HIV-Associated Cryptococcal Disease in Resource-Limited Settings: A Case for “Prevention Is Better Than Cure”?
Abstract
:1. Introduction
2. Epidemiology of Cryptococcal Disease
3. Cryptococcal Disease Diagnosis in Low Resource Settings
3.1. Conventional Methods
3.2. Immunodiagnosis of Cryptococcosis
4. Management of Cryptococcal Meningitis in Resource-Limited Settings
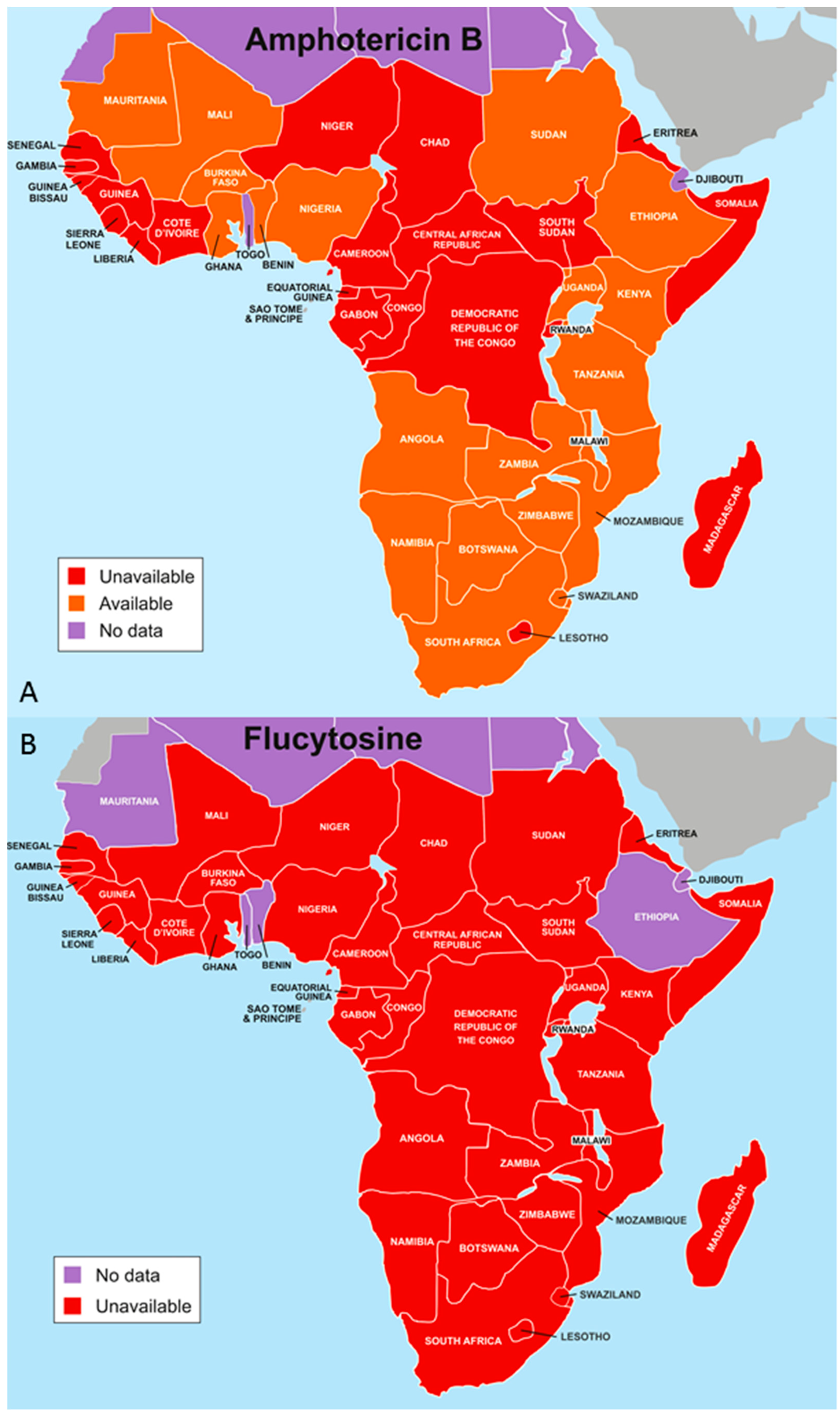
4.1. Optimal Antifungal Therapy
4.2. Antiretroviral Therapy Timing after Initiation of Antifungal Therapy
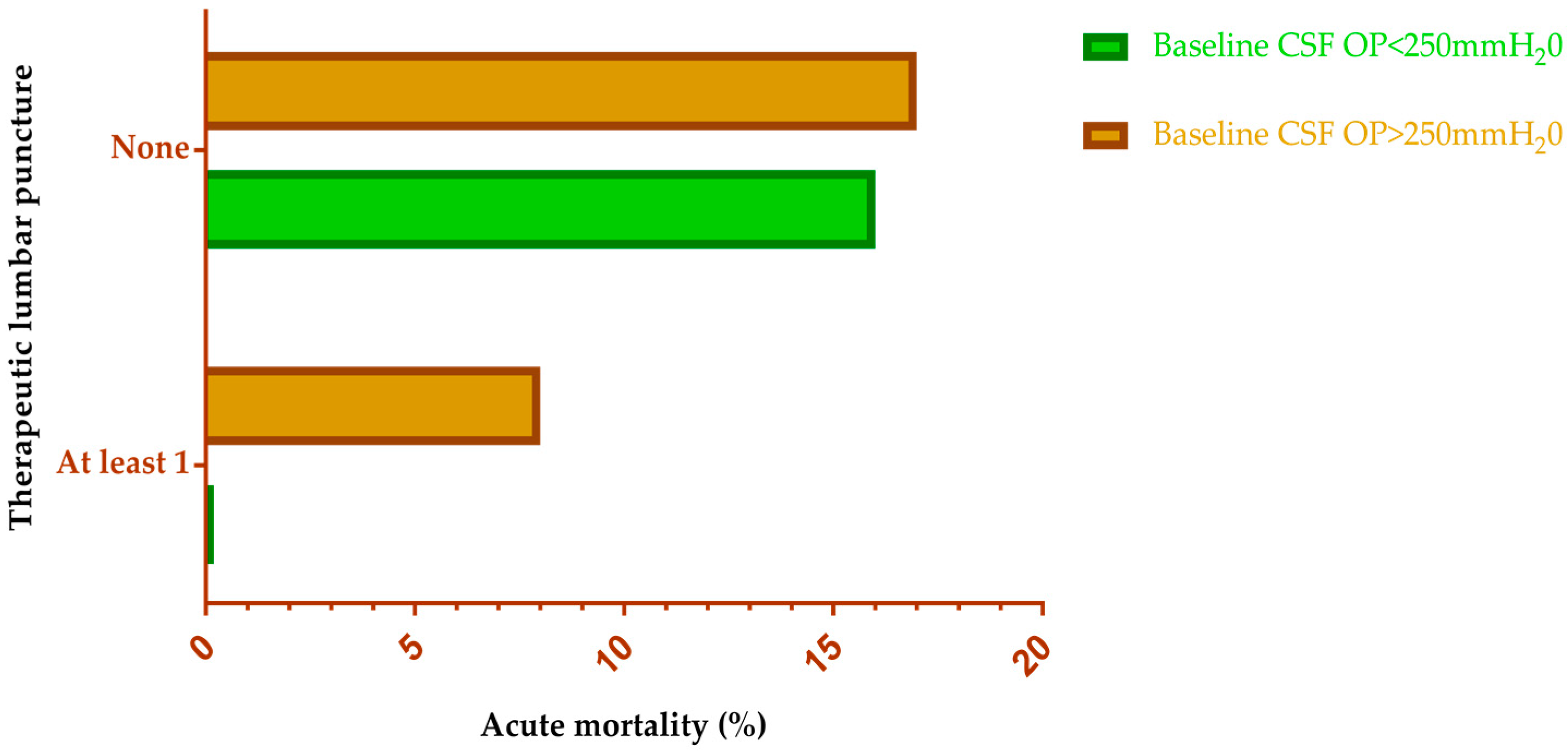
4.3. Management of Intracranial Pressure
4.4. Treatment Outcomes
4.4.1. Mortality
4.4.2. Relapses and Persistent Cryptococcal Disease
4.4.3. Cryptococcal Immune Reconstitution Inflammatory Syndrome (C-IRIS)
4.4.4. Sequelae of Cryptococcal Meningitis
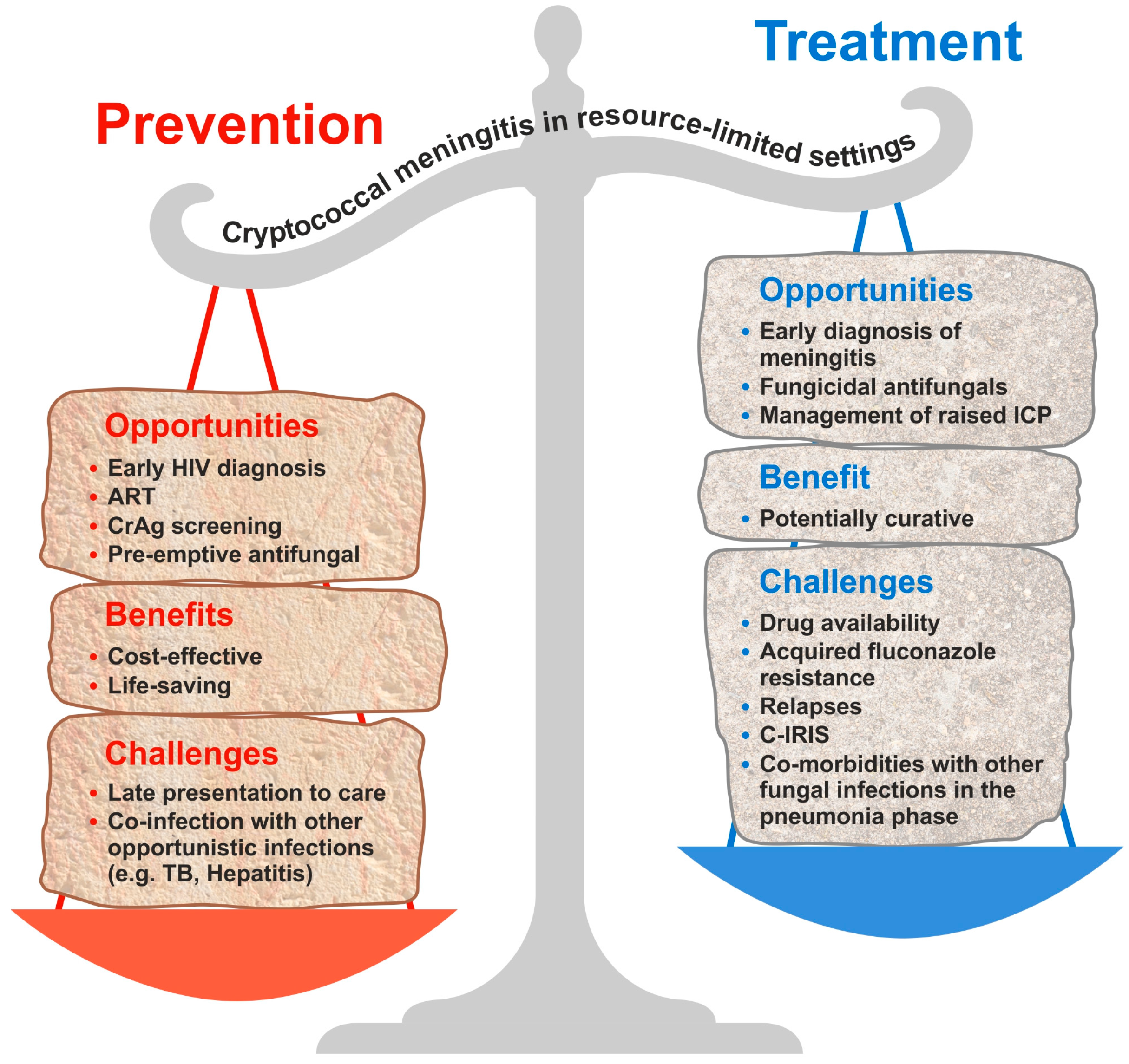
5. Antifungal Prophylaxis for Cryptococcal Meningitis
5.1. Primary Prophylaxis
5.2. Secondary Prophylaxis
6. Cryptococcal Antigen Screening
7. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Perfect, J.R.; Bicanic, T. Cryptococcosis diagnosis and treatment: What do we know now. Fungal Genet. Biol. 2015, 78, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev. Infect. Dis. 1991, 13, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global Burden of Fungal Diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S. New Insights into HIV/AIDS-Associated Cryptococcosis. Aids 2013, 2013, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Rachel, M.S.; Benjamin, J.P.; Joseph, N.J.; Nelesh, P.G.; Tom, M.C.; David, W.D.; Angela, L.; David, R.B. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 3099, 1–9. [Google Scholar] [CrossRef]
- Letang, E.; Müller, M.C.; Ntamatungiro, A.J.; Kimera, N.; Faini, D.; Furrer, H.; Battegay, M.; Tanner, M.; Hatz, C.; Boulware, D.R.; et al. Cryptococcal Antigenemia in Immunocompromised Human Immunodeficiency Virus Patients in Rural Tanzania: A Preventable Cause of Early Mortality. Open Forum Infect. Dis. 2015, 2, ofv046. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Perfect, J.R.; Cloud, G.A.; Larsen, R.A.; Pankey, G.A.; Lancaster, D.J.; Henderson, H.; Kauffman, C.A.; Haas, D.W.; Saccente, M.; et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001, 33, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Fishman, J.A.; Horn, D.; Anaissie, E.; Chang, C.; Olyaei, A.; Pfaller, M.; Steinbach, W.J.; Webster, K.M.; Marr, K.A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Pyrgos, V.; Seitz, A.E.; Steiner, C.A.; Prevots, D.R.; Williamson, P.R. Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS ONE 2013, 8, e56269. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.; Smith, R.M.; Chiller, T.M.; Detels, R.; French, A.; Margolick, J.; Klausner, J.D. Prevalence and correlates of cryptococcal antigen positivity among AIDS patients—United States, 1986–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Powderly, W.G.; Cloud, G.A.; Robinson, P.; Grieco, M.H.; Sharkey, P.K.; Thompson, S.E.; Sugar, A.M.; Tuazon, C.U.; Fisher, J.F. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N. Engl. J. Med. 1992, 326, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.A.; Bauer, M.; Leal, M.A.E.; Evans, S.G.; Holtom, P.D.; Diamond, D.M.; Leedom, J.M.; Larsen, R.A. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 1999, 28, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kambugu, A.; Meya, D.B.; Rhein, J.; O’Brien, M.; Janoff, E.N.; Ronald, A.R.; Kamya, M.R.; Mayanja-Kizza, H.; Sande, M.A.; Bohjanen, P.R.; et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin. Infect. Dis. 2008, 46, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Lessells, R.J.; Mutevedzi, P.C.; Heller, T.; Newell, M.-L. Poor long-term outcomes for cryptococcal meningitis in rural South Africa. S. Afr. Med. J. 2011, 101, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.B.; Zijlstra, E.E.; Mukaka, M.; Reiss, M.; Kamphambale, S.; Scholing, M.; Waitt, P.I.; Neuhann, F. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop. Med. Int. Health 2010, 15, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Meya, D.; Rajasingham, R.; Nalintya, E.; Tenforde, M.; Jarvis, J.N. Preventing Cryptococcosis—Shifting the Paradigm in the Era of Highly Active Antiretroviral Therapy. Curr. Trop. Med. Rep. 2015, 2, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Masur, H.; Brooks, J.T.; Benson, C.A.; Holmes, K.K.; Pau, A.K.; Kaplan, J.E.; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, 1308–1311. [Google Scholar] [PubMed]
- World Health Organization. Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: December 2011. 2011. Available online: http://apps.who.int/iris/handle/10665/44786 (accessed on 20 August 2017).
- Meya, D.B.; Manabe, Y.C.; Castelnuovo, B.; Cook, B.A.; Elbireer, A.M.; Kambugu, A.; Kamya, M.R.; Bohjanen, P.R.; Boulware, D.R. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count <or =100 cells/microL who start HIV therapy in resource-limited settings. Clin. Infect. Dis. 2010, 51, 448–455. [Google Scholar] [PubMed]
- Jarvis, J.N.; Lawn, S.D.; Wood, R.; Harrison, T.S. Cryptococcal Antigen Screening for Patients Initiating Antiretroviral Therapy: Time for Action. Clin. Infect. Dis. 2010, 51, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Desmet, P.; Kayembe, K.D.; De Vroey, C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. Aids 1989, 3, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Micol, R.; Lortholary, O.; Sar, B.; Laureillard, D.; Ngeth, C.; Dousset, J.-P.; Chanroeun, H.; Ferradini, L.; Guerin, P.J.; Dromer, F.; et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2007, 45, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Pongsai, P.; Atamasirikul, K.; Sungkanuparph, S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. J. Infect. 2010, 60, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa, O.F.; Dirisu, O.; Okuonghae, E. Cryptococcal antigenemia in anti-retroviral naïve AIDS patients: Prevalence and its association with CD4 cell count. Acta Med. Iran. 2012, 50, 344–347. [Google Scholar] [PubMed]
- Oladele, R.O.; Akanmu, A.S.; Nwosu, A.O.; Ogunsola, F.T.; Richardson, M.D.; Denning, D.W. Cryptococcal Antigenemia in Nigerian Patients With Advanced Human Immunodeficiency Virus: Influence of Antiretroviral Therapy Adherence. Open Forum Infect. Dis. 2016, 3, ofw055. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.S.; Kempker, R.R.; Tenna, A.; Smitson, C.; Berhe, N.; Fekade, D.; Blumberg, H.M.; Aseffa, A. High Prevalence of Cryptococcal Antigenemia among HIV-infected Patients Receiving Antiretroviral Therapy in Ethiopia. PLoS ONE 2013, 8, e58377. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F. A review of Odongo-Aginya stain: the other alternative to India ink. Trop. Dr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R.; Jarvis, J.N.; Panackal, A.A.; Fisher, M.C.; Molloy, S.F.; Loyse, A.; Harrison, T.S. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat. Rev. Neurol. 2016, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- French, N.; Gray, K.; Watera, C.; Nakiyingi, J.; Lugada, E.; Moore, M.; Lalloo, D.; Whitworth, J.G.; Gilks, C.F. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. Aids 2002, 16, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.R.; Noble, A.; Denning, D.W.; Stevens, D.A. Performance of Cryptococcus antigen latex agglutination kits on serum and cerebrospinal fluid specimens of AIDS patients before and after pronase treatment. J. Clin. Microbiol. 1991, 29, 333–339. [Google Scholar] [PubMed]
- Cassim, N.; Schnippel, K.; Coetzee, L.M.; Glencross, D.K. Establishing a cost-per-result of laboratorybased, reflex Cryptococcal antigenaemia screening (CrAg) in HIV+ patients with CD4 counts less than 100 cells/μl using a Lateral Flow Assay (LFA) at a typical busy CD4 laboratory in South Africa. PLoS ONE 2017, 12, e0171675. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Percival, A.; Bauman, S.; Pelfrey, J.; Meintjes, G.; Williams, G.N.; Longley, N.; Harrison, T.S.; Kozel, T.R. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2011, 53, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kozel, T.R.; Bauman, S.K. CrAg lateral flow assay for cryptococcosis. Expert Opin. Med. Diagn. 2012, 6, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A.; Crowe, S.M.; Garcia, M. Point-of-care testing. Curr. HIV/AIDS Rep. 2011, 8, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, M.D.; Mekha, N.; Baggett, H.C.; Surinthong, Y.; Autthateinchai, R.; Sawatwong, P.; Harris, J.R.; Park, B.J.; Chiller, T.; Balajee, S.A. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin. Infect. Dis. 2011, 53, 321–325. [Google Scholar] [CrossRef] [PubMed]
- McMullan, B.J.; Halliday, C.; Sorrell, T.C.; Judd, D.; Sleiman, S.; Marriott, D.; Olma, T.; Chen, S.C.A. Clinical Utility of the Cryptococcal Antigen Lateral Flow Assay in a Diagnostic Mycology Laboratory. PLoS ONE 2012, 7, e49541. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Rolfes, M.A.; Rajasingham, R.; von Hohenberg, M.; Qin, Z.; Taseera, K.; Schutz, C.; Kwizera, R.; Butler, E.K.; Meintjes, G.; et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg. Infect. Dis. 2014, 20, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Jespersen, D.J.; Bestrom, J.E.; Rollins, L.O. Comparison of four assays for the detection of cryptococcal antigen. Clin. Vaccine Immunol. 2012, 19, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Escandón, P.; Lizarazo, J.; Agudelo, C.I.; Chiller, T.; Castañeda, E. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Med. Mycol. 2013, 51, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Rugemalila, J.; Maro, V.P.; Kapanda, G.; Ndaro, A.J.; Jarvis, J.N. Cryptococcal antigen prevalence in HIV-infected Tanzanians: A cross-sectional study and evaluation of a point-of-care lateral flow assay. Trop. Med. Int. Health 2013, 18, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Kiiza, T.; Kwizera, R.; Kiggundu, R.; Velamakanni, S.; Meya, D.B.; Rhein, J.; Boulware, D.R. Evaluation of Fingerstick Cryptococcal Antigen Lateral Flow Assay in HIV-Infected Persons: A Diagnostic Accuracy Study. Clin. Infect. Dis. 2015, 61, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Klausner, J.D.; Vijayan, T.; Chiller, T. Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid. MLO Med. Lab. Obs. 2013, 45, 16. [Google Scholar]
- Magambo, K.A.; Kalluvya, S.E.; Kapoor, S.W.; Seni, J.; Chofle, A.A.; Fitzgerald, D.W.; Downs, J.A. Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J. Int. Aids Soc. 2014, 17, 19040. [Google Scholar] [CrossRef] [PubMed]
- Kabanda, T.; Siedner, M.J.; Klausner, J.D.; Muzoora, C.; Boulware, D.R. Point-of-Care Diagnosis and Prognostication of Cryptococcal Meningitis With the Cryptococcal Antigen Lateral Flow Assay on Cerebrospinal Fluid. Clin. Infect. Dis. 2014, 58, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Radice, A.; Galimberti, L.; Magni, C.; Fasan, M.; Parravicini, C. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J. Clin. Microbiol. 2005, 43, 5828–5829. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.J.; Dedicoat, M.J.; Lalloo, D.G. Treatment of cryptococcal meningitis in resource limited settings. Curr. Opin. Infect. Dis. 2009, 22, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Day, J.N.; Chau, T.T.H.; Wolbers, M.; Mai, P.P.; Dung, N.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Phong, N.D.; Thai, C.Q.; et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N. Engl. J. Med. 2013, 368, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Thangaraj, H.; Easterbrook, P.; Ford, N.; Roy, M.; Chiller, T.; Govender, N.; Harrison, T.S.; Bicanic, T. Cryptococcal meningitis: Improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 2013, 13, 629–637. [Google Scholar] [CrossRef]
- Govender, N.; Meintjes, G.; Bicanic, T.; Dawood, H.; Harrison, T.S.; Jarvis, J.N.; Karstaedt, A.S.; Maartens, G.; McCarthy, K.M.; Variava, E.; et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. S. Afr. J. HIV Med. 2013, 14, 76–86. [Google Scholar]
- Bicanic, T.; Wood, R.; Meintjes, G.; Rebe, K.; Brouwer, A.; Loyse, A.; Bekker, L.-G.; Jaffar, S.; Harrison, T. High-dose Amphotericin B with Flucytosine for the Treatment of Cryptococcal Meningitis in HIV-Infected Patients: A Randomized Trial. Clin. Infect. Dis. 2008, 47, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Longley, N.; Muzoora, C.; Taseera, K.; Mwesigye, J.; Rwebembera, J.; Chakera, A.; Wall, E.; Andia, I.; Jaffar, S.; Harrison, T.S. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin. Infect. Dis. 2008, 47, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Kartalija, M.; Kaye, K.; Tureen, J.H.; Liu, Q.; Tauber, M.G.; Elliott, B.R.; Sande, M.A. Treatment of experimental cryptococcal meningitis with fluconazole: Impact of dose and addition of flucytosine on mycologic and pathophysiologic outcome. J. Infect. Dis. 1996, 173, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.A.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.C.C.; Jackson, A.; Namarika, D.; Phulusa, J.; Kenala, J.; Kanyemba, C.; Jarvis, J.N.N.; Jaffar, S.; Hosseinipour, M.C.C.; Kamwendo, D.; et al. Combination Flucytosine and High-Dose Fluconazole Compared with Fluconazole Monotherapy for the Treatment of Cryptococcal Meningitis: A Randomized Trial in Malawi. Clin. Infect. Dis. 2010, 50, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Rolfes, M.A.; Birkenkamp, K.E.; Meya, D.B.; Boulware, D.R. Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis. PLoS Med. 2012, 9, e1001316. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Leeme, B.T.; Chofle, A.A. Ambition-CM: High-dose liposomal amphotericin for HIV-related cryptococcal meningitis. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Bozzette, S.A.; Larsen, R.A.; Chiu, J.; Leal, M.A.; Jacobsen, J.; Rothman, P.; Robinson, P.; Gilbert, G.; McCutchan, J.A.; Tilles, J. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N. Engl. J. Med. 1991, 324, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Katende, A.; Mbwanji, G.; Faini, D. Sertraline and high-dose fluconazole treatment of cryptococcal meningitis in Tanzania. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Boulware, D.R.; Meya, D.B.; Muzoora, C.; Rolfes, M.A.; Hullsiek, K.H.; Musubire, A.; Taseera, K.; Nabeta, H.W.; Schutz, C.; Williams, D.A.; et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014, 370, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Scriven, J.E.; Rhein, J.; Hullsiek, K.H.; Von Hohenberg, M.; Linder, G.; Rolfes, M.A.; Williams, D.A.; Taseera, K.; Meya, D.B.; Meintjes, G.; et al. Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J. Infect. Dis. 2015, 212, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Van Der Horst, C.; Saag, M.; Cloud, G.; Hamill, R.; Graybill, J.; Sobel, J.; Johnson, P.; Tuazon, C.; Kerkering, T.; Moskovitz, B.; et al. Treatment of Cryptococcal Meningitis Associated with the Acquired Immunodeficiency Syndrome. N. Engl. J. Med. 1997, 337, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Lawn, S.D.; Vogt, M.; Bangani, N.; Wood, R.; Harrison, T.S. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin. Infect. Dis. 2009, 48, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Armstrong, R.W.; Lewis, B.H.; Stevens, D.A. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. Am. J. Med. 1991, 91, 267–272. [Google Scholar] [CrossRef]
- Rolfes, M.A.; Hullsiek, K.H.; Rhein, J.; Nabeta, H.W.; Taseera, K.; Schutz, C.; Musubire, A.; Rajasingham, R.; Williams, D.A.; Thienemann, F.; et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin. Infect. Dis. 2014, 59, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Chirianni, A.; Esposito, V. HIV-related cryptococcal meningitis in resource-limited settings. HIV Ther. 2010, 4, 567–576. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Corbett, E.L.; Foster, O.; Ash, S.; Cohen, J. Raised intracranial pressure and visual complications in AIDS patients with cryptococcal meningitis. J. Infect. 1992, 24, 185–189. [Google Scholar] [CrossRef]
- Beardsley, J.; Wolbers, M.; Kibengo, F.M.; Ggayi, A.-B.M.; Kamali, A.; Cuc, N.T.K.; Binh, T.Q.; Chau, N.V.V.; Farrar, J.; Merson, L.; et al. Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N. Engl. J. Med. 2016, 374, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Harries, A.D.; Anglaret, X.; Myer, L.; Wood, R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. Aids 2008, 22, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Mayanja-Kizza, H.; Oishi, K.; Mitarai, S.; Yamashita, H.; Nalongo, K.; Watanabe, K.; Izumi, T.; Ococi-Jungala; Augustine, K.; Mugerwa, R.; et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin. Infect. Dis. 1998, 26, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, M.C.; Paschoal, R.C.; Melhem, M.S. AIDS-associated central nervous system cryptococcosis: A Brazilian case study. Aids 2007, 21, 1971–1972. [Google Scholar] [CrossRef] [PubMed]
- Mwaba, P.; Mwansa, J.; Chintu, C.; Pobee, J.; Scarborough, M.; Portsmouth, S.; Zumla, A. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad. Med. J. 2001, 77, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Wake, R.; Leeme, T.; Jarvis, J.N. HIV-Associated Cryptococcal Meningitis: Bridging the Gap Between Developed and Resource-Limited Settings. Curr. Clin. Microbiol. Rep. 2016, 3, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Leeme, T.B.; Patel, R.K.; Azzo, C. Mortality due to HIV-associated cryptococcal meningitis in Botswana in the art era. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Casadevall, A.; Spitzer, E.D.; Webb, D.; Rinaldi, M.G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob. Agents Chemother. 1993, 37, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Cloud, G.A.; Graybill, J.R. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. [Spanish] Comparacion de itraconazol vs fluconazol como tratamiento de mantenimiento para la meningitis criptococica relacionada con el SID. Enfermedades Infecc y Microbiol. 1999, 19, 213–214. [Google Scholar]
- Lortholary, O.; Poizat, G.; Zeller, V.; Neuville, S.; Boibieux, A.; Alvarez, M.; Dellamonica, P.; Botterel, F.; Dromer, F.; Chêne, G. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. Aids 2006, 20, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Musubire, A.K.; Meya, B.D.; Mayanja-Kizza, H.; Lukande, R.; Wiesner, L.D.; Bohjanen, P.; R Boulware, R.D. Challenges in diagnosis and management of Cryptococcal immune reconstitution inflammatory syndrome (IRIS) in resource limited settings. Afr. Health Sci. 2012, 12, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Haddow, L.J.; Colebunders, R.; Meintjes, G.; Lawn, S.D.; Elliott, J.H.; Manabe, Y.C.; Bohjanen, P.R.; Sungkanuparph, S.; Easterbrook, P.J.; French, M.A.; et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: Proposed clinical case definitions. Lancet Infect. Dis. 2010, 10, 791–802. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Boulware, D.R. Cryptococcus-Related Immune Reconstitution Inflammatory Syndrome(IRIS): Pathogenesis and Its Clinical Implications. Curr. Fungal Infect. Rep. 2011, 5, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Green, W.R.; Fox, R.; Polk, B.F.; Bartlett, J.G. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology 1989, 96, 1092–1099. [Google Scholar] [CrossRef]
- Bicanic, T.; Brouwer, A.E.; Meintjes, G.; Rebe, K.; Limmathurotsakul, D.; Chierakul, W.; Teparrakkul, P.; Loyse, A.; White, N.J.; Wood, R.; et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. Aids 2009, 23, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Chetchotisakd, P.; Sungkanuparph, S.; Thinkhamrop, B.; Mootsikapun, P.; Boonyaprawit, P. A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med. 2004, 5, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Parkes-Ratanshi, R.; Wakeham, K.; Levin, J.; Namusoke, D.; Whitworth, J.; Coutinho, A.; Mugisha, N.K.; Grosskurth, H.; Kamali, A.; Lalloo, D.G.; et al. Primary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: A double-blind, randomised, placebo-controlled trial. Lancet Infect. Dis. 2011, 11, 933–941. [Google Scholar] [CrossRef]
- Sungkanuparph, S.; Savetamornkul, C.; Pattanapongpaiboon, W. Primary Prophylaxis for Cryptococcosis With Fluconazole in Human Immunodeficiency Virus–Infected Patients With CD4 T-Cell Counts <100 Cells/μL and Receiving Antiretroviral Therapy. Clin. Infect. Dis. 2017, 64, 967–970. [Google Scholar] [PubMed]
- Patel, S.; Shin, G.Y.; Wijewardana, I.; Vitharana, S.R.; Cormack, I.; Pakianathan, M.; Harrison, T.S.; Bicanic, T. The prevalence of cryptococcal antigenemia in newly diagnosed HIV patients in a Southwest London cohort. J. Infect. 2013, 66, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Achan, B.; Hullsiek, K.H.; Mcdonald, T.R.; Okagaki, L.H.; Alhadab, A.A.; Akampurira, A.; Rhein, J.R.; Meya, D.B.; Boulware, D.R.; et al. Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 2015, 59, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Meintjes, G.; Williams, Z.; Rebe, K.; Harrison, T.S. Symptomatic relapse of HIV-associated cryptococcal meningitis in South Africa: The role of inadequate secondary prophylaxis. S. Afr. Med. J. 2010, 100, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.P.; Nakasujja, N.; Morawski, B.M.; Rajasingham, R.; Rhein, J.; Nalintya, E.; Williams, D.A.; Huppler Hullsiek, K.; Kiragga, A.; Rolfes, M.A.; et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: A comparison of three prospective cohorts. BMC Neurol. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Govender, N.; Chiller, T.; Park, B.J.; Longley, N.; Meintjes, G.; Bekker, L.G.; Wood, R.; Lawn, S.D.; Harrison, T.S. Cryptococcal antigen screening and preemptive therapy in patients initiating antiretroviral therapy in resource-limited settings: A proposed algorithm for clinical implementation. J. Int. Assoc. Physicians AIDS Care 2012, 11, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Bicanic, T.; Muzoora, C.; Brouwer, A.E.; Meintjes, G.; Longley, N.; Taseera, K.; Rebe, K.; Loyse, A.; Jarvis, J.; Bekker, L.-G.; et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: Analysis of a combined cohort of 262 patients. Clin. Infect. Dis. 2009, 49, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Wake, R.M.; Britz, E.; Sriruttan, C.; Rukasha, I.; Omar, T.; Spencer, D.C.; Nel, J.S.; Mashamaite, S.; Adelekan, A.; Chiller, T.M.; et al. High Cryptococcal Antigen Titers in Blood are Predictive of Subclinical Cryptococcal Meningitis among HIV-Infected Patients. Clin. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Morawski, B.; Boulware, D.; Nalintya, E. Pre-ART cryptococcal antigen titer associated with preemptive fluconazole failure. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 22–25 February 2016. [Google Scholar]
- Larson, B.A.; Rockers, P.C.; Bonawitz, R.; Sriruttan, C.; Glencross, D.K.; Cassim, N.; Coetzee, L.M.; Greene, G.S.; Chiller, T.M.; Vallabhaneni, S.; et al. Screening HIV-Infected patients with low cd4 counts for cryptococcal antigenemia prior to initiation of antiretroviral therapy: Cost effectiveness of alternative screening strategies in South Africa. PLoS ONE 2016, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.P.; Roy, M.; Mendes, J.F.; Zulu, T.G.; Chiller, T.M.; Karstaedt, A.S. Evaluation of screening and treatment of cryptococcal antigenaemia among HIV-infected persons in Soweto, South Africa. HIV Med. 2015, 16, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Durski, K.N.; Kuntz, K.M.; Yasukawa, K.; Virnig, B.A.; Meya, D.B.; Boulware, D.R. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J. Acquir. Immune Defic. Syndr. 2013, 63, e101–e108. [Google Scholar] [CrossRef] [PubMed]
- Micol, R.; Tajahmady, A.; Lortholary, O.; Balkan, S.; Quillet, C.; Dousset, J.-P.; Chanroeun, H.; Madec, Y.; Fontanet, A.; Yazdanpanah, Y. Cost-effectiveness of primary prophylaxis of AIDS associated cryptococcosis in Cambodia. PLoS ONE 2010, 5, e13856. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Harrison, T.S.; Lawn, S.D.; Meintjes, G.; Wood, R.; Cleary, S. Cost Effectiveness of Cryptococcal Antigen Screening as a Strategy to Prevent HIV-Associated Cryptococcal Meningitis in South Africa. PLoS ONE 2013, 8, e69288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.M.; Nguyen, T.A.; Ha, H.T.T.; Thang, P.H.; Thuy, C.; Lien, T.X.; Bui, H.T.; Le, T.H.; Struminger, B.; McConnell, M.S.; et al. Prevalence of cryptococcal antigenemia and cost-effectiveness of a cryptococcal antigen screening program—Vietnam. PLoS ONE 2013, 8, e62213. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Uganda | Nigeria |
|---|---|---|
| Population (2015) | 40.1 million | 181.2 million |
| Annual cases of cryptococcal meningitis | 12,211 * | 27,058 * |
| Annual mortality | 10,120 * | 24,972 * |
| Proportion of all AIDS deaths | 23% * | 14% * |
| Fluconazole | Available | Available |
| Amphotericin B | Available | Available (poor accessibility and unaffordable ) |
| Flucytosine | Not available | Not available |
| Lumbar puncture | Routinely done | Not done (adults) |
| Manometry | Routinely done | Not done |
| Cryptococcal lateral flow assays | Available | Not available |
| National cryptococcal screening and treatment program | Available | Not available |
| Expert physicians in cryptococcal disease management | >5 | 2 or 3 |
| Clinical trials on cryptococcal meningitis | >5 | None |
| Centre of excellence | Yes | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladele, R.O.; Bongomin, F.; Gago, S.; Denning, D.W. HIV-Associated Cryptococcal Disease in Resource-Limited Settings: A Case for “Prevention Is Better Than Cure”? J. Fungi 2017, 3, 67. https://doi.org/10.3390/jof3040067
Oladele RO, Bongomin F, Gago S, Denning DW. HIV-Associated Cryptococcal Disease in Resource-Limited Settings: A Case for “Prevention Is Better Than Cure”? Journal of Fungi. 2017; 3(4):67. https://doi.org/10.3390/jof3040067
Chicago/Turabian StyleOladele, Rita O., Felix Bongomin, Sara Gago, and David W. Denning. 2017. "HIV-Associated Cryptococcal Disease in Resource-Limited Settings: A Case for “Prevention Is Better Than Cure”?" Journal of Fungi 3, no. 4: 67. https://doi.org/10.3390/jof3040067





