Conundrum of Classifying Subtypes of Pulmonary Hypertension—Introducing a Novel Approach to Classify “Borderline” Patients in a Population with Severe Aortic Stenosis Undergoing TAVI
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Procedure of RHC
2.3. Subtypes of PH
2.4. Transthoracic Echocardiography
2.5. TAVI Procedure
2.6. Clinical Follow-Up and Study Endpoint
2.7. Statistical Analysis
3. Results
3.1. PH Subtypes of Study Collective
3.2. General Characteristics
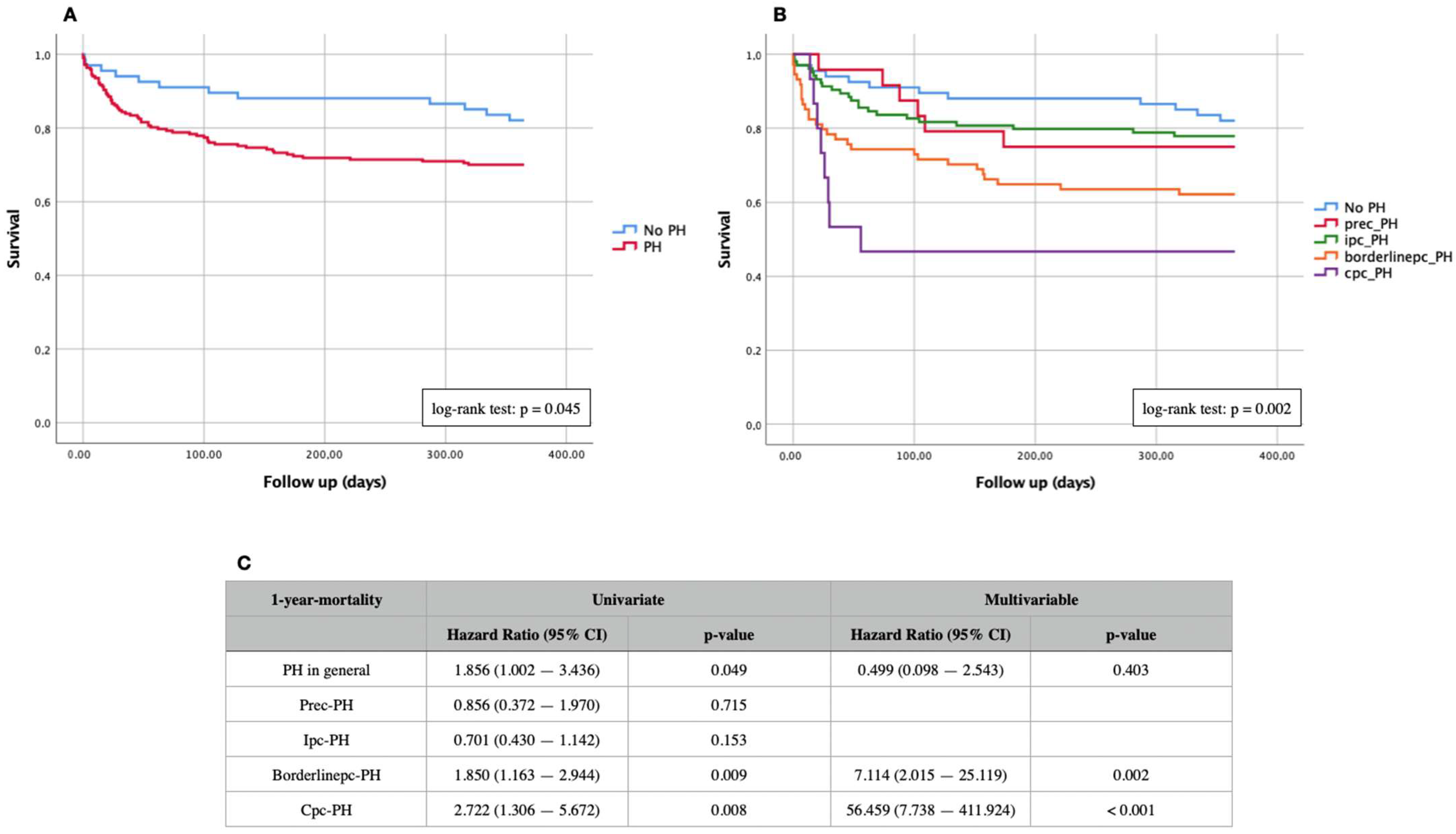
3.3. Kaplan–Meier Curves and Cox Proportional Hazard Regression
3.4. ROC Curves and Cut-Off Values
4. Discussion
4.1. PH as a Solitary Risk Factor in Patients Receiving TAVI?
4.2. Prec-PH and AS—Does This Constellation Fit Together?
4.3. Borderlinepc-PH—Is It Worth Being a New Subtype?
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, S.; Frangos, E.; Samaras, N.; Ellenberger, C.; Frangos, C.; Cikirikcioglu, M.; Bendjelid, K.; Frei, A.; Myers, P.; Licker, M.; et al. Transcatheter aortic valve implantation in nonagenarians: Effective and safe. Eur. J. Intern. Med. 2013, 24, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Panayiotides, I.M.; Nikolaides, E. Transcatheter aortic valve implantation (TAVI): Is it time for this intervention to be applied in a lower risk population? Clin. Med. Insights Cardiol. 2014, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Webb, J.G.; Cheung, A.; Ye, J.; Dumont, E.; Feindel, C.M.; Osten, M.; Natarajan, M.K.; Velianou, J.L.; Martucci, G.; et al. Transcatheter Aortic Valve Implantation for the Treatment of Severe Symptomatic Aortic Stenosis in Patients at Very High or Prohibitive Surgical Risk. Acute and Late Outcomes of the Multicenter Canadian Experience. J. Am. Coll. Cardiol. 2010, 55, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M. Pulmonary Vascular Remodeling in Pulmonary Hypertension. Cell Tissue Res. 2017, 367, 643–649. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yoshihisa, A.; Nakazato, K.; Yokokawa, T.; Misaka, T.; Oikawa, M.; Kobayashi, A.; Yamaki, T.; Kunii, H.; Ishida, T.; et al. Significance of pulmonary vascular resistance and diastolic pressure gradient on the new definition of combined post-capillary pulmonary hypertension. Int. Heart J. 2020, 61, 301–307. [Google Scholar] [CrossRef]
- Melby, S.J.; Moon, M.R.; Lindman, B.R.; Bailey, M.S.; Hill, L.L.; Damiano, R.J. Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2011, 141, 1424–1430. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. Registry of Transcatheter Aortic-Valve Implantation in High-Risk Patients. Surv. Anesthesiol. 2013, 57, 62–63. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tuller, D.; et al. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation insights from the new proposed pulmonary hypertension classification. Circ. Cardiovasc. Interv. 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Luçon, A.; Oger, E.; Bedossa, M.; Boulmier, D.; Verhoye, J.P.; Eltchaninoff, H.; Iung, B.; Leguerrier, A.; Laskar, M.; Leprince, P.; et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation study from the france 2 registry. Circ. Cardiovasc. Interv. 2014, 7, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Schewel, J.; Schmidt, T.; Kuck, K.H.; Frerker, C.; Schewel, D. Impact of Pulmonary Hypertension Hemodynamic Status on Long-Term Outcome After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Rickli, H.; Haager, P.K.; Joerg, L.; Weilenmann, D.; Brenner, R.; Taramasso, M.; Baier, P.; Maisano, F.; Maeder, M.T. Haemodynamic mechanisms and long-term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur. J. Heart Fail. 2019, 21, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kaple, R.; Sean, W.; Polydoros, K.; Amiran, B.; Micheas, Z.; Htoo, K.; Geoffrey, B.; Robert, M.; Richard, D.; Arash, S.; et al. Impact of etiology of pulmonary hypertension on post-procedural management and outcomes in patients undergoing transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2013, 61 (Suppl. 10), E1954. [Google Scholar] [CrossRef]
- Haskell, R.J.; French, W.J. Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting left ventricular end-diastolic pressure. Am. J. Cardiol. 1988, 61, 136–141. [Google Scholar] [CrossRef]
- Chen, A.; Ismail-Sayed, I.; Penupolu, S.; Bandyopadhyay, D. Discordance between pulmonary capillary wedge pressure (PCWP) and left ventricular end-diastolic pressure (LVEDP): A challenge in diagnosing pulmonary artery hypertension (PAH). Am. J. Respir. Crit. Care Med. 2017, 195, 4233. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617709241%0A (accessed on 8 March 2022). [CrossRef]
- Reddy YN, V.; El-Sabbagh, A.; Nishimura, R.A. Comparing pulmonary arterialwedge pressure and left ventricular end diastolic pressure for assessment of left-sided filling pressures. JAMA Cardiol. 2018, 3, 453–454. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Opotowsky, A.R.; Assad, T.R.; Xu, M.; Doss, L.N.; Farber-Eger, E.; Wells, Q.S.; Brittain, E.L. Features Associated With Discordance Between Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure in Clinical Practice: Implications for Pulmonary Hypertension Classification. Chest 2018, 154, 1099–1107. [Google Scholar] [CrossRef]
- Gerges, C.; Gerges, M.; Lang, M.B.; Zhang, Y.; Jakowitsch, J.; Probst, P.; Maurer, G.; Lang, I.M. Diastolic pulmonary vascular pressure gradient: A predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013, 143, 758–766. [Google Scholar] [CrossRef]
- Palazzini, M.; Dardi, F.; Manes, A.; Bacchi Reggiani, M.L.; Gotti, E.; Rinaldi, A.; Albini, A.; Monti, E.; Galiè, N. Pulmonary hypertension due to left heart disease: Analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur. J. Heart Fail. 2018, 20, 248–255. [Google Scholar] [CrossRef]
- Caravita, S.; Faini, A.; D’Araujo, S.C.; Dewachter, C.; Chomette, L.; Bondue, A.; Naeije, R.; Parati, G.; Vachiéry, J.L. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: Role of the pre-capillary component. PLoS ONE 2018, 13, e0199164. [Google Scholar] [CrossRef]
- Dragu, R.; Hardak, E.; Ohanyan, A.; Adir, Y.; Aronson, D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int. J. Cardiol. 2019, 290, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Overall Cohort | No PH | Prec-PH | Ipc-PH | Borderlinepc-PH | Cpc-PH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | p-Value | |
| General data | |||||||||||||
| Age (years) | 80.5 | 7.2 | 80.6 | 6.2 | 83.5 | 5.6 | 79.6 | 8.1 | 80.6 | 7.1 | 80.6 | 7.4 | 0.234 |
| Weight (kg) | 73.7 | 13.1 | 70.8 | 13.1 | 75.4 | 16.3 | 77.2 | 12.6 | 71.5 | 12.1 | 70.1 | 10.9 | 0.005 |
| Height (cm) | 164.4 | 8.9 | 162.9 | 8.9 | 164.7 | 9.0 | 166.0 | 8.8 | 163.1 | 8.5 | 166.0 | 9.6 | 0.102 |
| Creatinine (µmol/L) | 121.9 | 87.8 | 104.6 | 54.4 | 114.6 | 53.2 | 136.4 | 109.7 | 121.8 | 90.6 | 111.1 | 52.2 | 0.255 |
| C-reactive protein (mg/L) | 18.9 | 32.8 | 10.8 | 22.1 | 15.5 | 19.8 | 19.6 | 34.3 | 26.2 | 41.5 | 19.5 | 27.4 | 0.132 |
| Hemoglobin (mmol/L) | 7.5 | 1.1 | 7.5 | 1.0 | 7.7 | 0.9 | 7.2 | 1.1 | 7.5 | 1.2 | 7.7 | 1.4 | 0.218 |
| STS-Score | 4.1 | 2.5 | 3.5 | 2.1 | 4.7 | 3.0 | 4.1 | 2.8 | 4.3 | 2.2 | 4.0 | 2.8 | 0.267 |
| EuroScore II | 6.7 | 5.4 | 5.0 | 5.7 | 6.0 | 4.2 | 7.4 | 5.2 | 7.6 | 5.3 | 7.4 | 5.1 | 0.175 |
| Concomitant diseases | |||||||||||||
| Insulin-dependent diabetes (%) | 23.9 | 19.4 | 8.3 | 29.8 | 25.7 | 20.0 | 0.186 | ||||||
| Non-insulin-dependent diabetes (%) | 34.5 | 35.8 | 50.0 | 25.0 | 36.5 | 60.0 | 0.024 | ||||||
| Arterial hypertension (%) | 91.2 | 92.5 | 95.8 | 88.5 | 91.9 | 93.3 | 0.756 | ||||||
| Coronary heart disease - 1 vessel (%) | 21.8 | 20.9 | 16.7 | 23.1 | 21.6 | 26.7 | 0.950 | ||||||
| Coronary heart disease - 2 vessels (%) | 13.4 | 10.4 | 25.0 | 15.4 | 12.2 | 6.7 | 0.194 | ||||||
| Coronary heart disease - 3 vessels (%) | 15.5 | 14.9 | 4.2 | 13.5 | 23.0 | 13.3 | 0.206 | ||||||
| COPD (%) | 25.0 | 19.4 | 29.2 | 27.9 | 23.0 | 33.3 | 0.641 | ||||||
| Myocardial infarction (%) | 14.8 | 14.9 | 4.2 | 17.5 | 14.9 | 13.3 | 0.599 | ||||||
| Stroke (%) | 15.1 | 10.4 | 20.8 | 19.2 | 12.2 | 13.3 | 0.454 | ||||||
| Atrial fibrillation (%) | 52.6 | 34.9 | 40.0 | 66.7 | 52.4 | 71.4 | 0.023 | ||||||
| NYHA II | 11.3 | 17.9 | 20.8 | 8.7 | 5.4 | 13.3 | 0.072 | ||||||
| NYHA III | 60.2 | 62.7 | 62.5 | 58.7 | 62.2 | 46.7 | 0.844 | ||||||
| NYHA IV | 22.9 | 13.4 | 12.5 | 25.0 | 29.7 | 33.3 | 0.093 | ||||||
| Echocardiographic measurements | |||||||||||||
| EF (%) | 56.6 | 16.2 | 60.2 | 15.1 | 59.8 | 13.2 | 56.8 | 16.0 | 52.0 | 17.4 | 57.2 | 16.7 | 0.041 |
| LVEDD (mm) | 48.9 | 8.0 | 47.2 | 7.9 | 48.1 | 7.3 | 49.9 | 7.5 | 49.5 | 8.4 | 49.3 | 9.9 | 0.284 |
| LVESD (mm) | 32.5 | 9.5 | 30.0 | 9.3 | 32.6 | 9.6 | 33.0 | 9.3 | 33.9 | 9.5 | 34.8 | 11.6 | 0.286 |
| sPAP (mmHg) | 41.8 | 13.4 | 32.6 | 7.7 | 34.3 | 11.6 | 44.0 | 12.1 | 47.0 | 14.7 | 50.3 | 13.5 | <0.001 |
| AVA (cm2) | 0.7 | 0.2 | 0.7 | 0.2 | 0.6 | 0.2 | 0.7 | 0.2 | 0.6 | 0.2 | 0.7 | 0.2 | 0.012 |
| AV max (m/s) | 4.3 | 0.7 | 4.5 | 0.7 | 4.4 | 0.5 | 4.3 | 0.6 | 4.2 | 0.8 | 4.4 | 0.8 | 0.243 |
| AV dpmax (mmHg) | 78.5 | 26.3 | 82.8 | 25.6 | 79.4 | 15.5 | 75.1 | 23.0 | 78.2 | 32.3 | 81.1 | 30.2 | 0.470 |
| AV dpmean (mmHg) | 47.7 | 16.7 | 50.6 | 17.5 | 48.2 | 12.5 | 45.6 | 14.2 | 47.6 | 19.9 | 48.0 | 17.9 | 0.481 |
| Mitral regurgitation I° (%) | 49.6 | 63.6 | 62.5 | 45.5 | 32.4 | 66.7 | <0.001 | ||||||
| Mitral regurgitation II° (%) | 38.4 | 31.8 | 16.7 | 41.4 | 48.6 | 20.0 | 0.042 | ||||||
| Mitral regurgitation III° (%) | 8.4 | 3.1 | 16.7 | 9.1 | 14.9 | 6.7 | 0.076 | ||||||
| Tricuspid regurgitation I° (%) | 51.9 | 61.3 | 45.8 | 48.4 | 48.6 | 33.3 | 0.284 | ||||||
| Tricuspid regurgitation II° (%) | 32.7 | 30.6 | 29.2 | 32.3 | 29.7 | 46.7 | 0.791 | ||||||
| Tricuspid regurgitation III° (%) | 12.3 | 3.2 | 20.8 | 17.1 | 16.2 | 13.3 | 0.028 | ||||||
| Paravalvular regurgitation I° (%) | 37.9 | 38.1 | 60.0 | 39.6 | 30.0 | 37.5 | 0.524 | ||||||
| Paravalvular regurgitation II° (%) | 13.1 | 11.9 | 10.0 | 15.1 | 10.0 | 25.0 | 0.798 | ||||||
| Paravalvular regurgitation III° (%) | 0.7 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.006 | ||||||
| Measurements of RHC | |||||||||||||
| sPAP (mmHg) | 53.5 | 18.1 | 33.8 | 6.8 | 44.3 | 7.5 | 55.9 | 14.1 | 67.1 | 14.2 | 72.4 | 19.8 | <0.001 |
| mPAP (mmHg) | 34.1 | 12.0 | 19.6 | 3.7 | 27.6 | 2.9 | 36.3 | 8.2 | 42.9 | 8.7 | 50.1 | 11.3 | <0.001 |
| dPAP (mmHg) | 20.1 | 8.8 | 9.9 | 4.1 | 16.5 | 2.6 | 22.2 | 6.8 | 24.7 | 6.7 | 33.5 | 7.8 | <0.001 |
| mPCWP (mmHg) | 21.3 | 9.4 | 10.6 | 4.0 | 12.9 | 1.8 | 25.5 | 7.5 | 27.1 | 6.9 | 23.7 | 7.5 | <0.001 |
| DPG (mmHg) | −1.2 | 5.7 | −0.7 | 4.8 | 3.6 | 2.7 | −3.4 | 4.4 | −2.4 | 5.5 | 10.1 | 3.3 | <0.001 |
| PVR (WU) | 3.3 | 2.1 | 2.5 | 1.5 | 3.2 | 1.0 | 1.9 | 0.6 | 5.0 | 2.2 | 6.7 | 2.3 | <0.001 |
| TPG (mmHg) | 12.8 | 6.1 | 8.9 | 3.3 | 14.8 | 2.9 | 10.8 | 3.6 | 15.8 | 5.6 | 27.0 | 9.1 | <0.001 |
| CO (L/min) | 4.2 | 1.2 | 4.2 | 1.3 | 4.2 | 1.0 | 4.5 | 1.2 | 3.7 | 1.0 | 3.9 | 1.0 | <0.001 |
| Procedurale data | |||||||||||||
| Transfemoral approach (%) | 75.4 | 77.6 | 83.3 | 72.1 | 77.0 | 66.7 | 0.678 | ||||||
| CoreValve (%) | 22.3 | 17.9 | 29.2 | 25.0 | 21.6 | 20.0 | 0.800 | ||||||
| JenaValve (%) | 15.9 | 16.4 | 12.5 | 15.4 | 16.2 | 20.0 | 0.979 | ||||||
| Edwards (%) | 61.8 | 65.7 | 58.3 | 59.6 | 62.2 | 60.0 | 0.941 | ||||||
| Major vascular complications (%) | 11.3 | 11.9 | 8.3 | 9.6 | 12.2 | 20.0 | 0.786 | ||||||
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| General data | ||||
| Age (years) | 0.998 (0.967–1.030) | 0.894 | ||
| Weight (kg) | 0.989 (0.972–1.007) | 0.216 | ||
| Height (cm) | 1.000 (0.974–1.025) | 0.974 | ||
| Creatinine (µmol/L) | 1.001 (0.999–1.003) | 0.247 | ||
| C-reactive protein (mg/L) | 1.006 (1.000–1.011) | 0.039 | 1.000 (0.984–1.016) | 0.968 |
| Hemoglobin (mmol/L) | 0.899 (0.720–1.124) | 0.351 | ||
| STS-Score | 1.164 (1.093–1.240) | <0.001 | 1.114 (0.951–1.306) | 0.181 |
| EuroScore II | 1.081 (1.041–1.123) | <0.001 | 1.138 (1.049–1.235) | 0.002 |
| Concomitant diseases | ||||
| Insulin-dependent diabetes | 1.392 (0.854–2.268) | 0.184 | ||
| Non-insulin-dependent diabetes | 1.366 (0.866–2.155) | 0.179 | ||
| Arterial hypertension | 1.417 (0.572–3.508) | 0.451 | ||
| Coronary heart disease ≥ 2 vessels | 1.354 (0.844–2.171) | 0.209 | ||
| COPD | 1.939 (1.218–3.085) | 0.005 | 1.207 (0.380–3.836) | 0.750 |
| Myocardial infarction | 1.782 (1.040–3.054) | 0.036 | 1.202 (0.305–4.730) | 0.792 |
| Stroke | 1.959 (1.166–3.291) | 0.011 | 0.367 (0.079–1.718) | 0.203 |
| Atrial fibrillation | 1.619 (0.869–3.019) | 0.129 | ||
| NYHA ≥ III | 1.951 (0.786–4.843) | 0.150 | ||
| Echocardiographic measurements | ||||
| EF (%) | 0.990 (0.976–1.003) | 0.135 | ||
| LVEDD (mm) | 1.015 (0.988–1.044) | 0.273 | ||
| LVESD (mm) | 1.004 (0.974–1.035 | 0.788 | ||
| sPAP (mmHg) | 1.028 (1.012–1.044) | <0.001 | 0.974 (0.928–1.022) | 0.285 |
| AVA (cm2) | 0.231 (0.055–0.967) | 0.045 | 0.268 (0.014–5.192) | 0.384 |
| AV max (m/s) | 0.846 (0.605–1.182) | 0.327 | ||
| AV dpmax (mmHg) | 0.995 (0.985–1.004) | 0.249 | ||
| AV dpmean (mmHg) | 0.992 (0.978–1.006) | 0.267 | ||
| Mitral regurgitation ≥ II° | 1.319 (0.841–2.069) | 0.229 | ||
| Tricuspid regurgitation ≥ II° | 1.890 (1.166–3.065) | 0.010 | 3.119 (1.217–7.994) | 0.018 |
| Paravalvular regurgitation ≥ II° | 1.720 (0.792–3.733) | 0.170 | ||
| Measurements of RHC | ||||
| sPAP (mmHg) | 1.019 (1.008–1.030) | 0.001 | 1.015 (0.974–1.058) | 0.480 |
| mPAP (mmHg) | 1.032 (1.014–1.051) | 0.001 | 1.026 (0.874–1.203) | 0.756 |
| dPAP (mmHg) | 1.034 (1.009–1.059) | 0.008 | 0.912 (0.841–0.990) | 0.027 |
| mPCWP (mmHg) | 1.024 (1.001–1.047) | 0.041 | 1.043 (0.931–1.170) | 0.467 |
| DPG (mmHg) | 1.015 (0.973–1.059) | 0.480 | ||
| PVR (WU) | 1.167 (1.077–1.265) | <0.001 | 1.079 (0.867–1.344) | 0.494 |
| PVR > 3 (WU) | 2.414 (1.510–3.859) | <0.001 | 0.492 (0.093–2.615) | 0.406 |
| CO (L/min) | 0.859 (0.697–1.060) | 0.156 | ||
| Procedurale data | ||||
| Transfemoral approach | 0.540 (0.338–0.862) | 0.010 | 0.546 (0.230–1.297) | 0.171 |
| CoreValve | 0.990 (0.578–1.696) | 0.971 | ||
| JenaValve | 1.681 (0.981–2.880) | 0.059 | 0.478 (0.121–1.882) | 0.291 |
| Edwards | 0.702 (0.448–1.099) | 0.121 | ||
| Major vascular complications | 3.351 (1.953–5.749) | <0.001 | 4.194 (1.272–13.829) | 0.019 |
| AUC | p-Value | Cut-Off | Sensitivity | Specificity | Youden Index | |
|---|---|---|---|---|---|---|
| Measurements of RHC-overall cohort | ||||||
| sPAP (mmHg) | 0.620 | 0.002 | 49.50 | 0.69 | 0.53 | 0.22 |
| mPAP (mmHg) | 0.623 | 0.001 | 34.50 | 0.62 | 0.60 | 0.22 |
| dPAP (mmHg) | 0.599 | 0.010 | 15.50 | 0.79 | 0.60 | 0.19 |
| mPCWP (mmHg) | 0.586 | 0.036 | 16.50 | 0.77 | 0.40 | 0.16 |
| DPG (mmHg) | 0.511 | 0.772 | 1.50 | 0.36 | 0.73 | 0.09 |
| PVR (WU) | 0.631 | 0.001 | 3.15 | 0.59 | 0.67 | 0.26 |
| TPG (mmHg) | 0.575 | 0.053 | 12.50 | 0.55 | 0.60 | 0.16 |
| CO (L/min) | 0.431 | 0.082 | 2.45 | 0.99 | 0.05 | 0.03 |
| Measurements of RHC-prec-PH | ||||||
| sPAP (mmHg) | 0.519 | 0.894 | 37.50 | 1.00 | 0.17 | 0.17 |
| mPAP (mmHg) | 0.602 | 0.463 | 30.50 | 0.33 | 0.94 | 0.28 |
| dPAP (mmHg) | 0.611 | 0.424 | 15.50 | 0.83 | 0.50 | 0.33 |
| mPCWP (mmHg) | 0.486 | 0.920 | 14.50 | 0.33 | 0.83 | 0.17 |
| DPG (mmHg) | 0.542 | 0.764 | 8.50 | 0.17 | 1.00 | 0.17 |
| PVR (WU) | 0.711 | 0.157 | 3.30 | 1.00 | 0.67 | 0.67 |
| TPG (mmHg) | 0.551 | 0.714 | 13.50 | 0.83 | 0.33 | 0.17 |
| CO (L/min) | 0.267 | 0.118 | 2.95 | 1.00 | 0.06 | 0.06 |
| Measurements of RHC-ipc-PH | ||||||
| sPAP (mmHg) | 0.562 | 0.366 | 47.50 | 0.87 | 0.32 | 0.19 |
| mPAP (mmHg) | 0.551 | 0.457 | 33.50 | 0.74 | 0.48 | 0.22 |
| dPAP (mmHg) | 0.553 | 0.440 | 27.50 | 0.30 | 0.84 | 0.14 |
| mPCWP (mmHg) | 0.530 | 0.661 | 19.50 | 0.87 | 0.36 | 0.13 |
| DPG (mmHg) | 0.523 | 0.739 | -5.50 | 0.87 | 0.32 | 0.19 |
| PVR (WU) | 0.449 | 0.475 | 2.08 | 0.48 | 0.56 | 0.03 |
| TPG (mmHg) | 0.511 | 0.876 | 7.50 | 0.91 | 0.19 | 0.10 |
| CO (L/min) | 0.395 | 0.141 | 7.40 | 0.10 | 1.00 | 0.09 |
| Measurements of RHC-borderlinepc-PH | ||||||
| sPAP (mmHg) | 0.514 | 0.854 | 81.50 | 0.29 | 0.91 | 0.20 |
| mPAP (mmHg) | 0.513 | 0.854 | 50.50 | 0.29 | 0.89 | 0.18 |
| dPAP (mmHg) | 0.467 | 0.636 | 12.00 | 1.00 | 0.06 | 0.06 |
| mPCWP (mmHg) | 0.490 | 0.889 | 37.50 | 0.14 | 0.96 | 0.10 |
| DPG (mmHg) | 0.511 | 0.872 | 1.50 | 0.32 | 0.78 | 0.10 |
| PVR (WU) | 0.574 | 0.290 | 4.22 | 0.68 | 0.52 | 0.20 |
| TPG (mmHg) | 0.535 | 0.614 | 19.50 | 0.32 | 0.82 | 0.14 |
| CO (L/min) | 0.557 | 0.413 | 3.19 | 0.75 | 0.41 | 0.16 |
| Measurements of RHC-cpc-PH | ||||||
| sPAP (mmHg) | 0.857 | 0.021 | 67.00 | 1.00 | 0.86 | 0.86 |
| mPAP (mmHg) | 0.821 | 0.037 | 43.50 | 1.00 | 0.71 | 0.71 |
| dPAP (mmHg) | 0.643 | 0.355 | 38.50 | 0.50 | 0.86 | 0.36 |
| mPCWP (mmHg) | 0.482 | 0.908 | 16.50 | 1.00 | 0.14 | 0.14 |
| DPG (mmHg) | 0.821 | 0.037 | 9.50 | 0.75 | 0.86 | 0.61 |
| PVR (WU) | 0.729 | 0.156 | 4.74 | 1.00 | 0.50 | 0.50 |
| TPG (mmHg) | 0.898 | 0.013 | 23.50 | 1.00 | 0.86 | 0.86 |
| CO (L/min) | 0.429 | 0.643 | 2.92 | 0.88 | 0.29 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boxhammer, E.; Gharibeh, S.X.; Wernly, B.; Kelm, M.; Franz, M.; Kretzschmar, D.; Hoppe, U.C.; Lauten, A.; Lichtenauer, M. Conundrum of Classifying Subtypes of Pulmonary Hypertension—Introducing a Novel Approach to Classify “Borderline” Patients in a Population with Severe Aortic Stenosis Undergoing TAVI. J. Cardiovasc. Dev. Dis. 2022, 9, 294. https://doi.org/10.3390/jcdd9090294
Boxhammer E, Gharibeh SX, Wernly B, Kelm M, Franz M, Kretzschmar D, Hoppe UC, Lauten A, Lichtenauer M. Conundrum of Classifying Subtypes of Pulmonary Hypertension—Introducing a Novel Approach to Classify “Borderline” Patients in a Population with Severe Aortic Stenosis Undergoing TAVI. Journal of Cardiovascular Development and Disease. 2022; 9(9):294. https://doi.org/10.3390/jcdd9090294
Chicago/Turabian StyleBoxhammer, Elke, Sarah X. Gharibeh, Bernhard Wernly, Malte Kelm, Marcus Franz, Daniel Kretzschmar, Uta C. Hoppe, Alexander Lauten, and Michael Lichtenauer. 2022. "Conundrum of Classifying Subtypes of Pulmonary Hypertension—Introducing a Novel Approach to Classify “Borderline” Patients in a Population with Severe Aortic Stenosis Undergoing TAVI" Journal of Cardiovascular Development and Disease 9, no. 9: 294. https://doi.org/10.3390/jcdd9090294





