Ankle-Brachial Index and Arterial Stiffness, Modulate the Exertional Capacity of High-Frequency Training Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Cardio-Ankle-Vascular Index (CAVI)
2.3. Anthropometry and Body Composition
2.4. Exercise Treadmill-Stress-Testing
2.5. Statistical Analysis
3. Results
3.1. Type of Sport and Result of the Treadmill Stress Test
3.2. Ankle-Brachial Index and Result of the Treadmill Stress Test
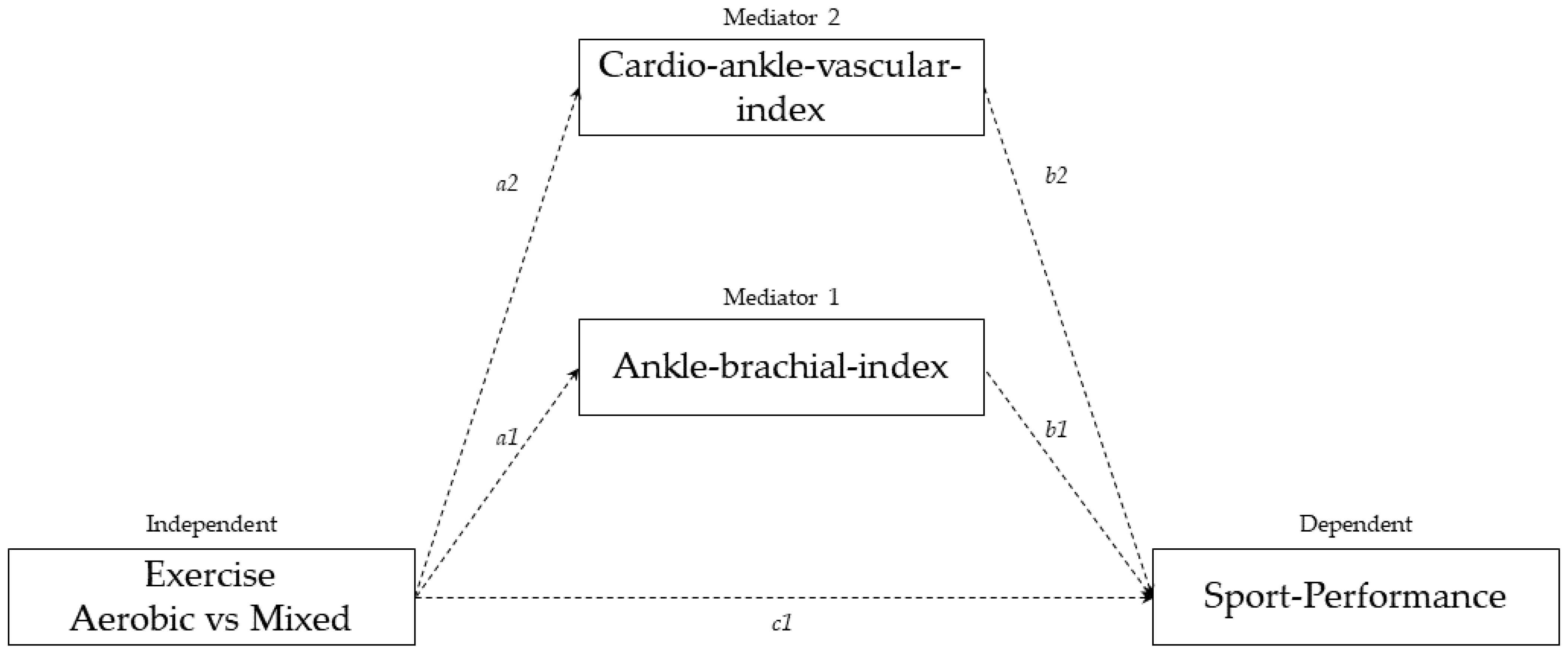
3.3. Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, C.; Barroso, R.; Beneke, R.; Bok, D.; Boullosa, D.; Casado, A.; Chamari, K.; Cortis, C.; de Koning, J.; Fusco, A.; et al. How to Succeed as an Athlete: What We Know, What We Need to Know. Int. J. Sports Physiol. Perform. 2022, 17, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Alejo, L.B.; Montalvo-Pérez, A.; Valenzuela, P.L.; Revuelta, C.; Ozcoidi, L.M.; de la Calle, V.; Mateo-March, M.; Lucia, A.; Santalla, A.; Barranco-Gil, D. Comparative analysis of endurance, strength and body composition indicators in professional, under-23 and junior cyclists. Front. Physiol. 2022, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaard, S.; Brocherie, F.; Jaspers, R.T. Under the Hood: Skeletal Muscle Determinants of Endurance Performance. Front. Sports Act. Living 2021, 3, 213. [Google Scholar] [CrossRef]
- van der Zwaard, S.; de Ruiter, C.J.; Jaspers, R.T.; de Koning, J.J. Anthropometric Clusters of Competitive Cyclists and Their Sprint and Endurance Performance. Front. Physiol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Woo, J.S.; Derleth, C.; Stratton, J.R.; Levy, W. The Influence of Age, Gender, and Training on Exercise Efficiency. J. Am. Coll. Cardiol. 2006, 47, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, A.B. Large Artery Stiffness: Implications For Exercise Capacity And Cardiovascular Risk. Clin. Exp. Pharmacol. Physiol. 2002, 29, 214–217. [Google Scholar] [CrossRef]
- Otsuki, T.; Maeda, S.; Iemitsu, M.; Saito, Y.; Tanimura, Y.; Ajisaka, R.; Miyauchi, T. Vascular endothelium-derived factors and arterial stiffness in strength- and endurance-trained men. Am. J. Physiol. Circ. Physiol. 2007, 292, H786–H791. [Google Scholar] [CrossRef]
- Tordi, N.; Colin, E.; Mourot, L.; Bouhaddi, M.; Regnard, J.; Laurant, P. Effects of resuming endurance training on arterial stiffness and nitric oxide production during exercise in elite cyclists. Appl. Physiol. Nutr. Metab. 2006, 31, 244–249. [Google Scholar] [CrossRef]
- Williams, P.T. Association between walking distance and percentiles of body mass index in older and younger men. Br. J. Sports Med. 2008, 42, 352–356. [Google Scholar] [CrossRef]
- Casey, D.P.; Pierce, G.L.; Howe, K.S.; Mering, M.C.; Braith, R.W. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur. J. Appl. Physiol. 2007, 100, 403–408. [Google Scholar] [CrossRef]
- Loimaala, A.; Groundstroem, K.; Rinne, M.; Nenonen, A.; Huhtala, H.; Parkkari, J.; Vuori, I. Effect of Long-Term Endurance and Strength Training on Metabolic Control and Arterial Elasticity in Patients With Type 2 Diabetes Mellitus. Am. J. Cardiol. 2009, 103, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Kubukeli, Z.N.; Noakes, T.D.; Dennis, S.C. Training Techniques to Improve Endurance Exercise Performances. Sports Med. 2002, 32, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of Exercise Modalities on Arterial Stiffness and Wave Reflection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, A.; Di Blasio, A.; Napolitano, G.; Ripari, P.; Paganelli, R.; Cipollone, F.; Angelo, D.I.; Andrea, D.B.; Giorgio, N.; Patrizio, R.; et al. High fat mass, low muscle mass, and arterial stiffness in a population of free-living healthy subjects: The “al passo con la tua salute” project. Medicine 2019, 98, e16172. [Google Scholar] [CrossRef]
- Kubozono, T.; Miyata, M.; Ueyama, K.; Nagaki, A.; Otsuji, Y.; Kusano, K.; Kubozono, O.; Tei, C. Clinical Significance and Reproducibility of New Arterial Distensibility Index. Circ. J. 2007, 71, 89–94. [Google Scholar] [CrossRef]
- Crawford, F.; Welch, K.; Andras, A.; Chappell, F.M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lockwood, C.M.; Hoffman, J.M.; West, S.G.; Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods 2002, 7, 83–104. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: A Systematic Review and Meta-Analysis. Sport. Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Hsu, C.L.; Best, J.R.; Davis, J.C.; Nagamatsu, L.S.; Wang, S.; Boyd, A.L.; Hsiung, G.R.; Voss, M.W.; Eng, J.J.; Liu-Ambrose, T. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br. J. Sports Med. 2018, 52, 184–191. [Google Scholar] [CrossRef]
- Miyachi, M.; Kawano, H.; Sugawara, J.; Takahashi, K.; Hayashi, K.; Yamazaki, K.; Tabata, I.; Tanaka, H. Unfavorable Effects of Resistance Training on Central Arterial Compliance. Circulation 2004, 110, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.J.; Pellinger, T.K.; Rosette, V.D.; Ortlip, A.T. Effects of a 12-Week Resistance Training Program on Arterial Stiffness. J. Strength Cond. Res. 2019, 1, 3281–3287. [Google Scholar] [CrossRef]
- Horiuchi, M.; Okita, K. Blood Flow Restricted Exercise and Vascular Function. Int. J. Vasc. Med. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stølen, T.; Chamari, K.; Castagna, C.; Wisløff, U. Physiology of Soccer. Sports Med. 2005, 35, 501–536. [Google Scholar] [CrossRef]
- McDermott, M.M.; Ferrucci, L.; Guralnik, J.M.; Dyer, A.R.; Liu, K.; Pearce, W.H.; Clark, E.; Liao, Y.; Criqui, M.H. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc. Med. 2010, 15, 251–257. [Google Scholar] [CrossRef]
- Krustrup, P.; Aagaard, P.; Nybo, L.; Petersen, J.; Mohr, M.; Bangsbo, J. Recreational football as a health promoting activity: A topical review. Scand. J. Med. Sci. Sports 2010, 20, 1–13. [Google Scholar] [CrossRef]
- Oral, O. Nitric oxide and its role in exercise physiology. J. Sports Med. Phys. Fitness 2021, 61, 1208–1211. [Google Scholar] [CrossRef]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021, 53, 280–294. [Google Scholar] [CrossRef]
- Krustrup, P.; Helge, E.W.; Hansen, P.R.; Aagaard, P.; Hagman, M.; Randers, M.B.; de Sousa, M.; Mohr, M. Effects of recreational football on women’s fitness and health: Adaptations and mechanisms. Eur. J. Appl. Physiol. 2018, 118, 11–32. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H. Effects of Endurance Training on Blood Pressure, Blood Pressure–Regulating Mechanisms, and Cardiovascular Risk Factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.R.; Gobel, F.L.; Jorgensen, C.R.; Wang, K.; Wang, Y.; Taylor, H.L. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 1974, 50, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
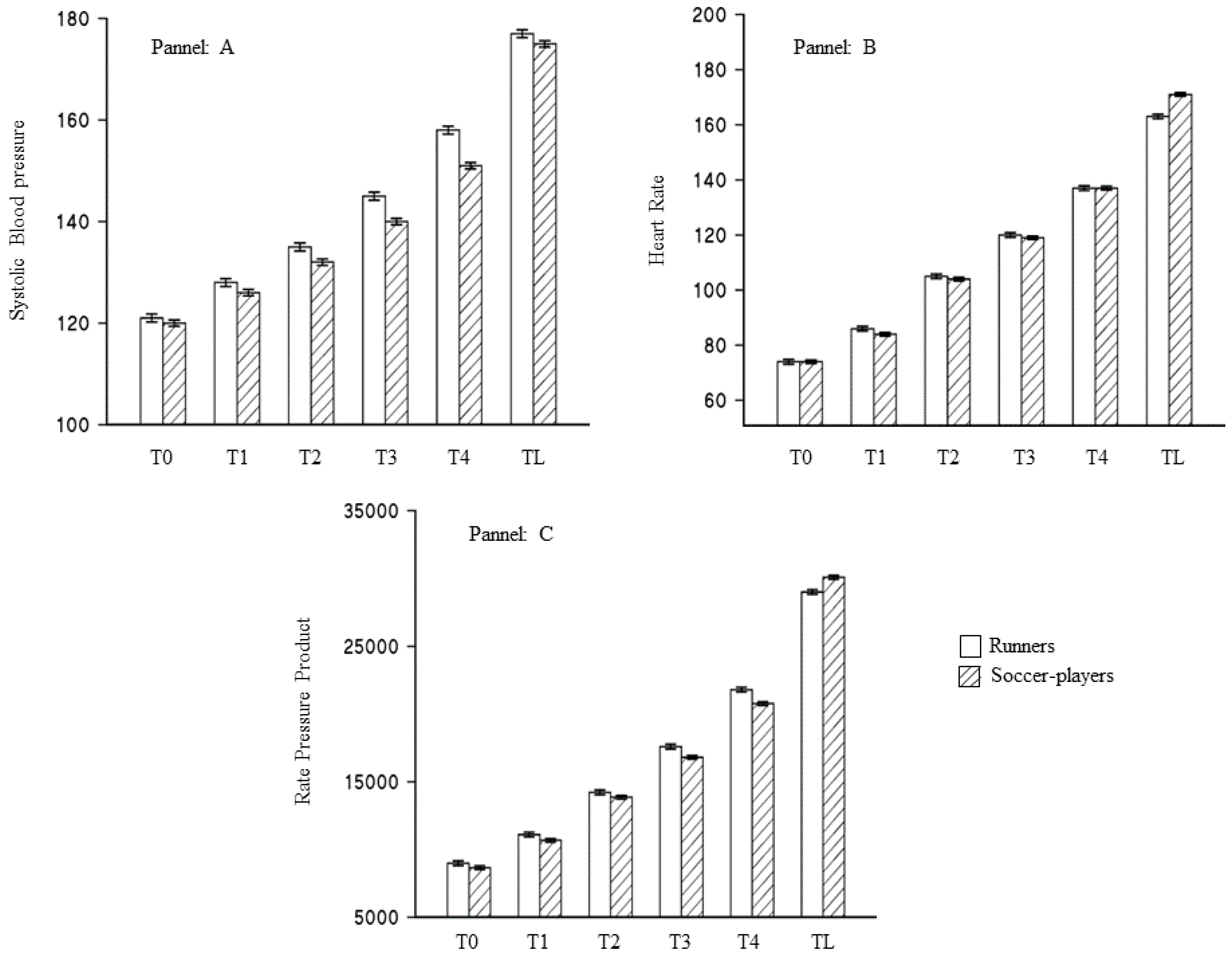
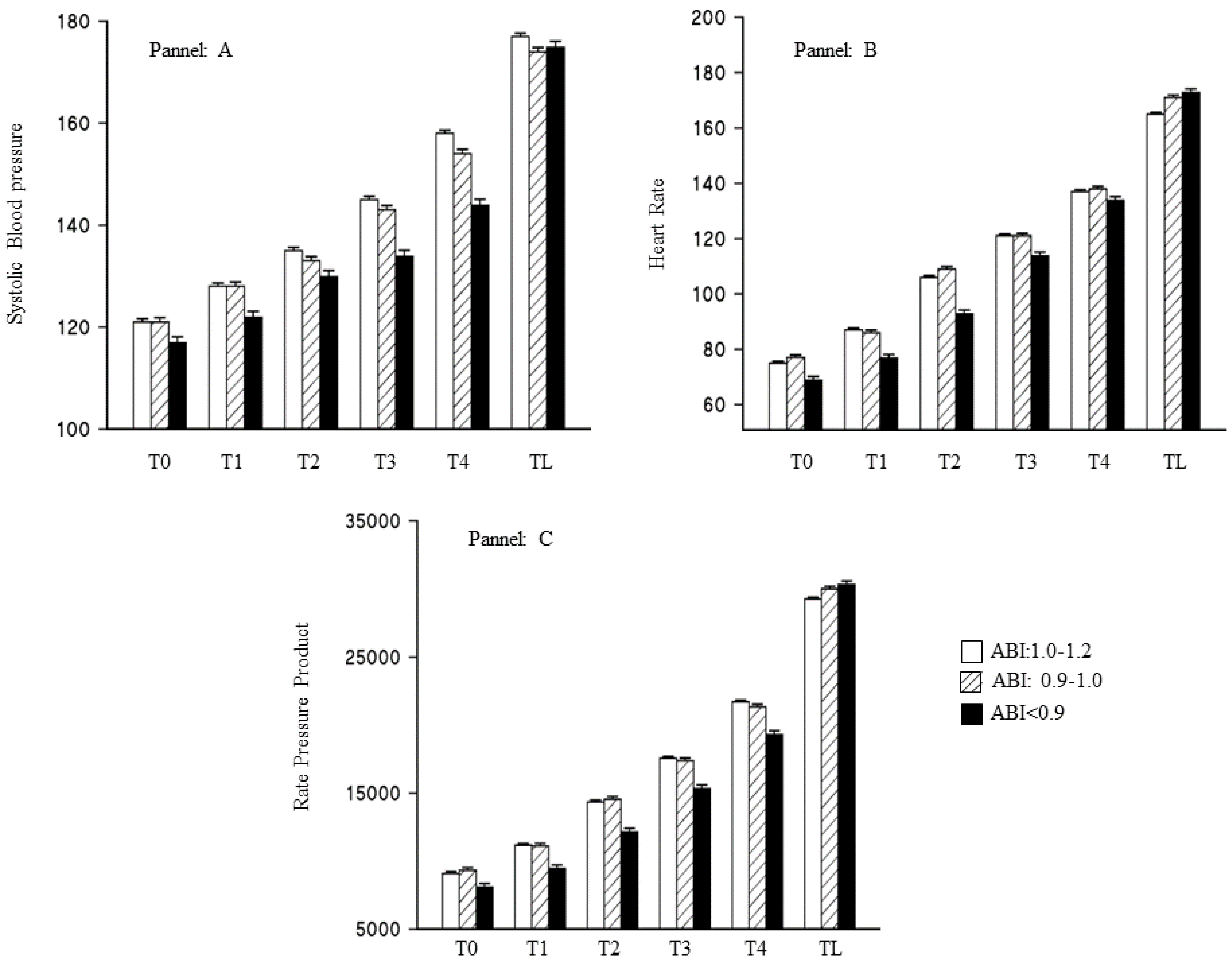
| Variables | Runners 92 | Soccer-Players 148 | p-Value |
|---|---|---|---|
| Age (years) | 23.52 (3.76) | 23.58 (3.91) | 0.91 |
| Height (m) | 1.77 (0.06) | 1.79 (0.06) | 0.63 |
| Weight (Kg) | 75.66 (6.34) | 75.33 (6.95) | 0.71 |
| Cardio ankle vascular index right | 6.01 (0.59) | 5.80 (0.64) | 0.01 |
| Cardio ankle vascular index left | 5.98 (0.63) | 5.75 (0.69) | 0.009 |
| SBP brachial right (mm/Hg) 1 | 124.55 (6.86) | 124.22 (6.64) | 0.71 |
| DBP brachial right (mm/Hg) 1 | 78.76 (9.53) | 69.10 (5.96) | 0.001 |
| SBP ankle right (mm/Hg) 1 | 129.54 (12.04) | 124.12 (13.29) | 0.002 |
| DBP ankle right (mm/Hg) 1 | 76.97 (10.74) | 69.56 (6.70) | 0.001 |
| Ankle-brachial index right | 1.04 (0.09) | 1.00 (0.10) | 0.009 |
| SBP brachial left (mm/Hg) 1 | 125.45 (5.38) | 125.14 (5.38) | 0.66 |
| DBP brachial left (mm/Hg) 1 | 78.18 (8.44) | 69.26 (6.10) | 0.001 |
| SBP ankle left (mm/Hg) 1 | 129.51 (8.97) | 122.91 (11.35) | 0.001 |
| DBP ankle left (mm/Hg) 1 | 77.66 (8.41) | 69.16 (6.72) | 0.001 |
| Ankle-brachial index left | 1.03 (0.07) | 0.98 (0.08) | 0.001 |
| Rest rate metabolism | 1964.89 (163.43) | 1988.87 (157.46) | 0.26 |
| Fat mass (percentage) | 14.18 (3.78) | 14.00 (3.37) | 0.70 |
| Muscle mass (percentage) | 81.49 (3.58) | 81.70 (3.20) | 0.64 |
| Path | Coefficient | Standard Error | p-Value | |
|---|---|---|---|---|
| Type of sport predicting ankle-brachial index(ABI) | a1 | −0.05 | 0.01 | <0.001 |
| Type of sport predicting arterial stiffness (CAVI) | a2 | −0.22 | 0.09 | 0.01 |
| Ankle-brachial index predicting time spent in the treadmill stress test | b1 | −11.33 | 1.50 | <0.001 |
| Arterial stiffness (CAVI) predicting time spent in the treadmill stress test | b2 | −1.14 | 0.18 | 0.008 |
| Direct effect of type of sport on time spent in the treadmill stress test | c1 | 1.48 | 0.24 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, R.; Sparvieri, E.; Di Blasio, A.; Barassi, G.; Murgia, M.; Ripari, P.; Di Iorio, A. Ankle-Brachial Index and Arterial Stiffness, Modulate the Exertional Capacity of High-Frequency Training Athletes. J. Cardiovasc. Dev. Dis. 2022, 9, 312. https://doi.org/10.3390/jcdd9090312
Pellegrino R, Sparvieri E, Di Blasio A, Barassi G, Murgia M, Ripari P, Di Iorio A. Ankle-Brachial Index and Arterial Stiffness, Modulate the Exertional Capacity of High-Frequency Training Athletes. Journal of Cardiovascular Development and Disease. 2022; 9(9):312. https://doi.org/10.3390/jcdd9090312
Chicago/Turabian StylePellegrino, Raffaello, Eleonora Sparvieri, Andrea Di Blasio, Giovanni Barassi, Massimiliano Murgia, Patrizio Ripari, and Angelo Di Iorio. 2022. "Ankle-Brachial Index and Arterial Stiffness, Modulate the Exertional Capacity of High-Frequency Training Athletes" Journal of Cardiovascular Development and Disease 9, no. 9: 312. https://doi.org/10.3390/jcdd9090312





