysis of steryl glycosides for their analysis in foods. Food Chem. 2014, 163, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Gardiner, D.M. Targeting pathogen sterols: Defence and counterdefence? PLoS Pathog. 2017, 13, e1006297. [Google Scholar] [CrossRef]
- Alim, D.; Sircaik, S.; Panwar, S. The Significance of Lipids to Biofilm Formation in Candida albicans: An Emerging Perspective. JoF 2018, 4, 140. [Google Scholar] [CrossRef] [Green Version]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Chalfant, C., Poeta, M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Wang, Y.; Wang, K.; Masso-Silva, J.A.; Rivera, A.; Xue, C. A Heat-Killed Cryptococcus Mutant Strain Induces Host Protection against Multiple Invasive Mycoses in a Murine Vaccine Model. Available online: https://mbio.asm.org/content/10/6/e02145-19 (accessed on 22 February 2020).
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [Green Version]
- Butts, A.; Krysan, D.J. Antifungal Drug Discovery: Something Old and Something New. PLoS Pathog. 2012, 8, e1002870. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.M.; Del Poeta, M. Lipid Metabolism in Model Fungi: The Achilles Heel of Fungal Pathogens. Reference Module in Life Sciences. 2018. Available online: https://f1000.com/prime/1001615 (accessed on 22 February 2020).
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of Sphingolipids in Microbial Pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Gibellini, F.; Smith, T.K. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Tams, R.N.; Cassilly, C.D.; Anaokar, S.; Brewer, W.T.; Dinsmore, J.T.; Chen, Y.L.; Patton-Vogt, J.; Reynolds, T.B. Overproduction of Phospholipids by the Kennedy Pathway Leads to Hypervirulence in Candida albicans. Front. Microbiol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farine, L.; Niemann, M.; Schneider, A.; Butikofer, P. Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Sci. Rep. 2015, 5, 16787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Hu, C.T.; Yu, J.H. Lipid Biosynthesis as an Antifungal Target. J. Fungi 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, X.L.; Zhang, T.; Wang, C.L.; Huang, Z.J.; Luo, X.; Deng, Y.H. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- McMaster, C.R. From yeast to humans-roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2018, 592, 1256–1272. [Google Scholar] [CrossRef] [Green Version]
- Rittershaus, P.C.; Kechichian, T.B.; Allegood, J.C.; Jr, A.H.M.; Hennig, M.; Luberto, C. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2007, 117, 1450. [Google Scholar] [CrossRef] [Green Version]
- Rella, A.; Mor, V.; Farnoud, A.M.; Singh, A.; Shamseddine, A.A.; Ivanova, E.; Carpino, N.; Montagna, M.T.; Luberto, C.; Del Poeta, M. Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: Potential applications for vaccine development. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shea, J.M.; Henry, J.L.; Del Poeta, M. Lipid metabolism in Cryptococcus neoformans. FEMS Yeast Res. 2006, 6, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.M.; Del Poeta, M.; Luberto, C. Sphingolipids as Regulators of the Phagocytic Response to Fungal Infections. Mediators of Inflammation 2015, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of the Structure of Natural Sterols and Sphingolipids on the Formation of Ordered Sphingolipid/Sterol Domains (Rafts): Comparison Of Cholesterol To Plant, Fungal, And Disease-Associated Sterols And Comparison Of Sphingomyelin, Cerebrosides, And Ceramide. J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [PubMed] [Green Version]
- Klemptner, R.L.; Sherwood, J.S.; Tugizimana, F.; Dubery, I.A.; Piater, L.A. Ergosterol, an orphan fungal microbe-associated molecular pattern (MAMP): Ergosterol as a MAMP. Mol. Plant Pathol. 2014, 15, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kalia, A. Pharmaceutic Prodigy of Ergosterol and Protein Profile of Ganoderma lucidum. In Biology of Macrofungi; Singh, B.P., Lallawmsanga Passari, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ghaffar, M.; Orr, C.; Webb, G. Antiphagocytic protein 1 increases the susceptibility of Cryptococcus neoformans to amphotericin B and fluconazole. PLoS ONE 2019, 14, e0225701. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Stanchev, L.D.; da Silva, V.K.A.; Nimrichter, L.; Pomorski, T.G.; Rodrigues, M.L. Role of lipid transporters in fungal physiology and pathogenicity. Comput. Struct. Biotechnol. J. 2019, 17, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Fessler, M.B. The intracellular cholesterol landscape: Dynamic integrator of the immune response. Trends Immunol. 2016, 37, 819–830. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, T.J.; Crowley, J.T.; Cusack, B.J.; Pathak, P.; Benach, J.; London, E.; Garcia-Monco, J.C.; Benach, J.L. Cholesterol Lipids of Borrelia burgdorferi Form Lipid Rafts and Are Required for the Bactericidal Activity of a Complement-Independent Antibody. Cell Host Microbe. 2010, 8, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Crowley, J.T.; Toledo, A.M.; LaRocca, T.J.; Coleman, J.L.; London, E.; Benach, J.L. Lipid Exchange between Borrelia burgdorferi and Host Cells. PLoS Pathog. 2013, 9, e1003109. [Google Scholar] [CrossRef] [Green Version]
- Du, S.-Y.; Wang, H.-J.; Cheng, H.-H.; Chen, S.-D.; Wang, L.H.-C.; Wang, W.-C. Cholesterol glucosylation by Helicobacter pylori delays internalization and arrests phagosome maturation in macrophages. J. Microbiol. Immunol. Infect. 2016, 49, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Wunder, C.; Churin, Y.; Winau, F.; Warnecke, D.; Vieth, M.; Lindner, B.; Zähringer, U.; Mollenkopf, H.-J.; Heinz, E.; Meyer, T.F. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat. Med. 2006, 12, 1030–1038. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30228244 (accessed on 22 February 2020).
- Alvarez, F.J.; Konopka, J.B. Identification of an N-Acetylglucosamine Transporter That Mediates Hyphal Induction in Candida albicans D. Mol. Biol. Cell 2007, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.W.; Konopka, J.B. Lipid Raft Polarization Contributes to Hyphal Growth in Candida albicans. Eukaryotic Cell 2004, 3, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, C.B.; Fraser, J.A.; Heitman, J. PAK Kinases Ste20 and Pak1 Govern Cell Polarity at Different Stages of Mating in Cryptococcus neoformans. MBoC 2004, 15, 4476–4489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, C.L.; Xu, K.; Sharpless, K.E.; Harris, S.D. MesA, a Novel Fungal Protein Required for the Stabilization of Polarity Axes in Aspergillus nidulans D. Mol. Biol. Cell 2004, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, N.; Diallinas, G.; Fischer, R. The role of flotillin FloA and stomatin StoA in the maintenance of apical sterol-rich membrane domains and polarity in the filamentous fungus Aspergillus nidulans: Flotillin and stomatin in Aspergillus nidulans. Mol. Microbiol. 2012, 83, 1136–1152. [Google Scholar] [CrossRef]
- Takeshita, N.; Higashitsuji, Y.; Konzack, S.; Fischer, R. Apical Sterol-rich Membranes Are Essential for Localizing Cell End Markers That Determine Growth Directionality in the Filamentous Fungus Aspergillus nidulans. MBoC 2008, 19, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Vellucci, V.F.; Kyc, S.; Hostetter, M.K. Simvastatin Inhibits Candida albicans Biofilm In Vitro. Pediatr. Res. 2009, 66, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Westermeyer, C.; Macreadie, I.G. Simvastatin reduces ergosterol levels, inhibits growth and causes loss of mtDNA in Candida glabrata. FEMS Yeast Res. 2007, 7, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Tejada-Simon, M.V.; Pestka, J.J. Production of Polyclonal Antibody against Ergosterol Hemisuccinate Using Freund’s and Titermax Adjuvants. J. Food Prot. 1998, 61, 1060–1063. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Spellberg, B.J.; Ibrahim, A.S.; Ritchie, J.A.; Currie, B.; Spitzer, E.D.; Edwards, J.E.; Casadevall, A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob. Agents Chemother. 1994, 38, 2029–2033. [Google Scholar] [CrossRef] [Green Version]
- Rella, A.; Farnoud, A.M.; Del Poeta, M. Plasma membrane lipids and their role in fungal virulence. Prog. Lipid Res. 2016, 61, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.R.A.; Mirzaian, M.; Akiyama, H.; Wisse, P.; Ferraz, M.J.; Gaspar, P.; Ghauharali-van der Vlugt, K.; Meijer, R.; Giraldo, P.; Alfonso, P.; et al. Glucosylated cholesterol in mammalian cells and tissues: Formation and degradation by multiple cellular β-glucosidases. J. Lipid Res. 2016, 57, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, J.; Colombo, A.C.; Zamith-Miranda, D.; Silva, V.K.A.; Allegood, J.C.; Casadevall, A.; Del Poeta, M.; Nosanchuk, J.D.; Kronstad, J.W.; Rodrigues, M.L. The putative flippase Apt1 is required for intracellular membrane architecture and biosynthesis of polysaccharide and lipids in Cryptococcus neoformans. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, T.; Zähringer, U.; Warnecke, D.C.; Fahl, A.; Knogge, W.; Heinz, E. Sterol glycosides and cerebrosides accumulate in Pichia pastoris, Rhynchosporium secalis and other fungi under normal conditions or under heat shock and ethanol stress: Glycolipids in yeasts and fungi. Yeast 2001, 18, 679–695. [Google Scholar] [CrossRef]
- Chang, W.; Li, Y.; Zhang, M.; Zheng, S.; Li, Y.; Lou, H. Solasodine-3-O-β-d-glucopyranoside kills Candida albicans by disrupting the intracellular vacuole. Food Chem. Toxicol. 2017, 106, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.N.; Porta, E.O.J.; Labadie, G.R. Convenient synthesis of the immunogenic glycolipid BbGL1. Steroids 2019, 141, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, D.; Erdmann, R.; Fahl, A.; Hube, B.; Müller, F.; Zank, T.; Zähringer, U.; Heinz, E. Cloning and Functional Expression of UGT Genes Encoding Sterol Glucosyltransferases from Saccharomyces cerevisiae, Candida albicans, Pichia pastoris, and Dictyostelium discoideum. J. Biol. Chem. 1999, 274, 13048–13059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Tani, M.; Ishibashi, Y.; Endo, I.; Okino, N.; Ito, M. Ergosteryl-β-glucosidase (Egh1) involved in sterylglucoside catabolism and vacuole formation in Saccharomyces cerevisiae. Glycobiology 2015, 25, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Sugai, M.; Takakuwa, N.; Ohnishi, M.; Urashima, T.; Oda, Y. Characterization of Sterol Lipids in Kluyveromyces lactis Strain M-16 Accumulating a High Amount of Steryl Glucoside. J. Oleo Sci. 2009, 58, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef]
- Warnecke, D. UDP-glucose:sterol glucosyltransferase: Cloning and functional expression in Escherichia coli. Available online: https://link.springer.com/article/10.1023%2FA%3A1005806119807 (accessed on 22 February 2020).
- Warnecke, D.C.; Heinz, E. Purification of a Membrane-Bound UDP-G1ucose:Sterol p-D- Clucosyltransferase Based on Its solubility in Diethyl Ether’ 7. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12232266 (accessed on 22 February 2020).
- Grösch, S.; Alessenko, A.V.; Albi, E. The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response. Mediat. Inflamm. 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimoto, S.; Murofushi, W.; Kai, H.; Ishida, Y.; Uchiyama, A.; Kobayashi, T.; Kobayashi, S.; Murofushi, H.; Murakami-Murofushi, K. Steryl Glucoside is a Lipid Mediator in Stress-responsive Signal Transduction. Cell Struct. Funct. 2002, 27, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Tiwari, M.; Singh, S.P.; Singh, S.; Trivedi, P.K.; Misra, P. Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci. Rep. 2016, 6, 25562. [Google Scholar] [CrossRef] [PubMed]
- Maitani, Y.; Nakamura, K.; Kawano, K. Application of Sterylglucoside-Containing Particles for Drug Delivery. CPB 2005, 6, 81–93. [Google Scholar] [CrossRef]
- Bouic, P.J.D.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; Van Jaarsveld, P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Donald, P.; Lamprecht, J.; Freestone, M.; Albrecht, C.F.; Bouic, P.J.D.; Kotze, D.; Van Jaarsveld, P. A randomised placebo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 1997, 1, 518–522. [Google Scholar]
- Chang, W.; Li, Y.; Zheng, S.; Zhang, M.; Gao, Y.; Lou, H. Solasodine-3-O-β-d-glucopyranoside is hydrolyzed by a membrane glucosidase into active molecule solasodine against Candida albicans. Food Chem. Toxicol. 2017, 109, 356–362. [Google Scholar] [CrossRef]
- Monari, C.; Bistoni, F.; Casadevall, A.; Pericolini, E.; Pietrella, D.; Kozel, T.R.; Vecchiarelli, A. Glucuronoxylomannan, a Microbial Compound, Regulates Expression of Costimulatory Molecules and Production of Cytokines in Macrophages. J. Infect. Dis. 2005, 191, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, J.; Oliveira, D.L.; Joffe, L.S.; Hu, G.; Gazos-Lopes, F.; Fonseca, F.L.; Almeida, I.C.; Frases, S.; Kronstad, J.W.; Rodrigues, M.L. Role of the Apt1 Protein in Polysaccharide Secretion by Cryptococcus neoformans. Eukaryotic Cell 2014, 13, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Stasyk, O.V.; Nazarko, T.Y.; Stasyk, O.G.; Krasovska, O.S.; Warnecke, D.; Nicaud, J.-M.; Cregg, J.M.; Sibirny, A.A. Sterol glucosyltransferases have different functional roles in Pichia pastoris and Yarrowia lipolytica. Cell Biol. Int. 2003, 27, 947–952. [Google Scholar] [CrossRef]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.; Pastor, V.; Chávez, Á.; Arró, M.; Boronat, A.; Flors, V.; Ferrer, A.; Altabella, T. Inactivation of UDP-Glucose Sterol Glucosyltransferases Enhances Arabidopsis Resistance to Botrytis cinerea. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, M. Immunological Functions of Steryl Glycosides. Arch. Immunol. Ther. Exp. 2012, 60, 351–359. [Google Scholar] [CrossRef]
- Shimamura, M.; Hidaka, H. Therapeutic Potential of Cholesteryl O-acyl-glucoside Found in Helicobacter pylori 6. 2012. Available online: https://www.researchgate.net/publication/6461223_Phagocytosis_and_persistence_of_Helicobacter_pylori (accessed on 22 February 2020).
- Piao, S.; Jin, X.L.; Yun, B.-Y.; Kim, N.; Cho, H.-S.; Fukuda, M.; Lee, H.; Ha, N.-C. Crystal structure and functional insight of HP0420-homolog from Helicobacter felis. Biochem. Biophys. Res. Commun. 2010, 394, 940–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, N.H.; Hong, S.-Y.; Huong, N.L.; Park, J.W. Biochemical Characterization of Recombinant UDP-Glucose: Sterol 3-O-Glycosyltransferase from Micromonospora rhodorangea ATCC 31603 and Enzymatic Biosynthesis of Sterol-3-O-β-Glucosides. J. Microbiol. Biotechnol. 2016, 26, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Sood, P.; Lenardon, M.D.; Milne, G.; Olson, J.; Jensen, G.; Wolf, J.; Casadevall, A.; Adler-Moore, J.; Gow, N.A.R. The Viscoelastic Properties of the Fungal Cell Wall Allow Traffic of AmBisome as Intact Liposome Vesicles. 2018. Available online: https://www.researchgate.net/publication/322961579_The_Viscoelastic_Properties_of_the_Fungal_Cell_Wall_Allow_Traffic_of_AmBisome_as_Intact_Liposome_Vesicles (accessed on 22 February 2020).
- Seki, J.; Okita, A.; Watanabe, M.; Nakagawa, T.; Honda, K.; Tatewaki, N.; Sugiyama, M. Plasma Lipoproteins as Drug Carriers: Pharmacological Activity and Disposition of the Complex of β-Sitosteryl-β-d-Glucopyranoside with Plasma Lipoproteins. J. Pharm. Sci. 1985, 74, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar] [CrossRef]
- Almeida, G.M.; Andrade, R.M.; Bento, C.A.M. The Capsular Polysaccharides of Cryptococcus neoformans Activate Normal CD4+ T Cells in a Dominant Th2 Pattern. J. Immunol. 2001, 167, 5845–5851. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan Cell Production Enhances the Virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef] [Green Version]
- Probert, M.; Zhou, X.; Goodall, M.; Johnston, S.A.; Bielska, E.; Ballou, E.R.; May, R.C. A Glucuronoxylomannan Epitope Exhibits Serotype-Specific Accessibility and Redistributes towards the Capsule Surface during Titanization of the Fungal Pathogen Cryptococcus neoformans. Infect. Immun. 2019, 87, e00731. [Google Scholar] [CrossRef] [Green Version]
- Trevijano-Contador, N.; de Oliveira, H.C.; García-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, Á.; Janbon, G.; Ariño, J.; Zaragoza, Ó. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans Extracellular Vesicles with the Cell Wall. Eukaryotic Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, G.; Rocha, J.D.B.; Oliveira, D.L.; Albuquerque, P.C.; Frases, S.; Santos, S.S.; Nosanchuk, J.D.; Gomes, A.M.O.; Medeiros, L.C.A.S.; Miranda, K.; et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans: Extracellular vesicles from Candida albicans. Cell Microbiol. 2015, 17, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.A.; Lee, C.K.; Huang, H.; Hester, M.M.; Liu, J.; Luckie, B.A.; Torres Santana, M.A.; Mirza, Z.; Khoshkenar, P.; Abraham, A.; et al. Vaccination with Recombinant Cryptococcus Proteins in Glucan Particles Protects Mice against Cryptococcosis in a Manner Dependent upon Mouse Strain and Cryptococcal Species. 2017. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29184017 (accessed on 22 February 2020).
- Specht, C.A.; Lee, C.K.; Huang, H.; Tipper, D.J.; Shen, Z.T.; Lodge, J.K.; Leszyk, J.; Ostroff, G.R.; Levitz, S.M. Protection against Experimental Cryptococcosis following Vaccination with Glucan Particles Containing Cryptococcus Alkaline Extracts. 2015. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01359/full (accessed on 22 February 2020).
- Reis, F.C.G.; Borges, B.S.; Jozefowicz, L.J.; Sena, B.A.G.; Garcia, A.W.A.; Medeiros, L.C.; Martins, S.T.; Honorato, L.; Schrank, A.; Vainstein, M.H.; et al. A Novel Protocol for the Isolation of Fungal Extracellular Vesicles Reveals the Participation of a Putative Scramblase in Polysaccharide Export and Capsule Construction in 4:15. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30894430 (accessed on 22 February 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Normile, T.G.; McEvoy, K.; Del Poeta, M. Steryl Glycosides in Fungal Pathogenesis: An Understudied Immunomodulatory Adjuvant. J. Fungi 2020, 6, 25. https://doi.org/10.3390/jof6010025
Normile TG, McEvoy K, Del Poeta M. Steryl Glycosides in Fungal Pathogenesis: An Understudied Immunomodulatory Adjuvant. Journal of Fungi. 2020; 6(1):25. https://doi.org/10.3390/jof6010025
Chicago/Turabian StyleNormile, Tyler G., Kyle McEvoy, and Maurizio Del Poeta. 2020. "Steryl Glycosides in Fungal Pathogenesis: An Understudied Immunomodulatory Adjuvant" Journal of Fungi 6, no. 1: 25. https://doi.org/10.3390/jof6010025





