Resuscitated Sudden Cardiac Arrest of a Neonate with Congenital LQT Syndrome-Associated Torsades de Pointes: A Case Report and Literature Review
Abstract
:1. Introduction
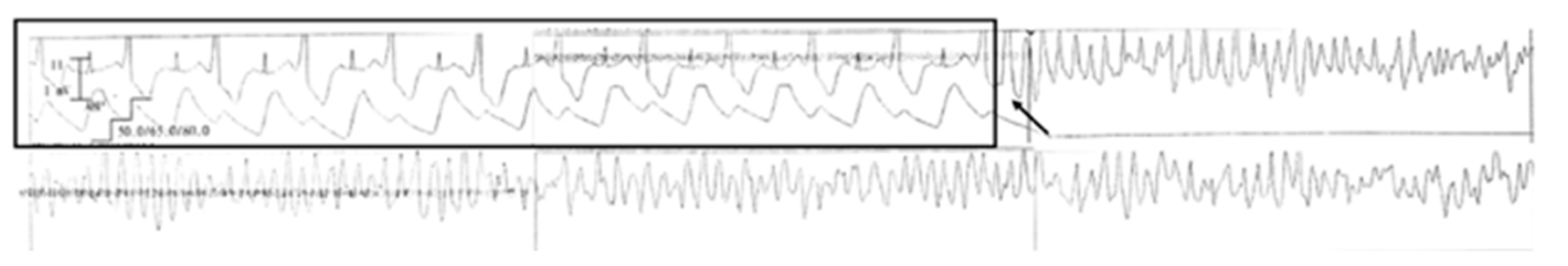
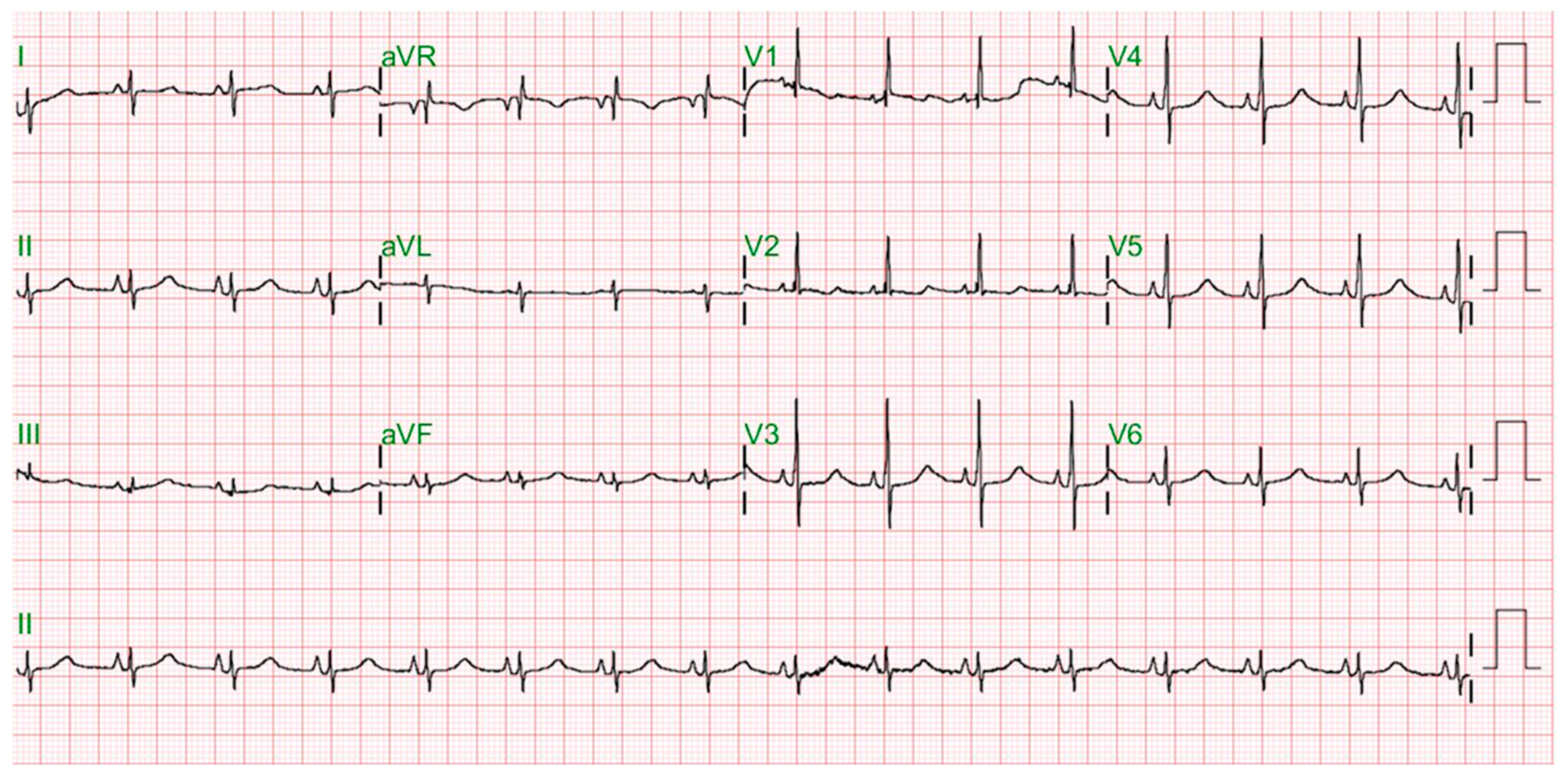
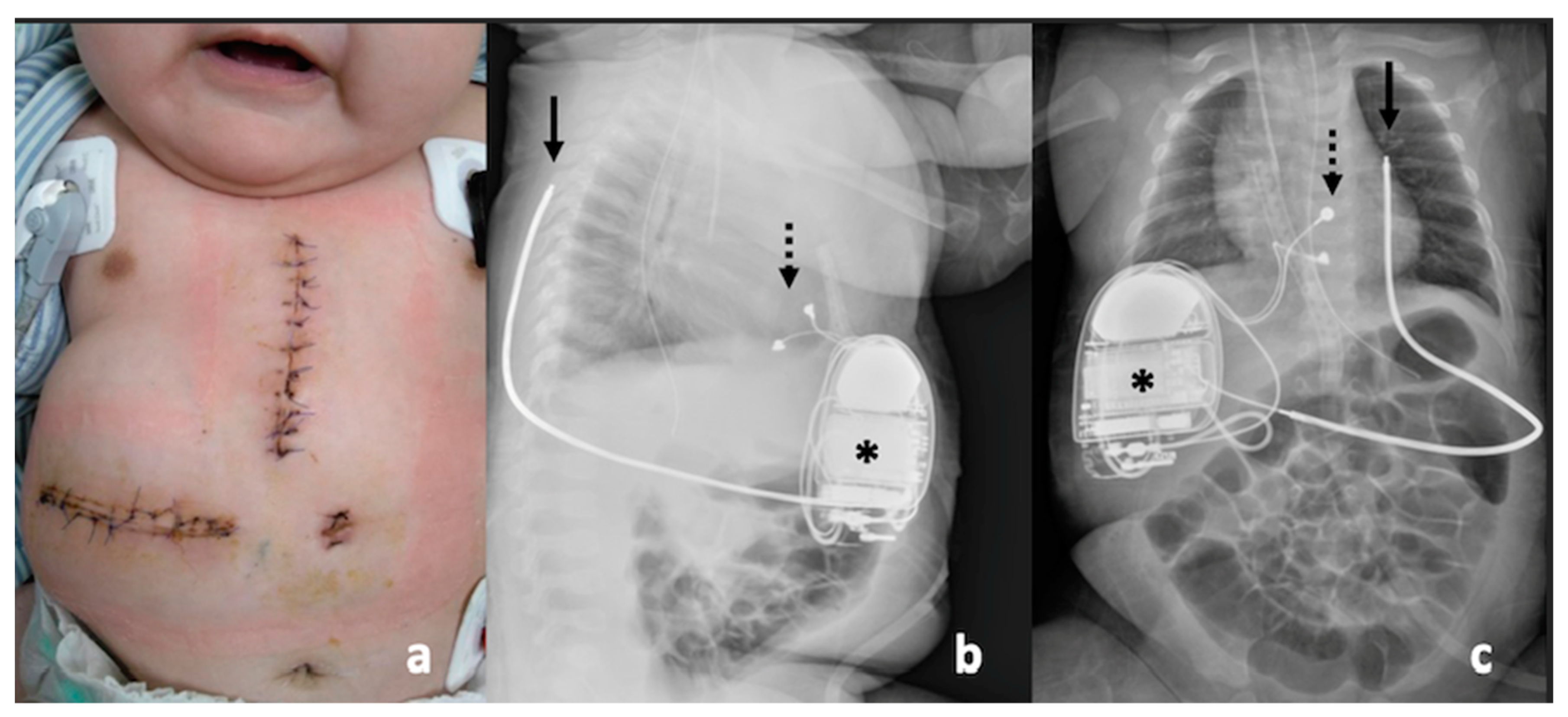
2. Case Report
3. Discussion
3.1. Linkage of SIDS and LQTS
3.2. Presentation of LQTS in Infancy
3.3. High-Risk Factors for Sudden Cardiac Death Due to LQTS in Infancy
3.4. Medical Treatment of LQTS in Infancy
3.5. ICD Implantation in Infancy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tester, D.J.; Ackerman, M.J. Sudden infant death syndrome: How significant are the cardiac channelopathies? Cardiovasc. Res. 2005, 67, 388–396. [Google Scholar] [CrossRef]
- Arnestad, M.; Crotti, L.; Rognum, T.O.; Insolia, R.; Pedrazzini, M.; Ferrandi, C.; Vege, A.; Wang, D.W.; Rhodes, T.E.; George, A.L., Jr.; et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation 2007, 115, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Hofbeck, M.; Ulmer, H.; Beinder, E.; Sieber, E.; Singer, H. Prenatal findings in patients with prolonged QT interval in the neonatal period. Heart 1997, 77, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Garson, A., Jr.; Dick, M., 2nd; Fournier, A.; Gillette, P.C.; Hamilton, R.; Kugler, J.D.; van Hare, G.F., 3rd; Vetter, V.; Vick, G.W., 3rd. The long QT syndrome in children. An international study of 287 patients. Circulation 1993, 87, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Klitzner, T.S. Sudden cardiac death in children. Circulation 1990, 82, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Wilde, A.A.M.; Amin, A.S.; Postema, P.G. Diagnosis, management, and therapeutic strategies for congenital long QT syndrome. Heart. 2022, 108, 332–338. [Google Scholar] [CrossRef]
- Moore, J.P.; Gallotti, R.G.; Shannon, K.M.; Bos, J.M.; Sadeghi, E.; Strasburger, J.F.; Wakai, R.T.; Horigome, H.; Clur, S.A.; Hill, A.C.; et al. Genotype Predicts Outcomes in Fetuses and Neonates with Severe Congenital Long QT Syndrome. JACC Clin. Electrophysiol. 2020, 6, 1561–1570. [Google Scholar] [CrossRef]
- Suzuki, S.; Motohashi, S.; Matsumoto, M. Surgical techniques for implanting implantable cardioverter defibrillators in children and infants. Surg. Today 2014, 44, 1801–1806. [Google Scholar] [CrossRef]
- Tester, D.J.; Wong, L.; Chanana, P.; Jaye, A.; Evans, J.M.; FitzPatrick, D.R.; Evans, M.J.; Fleming, P.; Jeffrey, I.; Cohen, M.C.; et al. Cardiac Genetic Predisposition in Sudden Infant Death Syndrome. J. Am. Coll. Cardiol. 2018, 71, 1217–1227. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Priori, S.G.; Dumaine, R.; Napolitano, C.; Antzelevitch, C.; Stramba-Badiale, M.; Richard, T.A.; Berti, M.R.; Bloise, R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N. Engl. J. Med. 2000, 343, 262–267. [Google Scholar] [CrossRef]
- Beinder, E.; Grancay, T.; Menéndez, T.; Singer, H.; Hofbeck, M. Fetal sinus bradycardia and the long QT syndrome. Am. J. Obstet. Gynecol. 2001, 185, 743–747. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Cuneo, B.F.; Etheridge, S.P.; Horigome, H.; Weng, H.Y.; Benson, D.W. Fetal heart rate predictors of long QT syndrome. Circulation 2012, 126, 2688–2695. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Etheridge, S.P.; Horigome, H.; Sallee, D.; Moon-Grady, A.; Weng, H.Y.; Ackerman, M.J.; Benson, D.W. Arrhythmia phenotype during fetal life suggests long-QT syndrome genotype: Risk stratification of perinatal long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2013, 6, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Gorgels, A.P.; Al Fadley, F.; Zaman, L.; Kantoch, M.J.; Al Halees, Z. The long QT syndrome with impaired atrioventricular conduction: A malignant variant in infants. J. Cardiovasc. Electrophysiol. 1998, 9, 1225–1232. [Google Scholar] [CrossRef]
- Pellegrino, A.; Ho, S.Y.; Anderson, R.H.; Hegerty, A.; Godman, M.J.; Michaelsson, M. Prolonged QT interval and the cardiac conduction tissues. Am. J. Cardiol. 1986, 58, 1112–1113. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Ushinohama, H.; Sato, S.; Tauchi, N.; Horigome, H.; Takahashi, H.; Sumitomo, N.; Kucho, Y.; Shiraishi, H.; Nomura, Y.; et al. Electrocardiographic screening of 1-month-old infants for identifying prolonged QT intervals. Circ. Arrhythm. Electrophysiol. 2013, 6, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Chih, W.L.; Lussier, E.C.; Chen, J.A.; Yeh, S.J.; Lin, S.M.; Chang, T.Y.; Chen, M.R. Physiological QT Interval Changes in Early Infancy Using Post-Menstrual and Post-Natal Age Calculation for Electrocardiogram Long QT Interval Screening in Taiwan. Acta Cardiol. Sin. 2022, 38, 73–83. [Google Scholar]
- Moss, A.J.; Schwartz, P.J.; Crampton, R.S.; Tzivoni, D.; Locati, E.H.; MacCluer, J.; Hall, W.J.; Weitkamp, L.; Vincent, G.M.; Garson, A., Jr. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991, 84, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Giudicessi, J.R.; Ackerman, M.J. Genotype- and phenotype-guided management of congenital long QT syndrome. Curr. Probl. Cardiol. 2013, 38, 417–455. [Google Scholar] [CrossRef] [Green Version]
- Wedekind, H.; Burde, D.; Zumhagen, S.; Debus, V.; Burkhardtsmaier, G.; Mönnig, G.; Breithardt, G.; Schulze-Bahr, E. QT interval prolongation and risk for cardiac events in genotyped LQTS-index children. Eur. J. Pediatr. 2009, 168, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Balbuena, D.; Deschênes, I. Long QT syndrome-Bench to bedside. Heart Rhythm O2 2021, 2, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Priori, S.G.; Spazzolini, C.; Moss, A.J.; Vincent, G.M.; Napolitano, C.; Denjoy, I.; Guicheney, P.; Breithardt, G.; Keating, M.T.; et al. Genotype-phenotype correlation in the long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation 2001, 103, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, T.; Shimizu, W.; Itoh, H.; Noda, T.; Miyamoto, Y.; Nagaoka, I.; Oka, Y.; Ashihara, T.; Ito, M.; Tsuji, K.; et al. Age- and genotype-specific triggers for life-threatening arrhythmia in the genotyped long QT syndrome. J. Cardiovasc. Electrophysiol. 2008, 19, 794–799. [Google Scholar] [CrossRef]
- Wilde, A.A.; Moss, A.J.; Kaufman, E.S.; Shimizu, W.; Peterson, D.R.; Benhorin, J.; Lopes, C.; Towbin, J.A.; Spazzolini, C.; Crotti, L.; et al. Clinical aspects of type 3 long-QT syndrome: An international multicenter study. Circulation 2016, 134, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Bankston, J.R.; Kass, R.S. Molecular determinants of local anesthetic action of beta-blocking drugs: Implications for therapeutic management of long QT syndrome variant 3. J. Mol. Cell. Cardiol. 2010, 48, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Chockalingam, P.; Crotti, L.; Girardengo, G.; Johnson, J.N.; Harris, K.M.; van der Heijden, J.F.; Hauer, R.N.; Beckmann, B.M.; Spazzolini, C.; Rordorf, R.; et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: Higher recurrence of events under metoprolol. J. Am. Coll. Cardiol. 2012, 60, 2092–2099. [Google Scholar] [CrossRef] [Green Version]
- Abu-Zeitone, A.; Peterson, D.R.; Polonsky, B.; McNitt, S.; Moss, A.J. Efficacy of different beta-blockers in the treatment of long QT syndrome. J. Am. Coll. Cardiol. 2014, 64, 1352–1358. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.J.; Silka, M.J.; Silva, J.; Balaji, S.; Beach, C.M.; Benjamin, M.N.; Berul, C.I.; Cannon, B.; Cecchin, F.; Cohen, M.I.; et al. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Cardiol. Young 2021, 31, 1738–1769. [Google Scholar] [CrossRef]
- Suzuki, S.; Watanabe, H.; Yoshii, S.; Kaga, S.; Honda, Y.; Ishikawa, N.; Matsumoto, M. Alternative technique for implanting an implantable cardioverter defibrillator in infants. Ann. Thorac. Surg. 2008, 86, 1701–1703. [Google Scholar] [CrossRef]
- Berul, C.I. Defibrillator indications and implantation in young children. Heart Rhythm 2008, 5, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Zahedivash, A.; Hanisch, D.; Dubin, A.M.; Trela, A.; Chubb, H.; Motonaga, K.S.; Goodyer, W.R.; Maeda, K.; Reinhartz, O.; Ma, M.; et al. Implantable Cardioverter Defibrillators in Infants and Toddlers: Indications, Placement, Programming, and Outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e010557. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, A.M.; Helvind, M.; Jensen, T.; Andersen, J.H.; Jacobsen, J.R.; Chen, X. Implantable cardioverter defibrillator in a 4-month-old infant with cardiac arrest associated with a vascular heart tumor. Pacing Clin. Electrophysiol. 2001, 24, 1699–1700. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, T.; Ruschewski, W.; Gonzalez y Gonzalez, M.; Walter, K.; Kroll, J.; Kampmann, C.; Heinemann, M.; Schneider, H.; Paul, T. ICD Implantation in infants and small children: The extracardiac technique. Pacing Clin. Electrophysiol. 2006, 29, 1319–1325. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-T.; Lee, P.-C.; Chen, Y.-H.; Yeh, S.-J.; Chen, M.-R.; Hsu, K.-H.; Chang, C.-I.; Lai, W.-T.; Hung, W.-L. Resuscitated Sudden Cardiac Arrest of a Neonate with Congenital LQT Syndrome-Associated Torsades de Pointes: A Case Report and Literature Review. J. Cardiovasc. Dev. Dis. 2022, 9, 184. https://doi.org/10.3390/jcdd9060184
Hsu Y-T, Lee P-C, Chen Y-H, Yeh S-J, Chen M-R, Hsu K-H, Chang C-I, Lai W-T, Hung W-L. Resuscitated Sudden Cardiac Arrest of a Neonate with Congenital LQT Syndrome-Associated Torsades de Pointes: A Case Report and Literature Review. Journal of Cardiovascular Development and Disease. 2022; 9(6):184. https://doi.org/10.3390/jcdd9060184
Chicago/Turabian StyleHsu, Yen-Teng, Pi-Chang Lee, Yu-Hsuan Chen, Shu-Jen Yeh, Ming-Ren Chen, Kung-Hong Hsu, Chung-I Chang, Wei-Ting Lai, and Wei-Li Hung. 2022. "Resuscitated Sudden Cardiac Arrest of a Neonate with Congenital LQT Syndrome-Associated Torsades de Pointes: A Case Report and Literature Review" Journal of Cardiovascular Development and Disease 9, no. 6: 184. https://doi.org/10.3390/jcdd9060184





