Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success
Abstract
:1. Introduction
2. In Vitro Antifungal Activity of Efinaconazole
3. Keratin Affinity and Transungual Penetration in Vitro
4. Transungual Penetration in Onychomycosis Patients
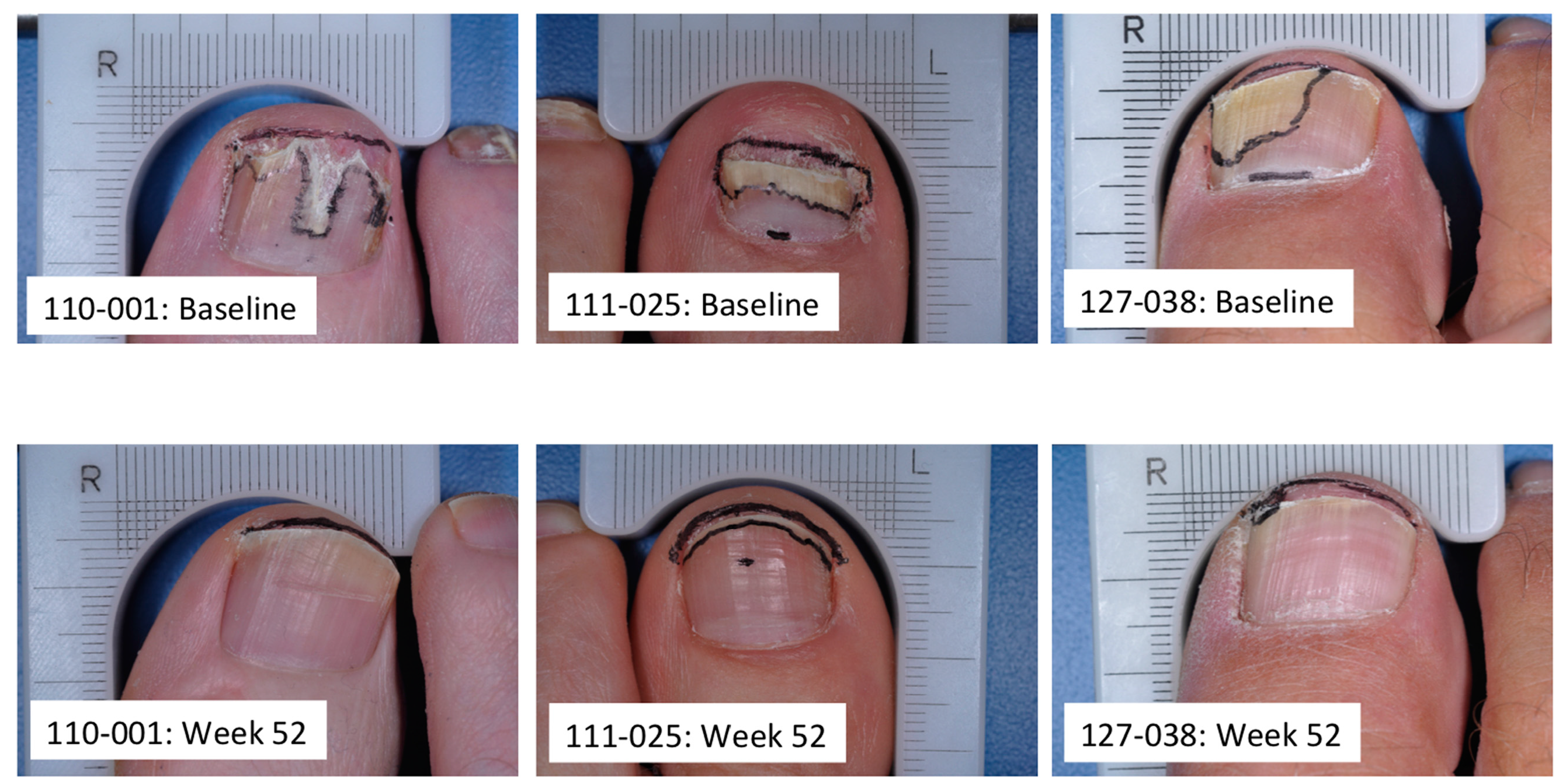
5. Subungual Penetration in Onychomycosis Patients
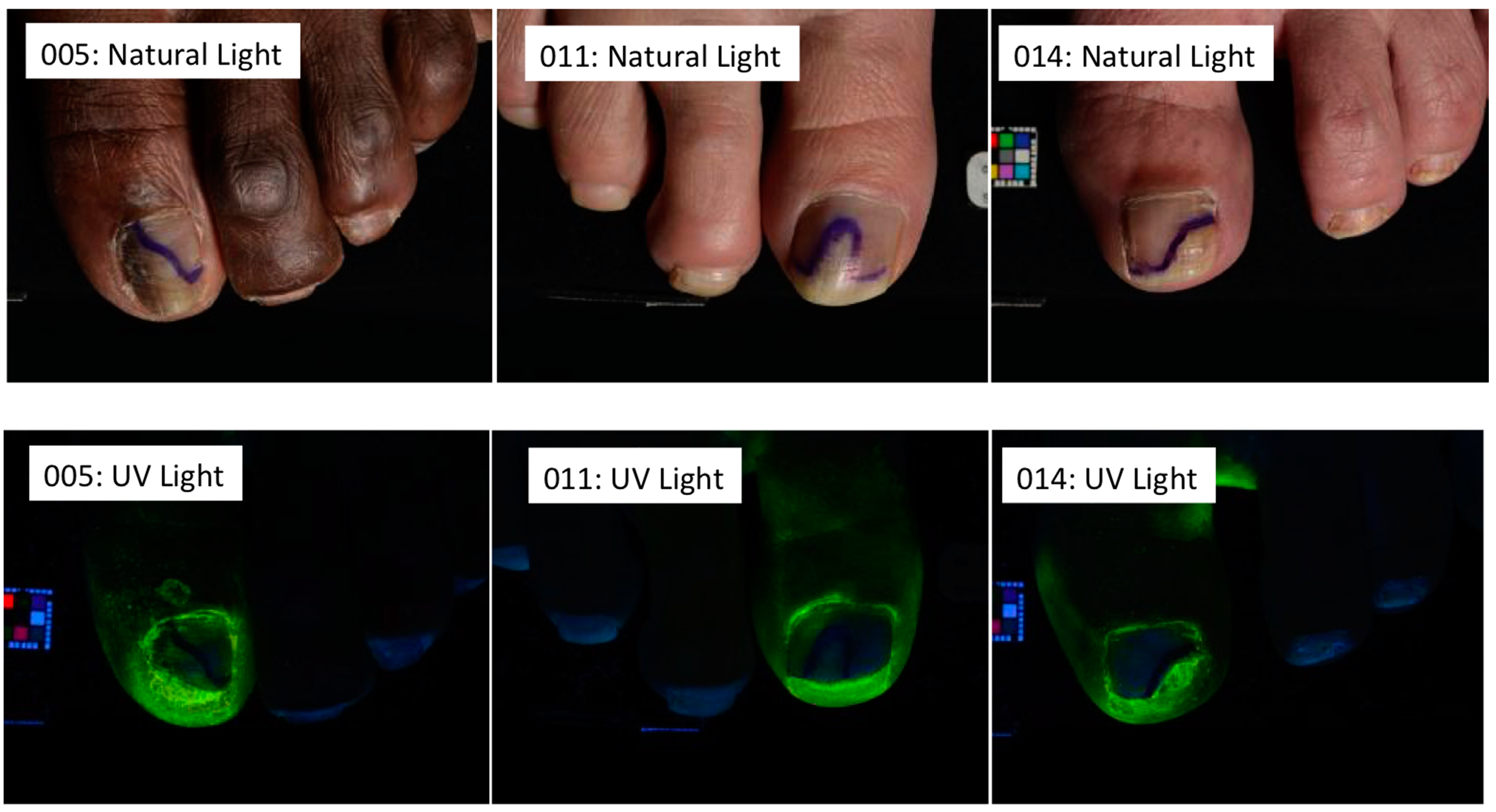
6. In Vitro Antifungal Activity under the Nail Plate
7. In Vitro Antifungal Activity in the Presence of Keratin
8. In Vivo Assessment of Efficacy in Guinea Pig Model of Onychomycosis
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scher, R.K.; Coppa, L.M. Advances in the diagnosis and treatment of onychomycosis. Hosp. Med. 1998, 34, 11–20. [Google Scholar]
- Crissey, J.T. Common dermatophyte infections. A simple diagnostic test and current management. Postgrad. Med. 1998, 103, 191–192; 197–200, 205. [Google Scholar] [CrossRef]
- Murdan, S. The nail: Anatomy, physiology, diseases and treatment. In Topical Nail Products and Ungual Drug Delivery; Murthy, S.N., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–36. [Google Scholar]
- Sparavigna, A.; Setaro, M.; Frisenda, L. Physical and microbiological properties of a new nail protective medical device. J. Plastic Dermatol. 2008, 4, 5–12. [Google Scholar]
- Gupta, A.K.; Joseph, W.S. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J. Am. Podiatr. Med. Assoc. 2000, 90, 495–501. [Google Scholar] [CrossRef]
- Baran, R.; Kaoukhov, A. Topical antifungal drugs for the treatment of onychomycosis: An overview of current strategies for monotherapy and combination therapy. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 21–29. [Google Scholar] [CrossRef]
- Lecha, M.; Effendy, I.; Feuilhade de Chauvin, M.; di Chiacchio, N.; Baran, R. Treatment options—Development of consensus guidelines. J. Eur. Acad. Dermatol. Venereol. 2005, 19 (Suppl. S1), 25–33. [Google Scholar] [CrossRef]
- Murdan, S. Drug delivery to the nail following topical application. Int. J. Pharm. 2002, 236, 1–26. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Berger, U.; Walker, B.; Schmidt, S.; Weindl, G.; Jäckel, A. Susceptibility testing of amorolfine, bifonazole and ciclopiroxolamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med. Mycol. 2009, 47, 753–758. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Yokoo, M.; Senda, H.; Kakehi, K. Therapeutic efficacy of topically applied KP-103 against experimental tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 2002, 46, 3797–3801. [Google Scholar] [CrossRef]
- Okeke, C.N.; Tsuboi, R.; Kawai, M.; Ogawa, H. Fluorometric assessment of in vitro antidermatophytic activities of antimycotics based on their keratin-penetrating power. J. Clin. Microbiol. 2000, 38, 489–491. [Google Scholar]
- Baraldi, A.; Jones, S.A.; Guesne, S.; Traynor, M.J.; McAuley, W.J.; Brown, M.B.; Murdan, S. Human nail plate modifications induced by onychomycosis: Implications for topical therapy. Pharm. Res. 2014, 32, 26–33. [Google Scholar] [CrossRef]
- Tosti, A.; Daniel, R.; Piraccini, B.M.; Iorizzo, M. Color Atlas of Nails; Springer: Berlin, Germany, 2010; pp. 15–22. [Google Scholar]
- Hamilton, J.B.; Terada, H.; Mestler, G.E. Studies of growth throughout the lifespan in Japanese: Growth and size of nails and their relationship to age, sex, heredity, and other factors. J. Geront. 1955, 10, 401–415. [Google Scholar] [CrossRef]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase 3 multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef]
- Bhatt, V.; Pillai, R. Efinaconazole topical solution 10%: Formulation development program of a new topical treatment of toenail onychomycosis. J. Pharm. Sci. 2015, 104, 2177–2182. [Google Scholar] [CrossRef]
- Gupta, A.K.; Jain, H.C.; Lynde, C.W.; Macdonald, P.; Cooper, E.A.; Summerbell, R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 2000, 43, 244–248. [Google Scholar] [CrossRef]
- Loo, D.S. Onychomycosis in the elderly: Drug treatment options. Drugs Aging 2007, 24, 293–302. [Google Scholar] [CrossRef]
- Foster, K.W.; Ghannoum, M.A.; Elewski, B.E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J. Am. Acad. Dermatol. 2004, 50, 748–752. [Google Scholar] [CrossRef]
- Vender, R.B.; Lynde, C.W.; Poulin, Y. Prevalence and epidemiology of onychomycosis. J. Cutan. Med. Surg. 2006, 10, S28–S33. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ricci, M.-J. Diagnosing onychomycosis. Dermatol. Clin. 2006, 24, 365–369. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Baran, R.; Summerbell, R.C. Nondermatophyte onychomycosis. Dermatol. Clin. 2003, 21, 257–268. [Google Scholar] [CrossRef]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012, 66, 494–502. [Google Scholar] [CrossRef]
- Summerbell, R.C. Epidemiology and ecology of onychomycosis. Dermatology 1997, 194, S32–S36. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S. Onychomycosis caused by nondermatophyte molds: Clinical features and response to treatment in 59 cases. J. Am. Acad. Dermatol. 2000, 42, 217–224. [Google Scholar] [CrossRef]
- Jo Siu, W.J.; Tatsumi, Y.; Senda, H.; Pillai, R.; Nakamura, T.; Sone, D.; Fothergill, A. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 1610–1606. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miyamoto, M.; Sugibayashi, K.; Morimoto, Y. Drug permeation through the three layers of the human nail plate. J. Pharm. Pharmacol. 1999, 51, 271–278. [Google Scholar] [CrossRef]
- Sugiura, K.; Sugimoto, N.; Hosaka, S.; Katafuchi-Nagashima, M.; Arakawa, Y.; Tatsumi, Y.; Jo Siu, W.; Pillai, R. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob. Agents Chemother. 2014, 58, 3837–3842. [Google Scholar] [CrossRef]
- Narasimha Murthy, S.; Wiskirchen, D.E.; Bowers, C.P. Iontophoretic drug delivery across human nail. J. Pharm. Sci. 2007, 96, 305–311. [Google Scholar] [CrossRef]
- Elewski, B.E.; Ghannoum, M.A.; Mayser, P.; Gupta, A.K.; Korting, H.C.; Shouey, R.J.; Baker, D.R.; Rich, P.A.; Ling, M.; Hugot, S.; et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: Results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 287–294. [Google Scholar] [CrossRef]
- Hui, X.; Shainhouse, Z.; Tanojo, H.; Anigbogu, A.; Markus, G.E.; Maibach, H.I.; Wester, R.C. Enhanced human nail drug delivery: Nail inner drug content assayed by new unique method. J. Pharm. Sci. 2002, 91, 189–195. [Google Scholar] [CrossRef]
- Sakamoto, M.; Sugimoto, N.; Kawabata, H.; Yamakawa, E.; Kodera, N.; Pillai, R.; Tatsumi, Y. Transungual delivery of efinaconazole: Its deposition in the nail of onychomycosis patients and in vitro fungicidal activity in human nails. J. Drugs Dermatol. 2014, 13, 1388–1392. [Google Scholar]
- Elewski, B.E.; Pollak, R.A.; Pillai, R.; Olin, J.T. Access of efinaconazole topical solution 10% to the infection site by spreading through the subungual space. J. Drugs Dermatol. 2014, 13, 1394–1398. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollak, R.A.; Siu, W.J.J.; Tatsumi, Y.; Pillai, R. Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success. J. Fungi 2015, 1, 107-114. https://doi.org/10.3390/jof1020107
Pollak RA, Siu WJJ, Tatsumi Y, Pillai R. Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success. Journal of Fungi. 2015; 1(2):107-114. https://doi.org/10.3390/jof1020107
Chicago/Turabian StylePollak, Richard A., William J. Jo Siu, Yoshiyuki Tatsumi, and Radhakrishnan Pillai. 2015. "Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success" Journal of Fungi 1, no. 2: 107-114. https://doi.org/10.3390/jof1020107
APA StylePollak, R. A., Siu, W. J. J., Tatsumi, Y., & Pillai, R. (2015). Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success. Journal of Fungi, 1(2), 107-114. https://doi.org/10.3390/jof1020107





