At the Crossroads of Minimally Invasive Mitral Valve Surgery—Benching Single Hospital Experience to a National Registry: A Plea for Risk Management Technology
Abstract
:1. Background
2. Methods
3. Results
3.1. Institutional Risk Management Performance
3.2. Anticipating Risks and Learning by Experience
3.2.1. Prevention and Recovery Information System for Monitoring and Analysis
3.2.2. Phases of Care Mortality Analysis
3.2.3. Failure Mode and Effects Analysis and Fault Tree Analysis
3.3. Risk Reduction by Patient Management and New Technologies
3.3.1. Access and Tools
3.3.2. Patient Management
3.3.3. Imaging and Planning
3.3.4. Data Management, Feedback and Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Girdauskas, E.; Pausch, J.; Harmel, E.; Gross, T.; Detter, C.; Sinning, C.; Kubitz, J.; Reichenspurner, H. Minimally invasive mitral valve repair for functional mitral regurgitation. Eur. J. Cardio-Thorac. Surg. 2019, 55, i17–i25. [Google Scholar] [CrossRef]
- Del Forno, B.; De Bonis, M.; Agricola, E.; Melillo, F.; Schiavi, D.; Castiglioni, A.; Montorfano, M.; Alfieri, O. Mitral valve regurgitation: A disease with a wide spectrum of therapeutic options. Nat. Rev. Cardiol. 2020, 17, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Van Wagtendonk, I.; Smits, M.; Merten, H.; Heetveld, M.J.; Wagner, C. Nature, causes and consequences of unintended events in surgical units. Br. J. Surg. 2010, 97, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Zegers, M.; De Bruijne, M.C.; De Keizer, B.; Merten, H.; Groenewegen, P.P.; Van Der Wal, G.; Wagner, C. The incidence, root-causes, and outcomes of adverse events in surgical units: Implication for potential prevention strategies. Patient Saf. Surg. 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lidén, K.; Ivert, T.; Sartipy, U. Death in low-risk cardiac surgery revisited. Open. Heart. 2020, 7, e001244. [Google Scholar] [CrossRef]
- Mejia, O.A.V.; Borgomoni, G.B.; Lima, E.G.; Guerreiro, G.P.; Dallan, L.R.; Silva, P.D.B.E.; Nakazone, M.A.; Junior, O.P.; Gomes, W.J.; de Oliveira, M.A.P.; et al. Most deaths in low-risk cardiac surgery could be avoidable. Sci. Rep. 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Fiocco, A.; Nadali, M.; Gerosa, G. Transapical mitral valve repair procedures: Primetime for microinvasive mitral valve surgery. J. Card. Surg. 2021, 1–9. [Google Scholar] [CrossRef]
- Netherlands Heart Registry Annual Report 2021. Page 116. Available online: https://dcvalliance.nl/news/item/nhr-annual-report-2021-published (accessed on 26 May 2022).
- Shannon, F.L.; Fazzalari, F.L.; Theurer, P.F.; Bell, G.F.; Sutcliffe, K.M.; Prager, R.L.; Michigan Society of Thoracic and Cardiovascular Surgeons. A Method to Evaluate Cardiac Surgery Mortality: Phase of Care Mortality Analysis. Ann. Thorac. Surg. 2012, 93, 36–43. [Google Scholar] [CrossRef]
- Driesen, B.E.; Baartmans, M.; Merten, H.; Otten, R.; Walker, C.; Nanayakkara, P.W.; Wagner, C. Root Cause Analysis Using the Prevention and Recovery Information System for Monitoring and Analysis Method in Healthcare Facilities: A Systematic Literature Review. J. Patient Saf. 2021, 18, 342–350. [Google Scholar] [CrossRef]
- Van Galen, L.S.; Struik, P.W.; Driesen, B.E.J.M.; Merten, H.; Ludikhuize, J.; Van Der Spoel, J.I.; Kramer, M.H.H.; Nanayakkara, P.W.B. Delayed Recognition of Deterioration of Patients in General Wards Is Mostly Caused by Human Related Monitoring Failures: A Root Cause Analysis of Unplanned ICU Admissions. PLoS ONE 2016, 11, e0161393. [Google Scholar] [CrossRef] [PubMed]
- Wubben, I.; van Manen, J.G.; Akker, B.J.V.D.; Vaartjes, S.R.; van Harten, W.H. Equipment-related incidents in the operating room: An analysis of occurrence, underlying causes and consequences for the clinical process. BMJ Qual. Saf. 2010, 19, e64. [Google Scholar] [CrossRef] [PubMed]
- Shannon, F.L.; Whitman, G.J.R.; Lobdell, K.W. On behalf of the STS Task-force on Quality Initiatives STS-Webinar Phase of Care MortalityAnalysis (POCMA). 2019. Available online: https://www.sts.org/sites/default/files/2019.12.11%20STS%20TQI%20Webinar_POCMA.pdf (accessed on 9 August 2022).
- Crawford, T.C.; Magruder, J.T.; Grimm, J.C.; Mandal, K.; Price, J.; Resar, J.; Chacko, M.; Hasan, R.K.; Whitman, G.; Conte, J.V. Phase of Care Mortality Analysis: A Unique Method for Comparing Mortality Differences Among Transcatheter Aortic Valve Replacement and Surgical Aortic Valve Replacement Patients. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Umana-Pizano, J.B.; Nissen, A.P.; Sandhu, H.K.; Miller, C.C.; Loghin, A.; Safi, H.J.; Eisenberg, S.B.; Estrera, A.L.; Nguyen, T.C. Acute Type A Dissection Repair by High-Volume Vs Low-Volume Surgeons at a High-Volume Aortic Center. Ann. Thorac. Surg. 2019, 108, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Umana-Pizano, J.B.; Nissen, A.P.; Nguyen, S.; Hoffmann, C.; Guercio, A.; De La Guardia, G.; Estrera, A.L.; Nguyen, T.C. Phase of Care Mortality Analysis According to Individual Patient Risk Profile. Ann. Thorac. Surg. 2019, 108, 531–535. [Google Scholar] [CrossRef]
- Smenes, B.T.; Pettersen, Ø.; Karlsen, Ø.; Stenseth, R.; Wahba, A. Phase of care mortality analysis and failure to rescue in a Norwegian cardiothoracic unit. Scand. Cardiovasc. J. 2019, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lago, P.; Bizzarri, G.; Scalzotto, F.; Parpaiola, A.; Amigoni, A.; Putoto, G.; Perilongo, G. Use of FMEA analysis to reduce risk of errors in prescribing and administering drugs in paediatric wards: A quality improvement report. BMJ Open 2012, 2, e001249. [Google Scholar] [CrossRef]
- Ryan, J.W.; Murray, A.S.; Gilligan, P.J.; Bisset, J.M.; Nolan, C.; Doyle, A.; Emerson, B.; Galvin, J.M.; Murray, J.G. MRI safety management in patients with cardiac implantable electronic devices: Utilizing failure mode and effects analysis for risk optimization. Int. J. Qual. Health Care 2020, 32, 431–437. [Google Scholar] [CrossRef]
- Pirouzi, M.; Gorji, H.A.; Ravaghi, H.; Afshari, A. Health Care Failure Mode and Effect Analysis in the Operating Room Setting. Qual. Manag. Health Care 2020, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- McElroy, L.M.; Khorzad, R.; Nannicelli, A.P.; Brown, A.R.; Ladner, D.P.; Holl, J.L. Failure mode and effects analysis: A comparison of two common risk prioritisation methods. BMJ Qual. Saf. 2015, 25, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ruijters, E.; Stoelinga, M. Fault tree analysis: A survey of the state-of-the-art in modeling, analysis and tools. Comput. Sci. Rev. 2015, 15–16, 29–62. [Google Scholar] [CrossRef]
- Westaby, S.; De Silva, R.; Petrou, M.; Bond, S.; Taggart, D. Surgeon-specific mortality data disguise wider failings in delivery of safe surgical services. Eur. J. Cardio-Thorac. Surg. 2014, 47, 341–345. [Google Scholar] [CrossRef]
- Yarmohammadian, M.H.; Rezaei, F.; Haghshenas, A.; Fallah, A.; Ferdosi, M. Revised risk priority number in failure mode and effects analysis model from the perspective of healthcare system. Int. J. Prev. Med. 2018, 9, 7. [Google Scholar] [CrossRef]
- Tang, B.; Cuschieri, A. Objective assessment of surgical operative performance by observational clinical human reliability analysis (OCHRA): A systematic review. Surg. Endosc. 2020, 34, 1492–1508. [Google Scholar] [CrossRef]
- Chitwood, W.R., Jr. Robotic mitral valve surgery: Overview, methodology, results, and perspective. Ann. Cardiothorac. Surg. 2016, 5, 544–555. [Google Scholar] [CrossRef]
- Bonatti, J.; Kiaii, B.; Alhan, C.; Cerny, S.; Torregrossa, G.; Bisleri, G.; Komlo, C.; Guy, T.S. The role of robotic technology in minimally invasive surgery for mitral valve disease. Expert Rev. Med Devices 2021, 18, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Chaplin, G.; Chan, J.S.K.; Eriksen, P.; MacCarthy-Ofosu, B.; Theologou, T.; Muir, A.D. The Future of Open Heart Surgery in the Era of Robotic and Minimal Surgical Interventions. Hear. Lung Circ. 2020, 29, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Loberman, D.; Pelletier, M.P.; Yazdchi, F.; Aranki, S.F.; Preisler, Y.; Mohr, R.; Ziv-Baran, T. Myocardial preservation methods in isolated minimal invasive mitral valve surgery: Society of Thoracic Surgeons (STS) database outcomes. J. Card. Surg. 2020, 35, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.A.; Chemtob, R.A.; Lopez, D.C.; Gillinov, A.M.; Wierup, P.; Mick, S.L. Open sternum, cooler heart: The effect of surgical approach on myocardial temperature. JTCVS Tech. 2020, 1, 41–42. [Google Scholar] [CrossRef]
- Momin, A.A.; Toth, A.J.; Gillinov, A.M.; Wierup, P.; Mick, S.L. Exploring ventricular dysfunction and poor venous drainage during robotic mitral valve surgery. J. Card. Surg. 2020, 35, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, G.; Nadali, M.; Longinotti, L.; Ponzoni, M.; Caraffa, R.; Fiocco, A.; Pradegan, N.; Besola, L.; D’Onofrio, A.; Bizzotto, E.; et al. Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes. Ann. Cardiothorac. Surg. 2021, 10, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, K.M.; Stamm, C.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Montagner, M.; Shafti, T.Z.N.; Jacobs, S.; Falk, V.; Kempfert, J. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interv. Cardiol. 2018, 13, 14–19. [Google Scholar] [CrossRef]
- Nia, P.S.; Olsthoorn, J.R.; Heuts, S.; van Kuijk, S.M.J.; Vainer, J.; Streukens, S.; Schalla, S.; Segers, P.; Barenbrug, P.; Crijns, H.J.G.M.; et al. Effect of a dedicated mitral heart team compared to a general heart team on survival: A retrospective, comparative, non-randomized interventional cohort study based on prospectively registered data. Eur. J. Cardio-Thorac. Surg. 2021, 60, 263–273. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kempfert, J.; Jacobs, S.; Stamm, C.; Akansel, S.; Kofler, M.; Sündermann, S.H.; Shafti, T.Z.N.; Jakobs, K.; Holzendorf, S.; et al. Mitral valve surgery: Current status and future prospects of the minimally invasive approach. Expert Rev. Med Devices 2021, 18, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, K.M.; Van Kampen, A.; Kofler, M.; Richter, G.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Kurz, S.D.; Jacobs, S.; Falk, V.; et al. Minimally invasive surgical aortic valve replacement: The RALT approach. J. Card. Surg. 2020, 35, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, K.M.; Kofler, M.; Shafti, T.Z.N.; El Al, A.A.; van Kampen, A.; Amabile, A.; Torregrossa, G.; Kempfert, J.; Falk, V.; Balkhy, H.H.; et al. Minimally Invasive Coronary Revascularisation Surgery: A Focused Review of the Available Literature. Interv. Cardiol. 2021, 16, e08. [Google Scholar] [CrossRef]
- Ando, M.; Funamoto, M.; Cameron, D.E.; Sundt, T.M. Concomitant surgical closure of left atrial appendage: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2018, 156, 1071–1080.e2. [Google Scholar] [CrossRef]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Jacobs, S.; Falk, V.; Unbehaun, A.; Kempfert, J. The MANTA Vascular Closure Device for Percutaneous Femoral Vessel Cannulation in Minimally Invasive Surgical Mitral Valve Repair. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2020, 15, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, A.; Herregods, M.C.; Verbrugghe, P.; Lamberigts, M.; Vanassche, T.; Meyns, B.; Oosterlinck, W.; Rega, F.; Adriaenssens, T.; Van Hoof, L.; et al. Antithrombotic Treatment After Surgical and Transcatheter Heart Valve Repair and Replacement. Front. Cardiovasc. Med. 2021, 8, 702780. [Google Scholar] [CrossRef] [PubMed]
- Van der Wall, S.J.; Olsthoorn, J.R.; Heuts, S.; Klautz, R.J.M.; Tomsic, A.; Jansen, E.K.; Vonk, A.B.A.; Nia, P.S.; Klok, F.A.; Huisman, M.V. Antithrombotic therapy after mitral valve repair: VKA or aspirin? J. Thromb. Thrombolysis 2018, 46, 473–481. [Google Scholar] [CrossRef]
- Noohi, F.; Sadeghipour, P.; Kordrostami, S.; Shafe, O.; Maleki, M.; Kyavar, M.; Bakhshandeh, H.; Rezaei, Y.; Rokni, M.; Moosavi, J.; et al. Rivaroxaban in patients undergoing surgical mitral valve repair. J. Thromb. Thrombolysis 2020, 49, 475–479. [Google Scholar] [CrossRef]
- Yu, S.; Valencia, M.B.; Roques, V.; Aljure, O.D. Regional analgesia for minimally invasive cardiac surgery. J. Card. Surg. 2019, 34, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.C.; Shannon, F.L.; Bolling, S.F.; Romano, M.A.; Sakwa, M.P.; Trescot, A.; Shi, L.; Johnson, R.L.; Starnes, V.A.; Grehan, J.F. Intercostal Cryo Nerve Block in Minimally Invasive Cardiac Surgery: The Prospective Randomized FROST Trial. Pain Ther. 2021, 10, 1579–1592. [Google Scholar] [CrossRef]
- Pieri, M.; De Simone, A.; Rose, S.; De Domenico, P.; Lembo, R.; Denaro, G.; Landoni, G.; Monaco, F. Trials Focusing on Prevention and Treatment of Delirium After Cardiac Surgery: A systematic Review of Randomized Evidence. J. Cardiothorac. Vasc. Anesthesia 2020, 34, 1641–1654. [Google Scholar] [CrossRef]
- Chen, H.; Mo, L.; Hu, H.; Ou, Y.; Luo, J. Risk factors of postoperative delirium after cardiac surgery: A meta-analysis. J. Cardiothorac. Surg. 2021, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Numan, T.; Boogaard, M.V.D.; Kamper, A.; Rood, P.; Peelen, L.; Slooter, A.; Abawi, M.; Claassen, J.A.; Coesmans, M.; Dautzenberg, P.; et al. Delirium detection using relative delta power based on 1-minute single-channel EEG: A multicentre study. Br. J. Anaesth. 2019, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2021, 19, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Dalal, H.M.; Doherty, P.; McDonagh, S.T.; Paul, K.; Taylor, R.S. Virtual and in-person cardiac rehabilitation. BMJ 2021, 373, n1270. [Google Scholar] [CrossRef]
- Melillo, F.; Boccellino, A.; Ingallina, G.; Ancona, F.; Capogrosso, C.; Napolano, A.; Stella, S.; Agricola, E. Multimodality imaging for preprocedural planning of percutaneous mitral valve repair: A comprehensive review. Mini-Invasive Surg. 2020, 4, 81. [Google Scholar] [CrossRef]
- Nia, P.S.; Heuts, S.; Daemen, J.; Luyten, P.; Vainer, J.; Hoorntje, J.; Cheriex, E.; Maessen, J. Preoperative planning with three-dimensional reconstruction of patient’s anatomy, rapid prototyping and simulation for endoscopic mitral valve repair. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 163–168. [Google Scholar] [CrossRef]
- Daemen, J.H.T.; Heuts, S.; Olsthoorn, J.R.; Maessen, J.G.; Nia, P.S. Mitral valve modelling and three-dimensional printing for planning and simulation of mitral valve repair. Eur. J. Cardio-Thorac. Surg. 2019, 55, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Ooms, J.F.; Ceusters, J.; Hirsch, A.; Budde, R.P.; Van Mieghem, N.M. Accuracy of three-dimensional computational modeling in prediction of the dynamic neo left ventricular outflow tract with transcatheter mitral valve replacement. Int. J. Cardiol. 2021, 336, 93–96. [Google Scholar] [CrossRef]
- Ooms, J.F.; Wang, D.D.; Rajani, R.; Redwood, S.; Little, S.H.; Chuang, M.L.; Popma, J.J.; Dahle, G.; Pfeiffer, M.; Kanda, B.; et al. Computed Tomography–Derived 3D Modeling to Guide Sizing and Planning of Transcatheter Mitral Valve Interventions. JACC: Cardiovasc. Imaging 2021, 14, 1644–1658. [Google Scholar] [CrossRef]
- Sacks, M.S.; Drach, A.; Lee, C.-H.; Khalighi, A.H.; Rego, B.V.; Zhang, W.; Ayoub, S.; Yoganathan, A.P.; Gorman, R.C.; Gorman, J.H., III. On the Simulation of Mitral Valve Function in Health, Disease, and Treatment. J. Biomech. Eng. 2019, 141, 070804. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Geirsson, A.; Bonde, P.N. Mathematical Blueprint of a Mitral Valve. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.; Choi, A.; McPherson, D.D.; Kim, H. Personalized Computational Modeling of Mitral Valve Prolapse: Virtual Leaflet Resection. PLoS ONE 2015, 10, e0130906. [Google Scholar] [CrossRef] [PubMed]
- Ginty, O.K.; Moore, J.T.; Eskandari, M.; Carnahan, P.; Lasso, A.; Jolley, M.A.; Monaghan, M.; Peters, T.M. Dynamic, patient-specific mitral valve modelling for planning transcatheter repairs. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Makhdom, F.; Hage, A.; Manian, U.; Ginty, O.; Losenno, K.L.; Kiaii, B.; Chu, M.W. Echocardiographic Method to Determine the Length of Neochordae Reconstruction for Mitral Repair. Ann. Thorac. Surg. 2021, 111, 519–528. [Google Scholar] [CrossRef]
- Khalighi, A.H.; Rego, B.V.; Drach, A.; Gorman, R.C.; Gorman, J.H.; Sacks, M.S. Development of a Functionally Equivalent Model of the Mitral Valve Chordae Tendineae Through Topology Optimization. Ann. Biomed. Eng. 2019, 47, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, A.C. The vagaries of patient selection in cardiovascular surgery. J. Thorac. Cardiovasc. Surg. 2016, 152, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Elfanagely, O.; Toyoda, Y.; Othman, S.; Mellia, J.A.; Basta, M.; Liu, T.; Kording, K.; Ungar, L.; Fischer, J.P. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J. Surg. Res. 2021, 264, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Tat, E.; Bhatt, D.L.; Rabbat, M.G. Addressing bias: Artificial intelligence in cardiovascular medicine. Lancet Digit. Health 2020, 2, e635–e636. [Google Scholar] [CrossRef]
- Lancet, T. Public reporting of surgical outcomes. Lancet 2011, 377, 1126. [Google Scholar] [CrossRef]
- Treasure, T.; King, A.; Lemp, L.H.; Golesworthy, T.; Pepper, J.; Takkenberg, J.J. Developing a shared decision support framework for aortic root surgery in Marfan syndrome. Heart 2018, 104, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Doll, J.A.; Jones, W.S.; Lokhnygina, Y.; Culpepper, S.; Parks, R.L.; Calhoun, C.; Au, D.H.; Patel, M.R. PREPARED Study: A Study of Shared Decision-Making for Coronary Artery Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005244. [Google Scholar] [CrossRef]
- Coylewright, M.; O’Neill, E.; Sherman, A.; Gerling, M.; Adam, K.; Xu, K.; Grande, S.W.; Dauerman, H.L.; Dodge, S.E.; Sobti, N.K.; et al. The Learning Curve for Shared Decision-making in Symptomatic Aortic Stenosis. JAMA Cardiol. 2020, 5, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Perpetua, E. Incorporating the Patient Voice into Shared Decision-Making for the Treatment of Aortic Stenosis. JAMA Cardiol. 2020, 5, 380. [Google Scholar] [CrossRef]
- Coylewright, M.; Forrest, J.K.; McCabe, J.M.; Nazif, T.M. TAVR in Low-Risk Patients. J. Am. Coll. Cardiol. 2020, 75, 1208–1211. [Google Scholar] [CrossRef]
- Percy, E.; Hirji, S.; Yazdchi, F.; McGurk, S.; Kiehm, S.; Cook, R.; Kaneko, T.; Shekar, P.; Pelletier, M.P. Long-Term Outcomes of Right Minithoracotomy Versus Hemisternotomy for Mitral Valve Repair. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2020, 15, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Olsthoorn, J.R.; Heuts, S.; Houterman, S.; Maessen, J.G.; Nia, P.S.; Bramer, S.; van Boven, W.J.P.; Vonk, A.B.A.; Koene, B.M.J.A.; Bekkers, J.A.; et al. Effect of minimally invasive mitral valve surgery compared to sternotomy on short- and long-term outcomes: A retrospective multicentre interventional cohort study based on Netherlands Heart Registration. Eur. J. Cardio-Thorac. Surg. 2021, 61, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Van Veghel, D.; Marteijn, M.; De Mol, B.; Measurably Better Study Group (The Netherlands) and Advisory Board. First results of a national initiative to enable quality improvement of cardiovascular care by transparently reporting on patient-relevant outcomes. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; De Kroon, T.L.; Post, M.C.; Kelder, J.C.; Schut, K.F.; Saouti, N.; Van Putte, B.P. Minimally invasive mitral valve surgery: A systematic safety analysis. Open Hear. 2020, 7, e001393. [Google Scholar] [CrossRef] [PubMed]
- Nia, P.S.; Daemen, J.H.; Maessen, J.G. Development of a high-fidelity minimally invasive mitral valve surgery simulator. J. Thorac. Cardiovasc. Surg. 2019, 157, 1567–1574. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Holfeld, J.; Pölzl, G.; Metzler, B.; Hintringer, F.; Adukauskaite, A.; Stijnen, M.; van Tuijl, S.; Müller, L.; Grimm, M.; et al. Beating heart porcine high-fidelity simulator for the training of edge-to-edge mitral valve repair. Multimedia Man. Cardio-Thorac. Surg. 2018. [Google Scholar] [CrossRef]
- Leopaldi, A.M.; Wrobel, K.; Speziali, G.; van Tuijl, S.; Drasutiene, A.; Chitwood, W.R. The dynamic cardiac biosimulator: A method for training physicians in beating-heart mitral valve repair procedures. J. Thorac. Cardiovasc. Surg. 2018, 155, 147–155. [Google Scholar] [CrossRef]
- Jung, J.J.; Kashfi, A.; Sharma, S.; Grantcharov, T. Characterization of device-related interruptions in minimally invasive surgery: Need for intraoperative data and effective mitigation strategies. Surg. Endosc. 2019, 33, 717–723. [Google Scholar] [CrossRef] [PubMed]

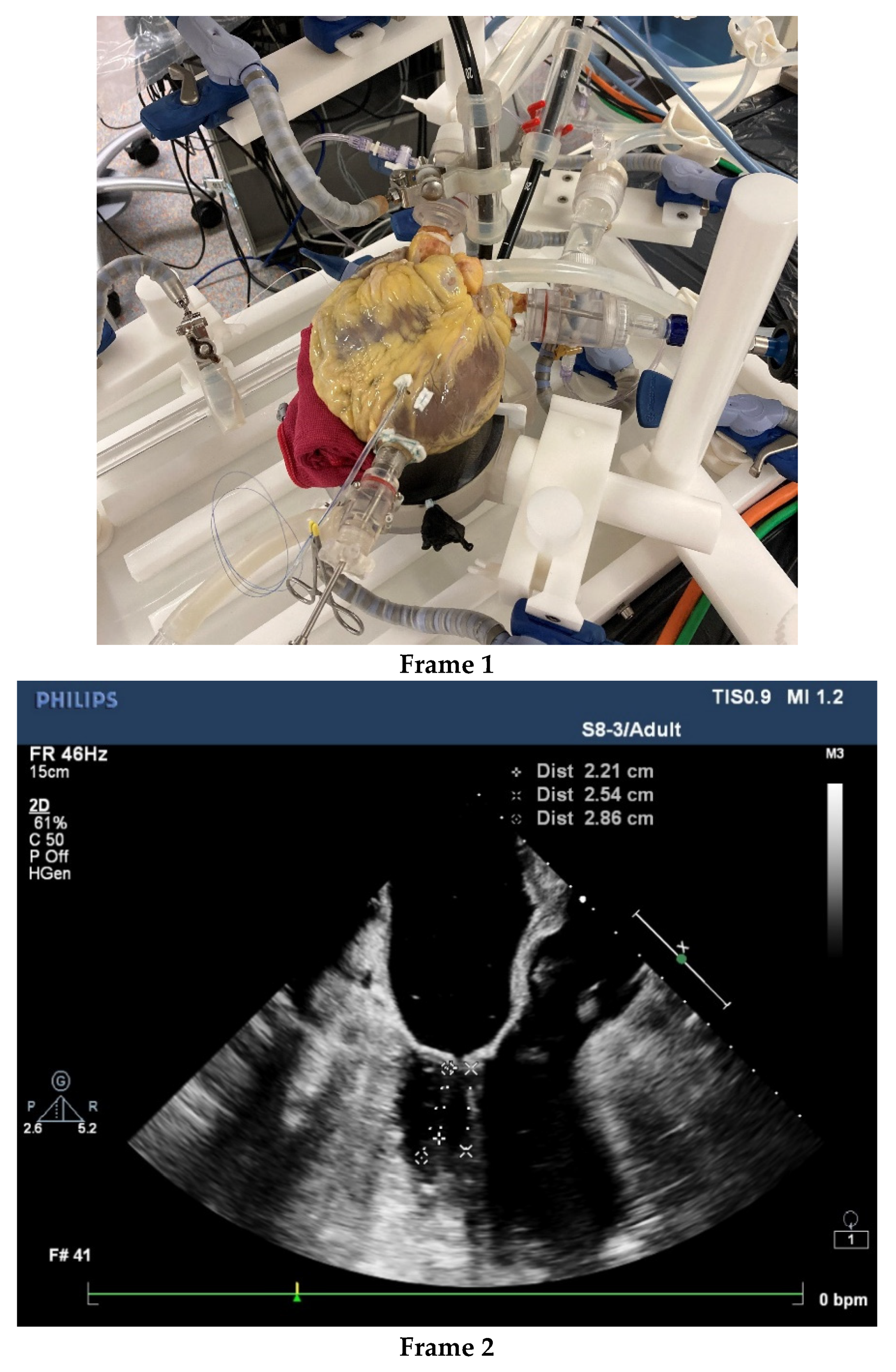
| 2016–2020 n = 372 | |
|---|---|
| 120-day mortality | 1.1% |
| 1-year mortality | 1.9% |
| Stroke | 0.3% |
| Postoperative mitral insufficiency (mild or severe) | 1.4% |
| EuroSCORE II (mean) | 3.56% |
| Rethoracotomy | 2.2% |
| Previous cardiac surgery | 10.8% |
| Age > 75 years | 20.4% |
| Description | ES II | Preoperative | Perioperative | Postoperative | Discharge | Avoidable |
|---|---|---|---|---|---|---|
| Postoperative bleeding | 1.84% | * d0 | Y | |||
| Fragile patient with high-risk profile | 6.9% | * | * d6 | Y | ||
| Epicardial vein injury | 0.89% | * d0 | Y | |||
| Fragile 86 year-old patient under Warfarin | 1.40% | * | * d2 | N | ||
| Fragile patient with Lupus, previous right lobectomy and under Warfarin, LMWH and Aspirin | 1.96% | * | * d22 | Y | ||
| Spontaneous Pectoral muscular bleeding under Warfarin, LMWH and Aspirin | 11.25% | * d5 | ? | |||
| Pectoral muscular bleeding | 2.41% | * d2 | ? | |||
| Diagnostic error Minimally invasive mitral surgery undiagnosed ventricular rupture | 42.68% | * | * d0 | Y |
| Description | Age | ES II | Preoperative | Perioperative | Postoperative | Discharge | Avoidable |
|---|---|---|---|---|---|---|---|
| Very high-risk case-bleeding and mitral annular rupture | 66 | 12.47% | * | † d0 | N | ||
| High-risk, delirium, MOF | 77 | 5.08% | * | † d112 | N | ||
| Heart failure, low preoperative EF | 71 | 17.76% | * | † d21 | N | ||
| OHCA 1 day after discharge | 69 | 6.41% | * | † d9 | Y/N | ||
| Surgery complicated by iatrogenic catheter perforation, MOF | 79 | 5.89% | * | † d37 | Y | ||
| Very high-risk re-redo | 80 | 81.32% | * | † d20 | N | ||
| MOF Papillary muscle rupture with preoperative undiagnosed LV wall perforation | 78 | 42.68% | * | * | † d42 | Y |
| Prevention of Adverse Surgical Events—Downsides |
|---|
| Preoperative adequate CT scan assessment—no evidence of cost-effectiveness Vascular closure device femoral—no evidence of cost-effectiveness Transapical Micro-invasiveness—limited indications Structural mini-invasiveness program—scale/specialists vs. generalists Specialized multidisciplinary heart teams—ineffective presence/time-consuming Atrial appendage closure—costs and selective indication Patient-tailored pre- and postop. anticoagulant therapy—uncertainty and difficult protocol Intercostal Block for pain relief—expensive/nerve injury Delirium detection—possible overtreatment and no evidence of cost-effectiveness Computer-assisted modeling and imaging—expensive/time-consuming Patient-tailored rehabilitation—time-consuming/no evidence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchieri, R.; van de Wetering, B.; van Tuijl, S.; Mousavi, I.; Riezebos, R.; de Mol, B. At the Crossroads of Minimally Invasive Mitral Valve Surgery—Benching Single Hospital Experience to a National Registry: A Plea for Risk Management Technology. J. Cardiovasc. Dev. Dis. 2022, 9, 261. https://doi.org/10.3390/jcdd9080261
Cocchieri R, van de Wetering B, van Tuijl S, Mousavi I, Riezebos R, de Mol B. At the Crossroads of Minimally Invasive Mitral Valve Surgery—Benching Single Hospital Experience to a National Registry: A Plea for Risk Management Technology. Journal of Cardiovascular Development and Disease. 2022; 9(8):261. https://doi.org/10.3390/jcdd9080261
Chicago/Turabian StyleCocchieri, Riccardo, Bertus van de Wetering, Sjoerd van Tuijl, Iman Mousavi, Robert Riezebos, and Bastian de Mol. 2022. "At the Crossroads of Minimally Invasive Mitral Valve Surgery—Benching Single Hospital Experience to a National Registry: A Plea for Risk Management Technology" Journal of Cardiovascular Development and Disease 9, no. 8: 261. https://doi.org/10.3390/jcdd9080261






