Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Training Effects
Abstract
:1. Introduction
2. Spectral Analysis of HRV in Patients with CHF
3. Nuclear Imaging with 123I-Metaiodobenzylguanidine in Patients with CHF
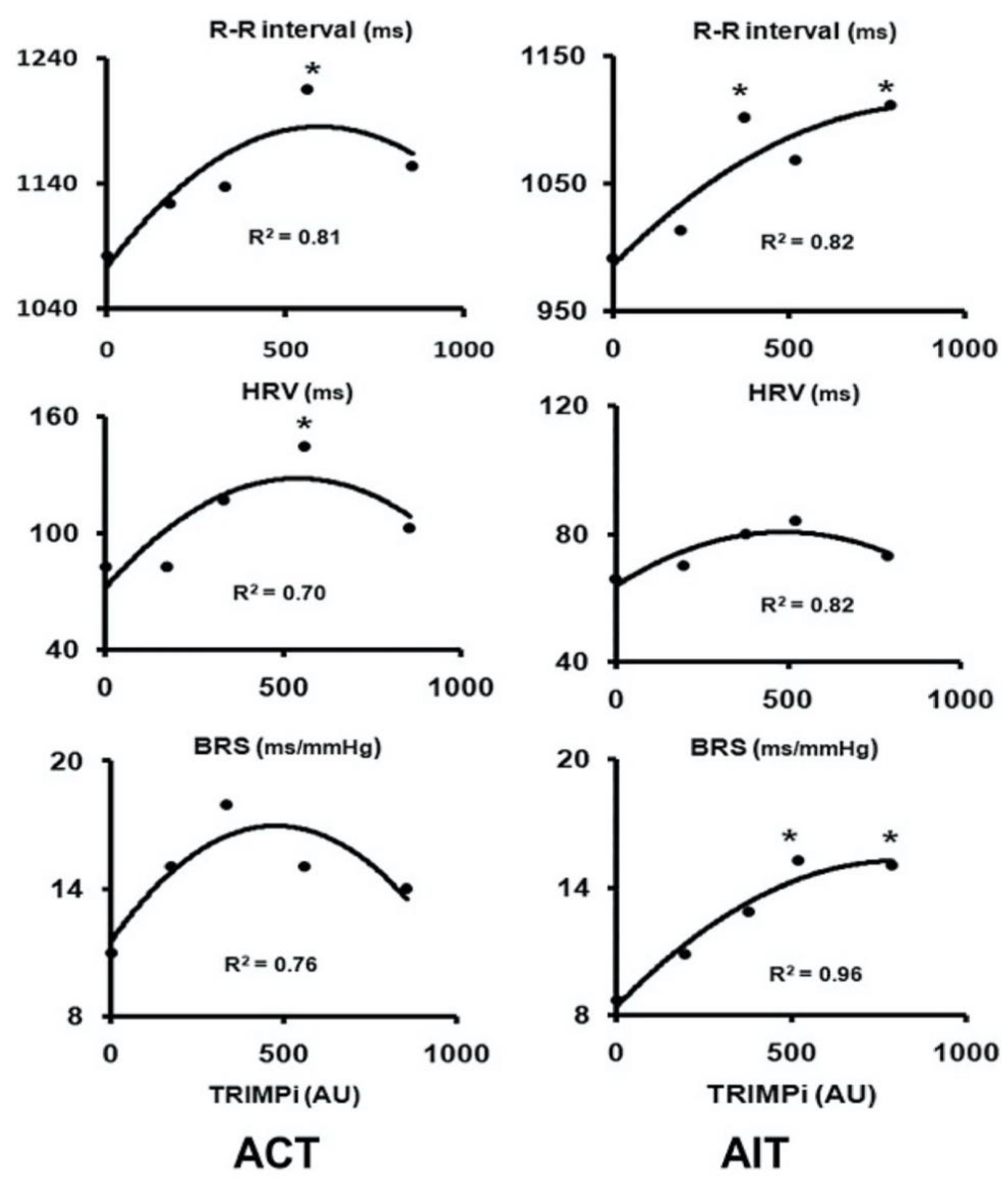
4. Possible Applications and Perspectives of MIBG Nuclear Imaging
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Floras, J.S. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J. Am. Coll. Cardiol. 1993, 22, 72A–84A. [Google Scholar] [CrossRef] [Green Version]
- Cohn, J.N.; Levine, B.T.; Olivari, M.T.; Garberg, V.; Lura, D.; Francis, G.S.; Simon, A.B.; Rector, T. Plasma norepinephrine as a guide to prognosis in patients with chronic heart failure. N. Engl. J. Med. 1984, 311, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M. The neurohumoral hypothesis: A theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 1992, 20, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standard of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, J.; Van Veldhuisen, D.J.; In’T Veld, A.J.M.; Haaksma, J.; Dijk, W.A.; Visser, K.R.; Boomsma, F.; Dunselman, P.H.J.M.; Lie, K.I. Prognostic value of heart rate variability during long-term follow-up in patients with mild to moderate heart failure. J. Am. Coll. Cardiol. 1996, 28, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Anker, S.D.; Chua, T.P.; Szelemej, R.; Piepoli, M.; Adamopoulos, S.; Webb-Peploe, K.; Harrington, D.; Banasiak, W.; Wrabec, K.; et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic cardiomyopathy. Am. J. Cardiol. 1997, 79, 1645–1650. [Google Scholar] [CrossRef]
- Nolan, J.; Batin, P.D.; Andrews, R.; Lindsay, S.J.; Brooksby, P.; Mullen, M.; Baig, W.; Flapan, A.D.; Cowley, A.; Prescott, R.J.; et al. Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom Heart Failure Evaluation Assessment of Risk trial. Circulation 1998, 98, 1510–1516. [Google Scholar] [CrossRef] [Green Version]
- Fauchier, L.; Babuty, D.; Cosnay, P.; Fauchier, J.P. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 1999, 33, 1203–1207. [Google Scholar] [CrossRef] [Green Version]
- Bonaduce, D.; Petretta, M.; Marciano, F.; Vicario, M.L.; Apicella, C.; Rao, M.A.; Nicolai, E.; Volpe, M. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. Am. Heart J. 1999, 138, 273–284. [Google Scholar] [CrossRef]
- Wieland, D.M.; Brown, L.E.; Rogers, W.L.; Worthington, K.C.; Wu, J.L.; Clinthorne, N.H.; Otto, C.A.; Swanson, D.P.; Beierwaltes, W.H. Myocardial imaging with a radioiodinated norepinephrine storage analogue. J. Nucl. Med. 1981, 22, 22–31. [Google Scholar]
- Henderson, E.S.; Kahn, J.K.; Corbett, J.R.; Corbett, J.R.; Jansen, D.E.; Pippin, J.J.; Kulkarni, P.; Ugolini, V.; Akers, M.S.; Hansen, C.; et al. Abnormal I-123 metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988, 78, 1192–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlet, P.; Valette, H.; Dubois-Rande, J.-L.; Moyse, D.; Duboc, D.; Dove, P.; Bourguignon, M.H.; Benvenuti, C.; Duval, A.M.; Agostini, D.; et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J. Nucl. Med. 1992, 33, 471–477. [Google Scholar] [PubMed]
- Cohen-Solal, A.; Esanu, Y.; Logeart, D.; Pessione, F.; Dubois, C.; Dreyfus, G.; Gourgon, R.; Merlet, P. Cardiac metaiodobenzylguanidine uptake in patients with moderate heart failure: Relationship with peak oxygen uptake and prognosis. J. Am. Coll. Cardiol. 1999, 33, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Imamura, Y.; Fukuyama, T.; Mochizuki, T.; Miyagawa, M.; Watanabe, K. Prognostic value of I-123 metaiodobenzylguanidine imaging and cardiac natriuretic peptide levels in patients with left ventricular dysfunction resulting from cardiomyopathy. Jpn. Circ. J. 2001, 65, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Ogita, H.; Shimonagata, T.; Fukunami, M.; Kumagai, K.; Yamada, T.; Asano, Y.; Hirataa, A.; Asaia, M.; Kusuokab, H.; Horic, M.; et al. Prognostic significance of cardiac I-123 metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: A prospective study. Heart 2001, 86, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Somsen, G.A.; Szabo, B.M.; Van Veldhuisen, D.J.; de Milliano, P.A.R.; de Groot, C.A.; Lie, K.I. Comparison between iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability for the assessment of cardiac sympathetic activity in mild to moderate heart failure. Am. Heart J. 1997, 134, 456–458. [Google Scholar] [CrossRef]
- Kurata, C.; Shouda, S.; Mikami, T.; Uehara, A.; Ishikawa, K.; Tawarahara, K.; Nakano, T.; Matoh, F.; Takeuchi, K. Metaiodobenzylguanidine and heart rate variability in heart failure. Jpn. Circ. J. 1998, 62, 770–772. [Google Scholar] [CrossRef] [Green Version]
- Lotze, U.; Kober, A.; Kaepplinger, S.; Neubauer, S.; Gottschild, D.; Figulla, H.R. Cardiac sympathetic activity as measured by myocardial 123-Imetaiodobenzylguanidine uptake and heart rate variability in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 1548–1551. [Google Scholar] [CrossRef]
- Shofer, J.; Spielmann, R.; Schuchert, A.; Weber, K.; Schluter, M. Iodine-123 metaiodobenzylguanidine scintigraphy: A noninvasive method to demonstrate myocardial adrenergic nerve system disintegrity in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 1988, 12, 1252–1258. [Google Scholar] [CrossRef] [Green Version]
- Imamura, Y.; Ando, H.; Mitsuoka, W.; Egashira, S.; Masaki, H.; Ashihara, T.; Fukuyama, T. Iodine-123 metaiodobenzylguanidine images reflect intense myocardial adrenergic nervous activity in congestive heart failure independent of underlying cause. J. Am. Coll. Cardiol. 1995, 26, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Capucci, A. Autonomic nervous system in the genesis of arrhythmias in chronic heart failure: Implication for risk stratification. Minerva Cardioangiol. 2007, 55, 325–333. [Google Scholar] [PubMed]
- Billman, G.E. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1171–H1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortara, A.; La Rovere, M.T.; Pinna, G.D.; Prpa, A.; Maestri, R.; Febo, O.; Pozzoli, M.; Opasich, C.; Tavazzi, L. Arterial baroreflex modulation of heart rate in chronic heart failure: Clinical and hemodynamic correlates and prognostic implications. Circulation 1997, 96, 3450–3458. [Google Scholar] [CrossRef]
- Perrone, M.A.; Volterrani, M.; Manzi, V.; Barchiesi, F.; Iellamo, F. Heart rate variability modifications in response to different types of exercise training in athletes. J Sports Med Phys Fitness 2021, 61, 1411–1415. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef]
- Coats, A.J.; Adamopoulos, S.; Radaelli, A.; McCance, A.; Meyer, T.E.; Bernardi, L.; Solda, P.L.; Davey, P.; Ormerodand, O.; Forfar, C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992, 85, 2119–2131. [Google Scholar] [CrossRef] [Green Version]
- Gademan, M.G.; Swenne, C.A.; Verwey, H.F.; Van Der Laarse, A.; Maan, A.C.; Van De Vooren, H.; Van Pelt, J.; Van Exel, H.J.; Lucas, C.M.H.B.; Cleuren, G.V.J.; et al. Effect of exercise training on autonomic derangement and neurohumoral activation in chronic heart failure. J. Card. Fail. 2007, 13, 294–303. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Corrà, U.; Benzer, W.; Bjarnason-Wehrens, B.; Dendale, P.; Gaita, D.; McGee, H.; Mendes, M.; Niebauer, J.; Zwisler, A.-D.O.; et al. Secondary prevention through cardiac rehabilitation: From knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev Rehabil. 2010, 17, 1–17. [Google Scholar] [CrossRef]
- Piña, I.L.; Apstein, C.S.; Balady, G.J.; Belardinelli, R.; Chaitman, B.R.; Duscha, B.D.; Fletcher, B.J.; Fleg, J.L.; Muers, J.N.; Sullivan, M.J. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003, 107, 1210–1225. [Google Scholar] [CrossRef]
- Iellamo, F.; Manzi, V.; Caminiti, G.; Sposato, B.; Massaro, M.; Cerrito, A.; Rosano, G.; Volterrani, M. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually-tailored exercise training in patients with heart failure. Int. J. Cardiol. 2013, 166, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Legramante, J.M.; Pigozzi, F.; Spataro, A.; Norbiato, G.; Lucini, D.; Pagani, M. Conversion from vagal to sympathetic predominance with strenuous training in high performance world class athletes. Circulation 2002, 105, 2719–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iellamo, F.; Manzi, V.; Caminiti, G.; Vitale, C.; Massaro, M.; Cerrito, A.; Rosano, G.; Volterrani, M. Validation of rate of perceived exertion-based exercise training in patients with heart failure: Insights from autonomic nervous system adaptations. Int. J. Cardiol. 2014, 176, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Manzi, V.; Castagna, C.; Padua, E.; Lombardo, M.; D’Ottavio, S.; Massaro, M.; Volterrani, M.; Iellamo, F. Dose-response relationship of autonomic nervous system responses to individualized training impulse in marathon runners. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1733–H1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iellamo, F.; Volterrani, M.; Di Gianfrancesco, A.; Fossati, C.; Casasco, M. The Effect of Exercise Training on Autonomic Cardiovascular Regulation: From Cardiac Patients to Athletes. Curr. Sports Med. Rep. 2018, 17, 473–479. [Google Scholar] [CrossRef]
- Verschure, D.O.; Lutter, R.; van Eck-Smit, B.L.F.; Somsen, G.A.; Verberne, H.J. Myocardial 123I-mIBG scintigraphy in relation to markers of inflammation and long-term clinical outcome in patients with stable chronic heart failure. J. Nucl. Cardiol. 2018, 25, 845–853. [Google Scholar] [CrossRef]
- Carrió, I.; Cowie, M.R.; Yamazaki, J.; Udelson, J.; Camici, P.G. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc. Imaging 2010, 3, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Carrio, I. Cardiac neurotransmission imaging. J. Nucl. Med. 2001, 42, 1062–1076. [Google Scholar]
- Agostini, D.; Carrio, I.; Verberne, H.J. How to use myocardial 123I-MIBG scintigraphy in chronic heart failure. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 555–559. [Google Scholar] [CrossRef] [Green Version]
- McGhie, A.I.; Corbett, J.R.; Akers, M.S.; Kulkarni, P.; Sills, M.N.; Kremers, M.; Buja, L.M.; Durant-Reville, M.; Parkey, R.W.; Willerson, J.T. Regional cardiac adrenergic function using I-123 meta-iodobenzylguanidine tomographic imaging after acute myocardial infarction. Am. J. Cardiol. 1991, 67, 236–242. [Google Scholar] [CrossRef]
- Nakata, T.; Nagao, K.; Tsuchihashi, K.; Hashimoto, A.; Tanaka, S.; Iimura, O. Regional cardiac sympathetic nerve dysfunction and the diagnostic efficacy of metaiodobenzylguanidine tomography in stable coronary artery disease. Am. J. Cardiol. 1996, 78, 292–297. [Google Scholar] [CrossRef]
- Estorch, M.; Flotats, A.; Serra-Grima, R.; Mari, C.; Prat, T.; Martín, J.C.; Bernà, L.; Catafau, A.M.; Tembl, A.; Carrió, I. Influence of exercise rehabilitation on myocardial perfusion and sympathetic heart innervation in ischemic heart disease. Eur. J. Nucl. Med. 2000, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu-Leen, A.C.; Scholte, A.J.H.A.; Jacobson, A.F. 123I-MIBG SPECT for Evaluation of Patients with Heart Failure. J. Nucl. Med. 2015, 56, 25S–30S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.A.; Dubrey, S.W.; McIntyre, H.F.; Walker, D.M.; Hardman, S.M.; Sutton, G.C.; McDonagh, T.A.; Cowie, M.R. Mode of death in patients with newly diagnosed heart failure in the general population. Eur. J. Heart Fail. 2008, 10, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, T.; Nakata, T.; Hashimoto, A.; Yuda, S.; Tsuchihashi, K.; Travin, M.I.; Shimamoto, K. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac death. J. Nucl. Med. 2001, 42, 1757–1767. [Google Scholar]
- Yamada, T.; Shimonagata, T.; Fukunami, M.; Kumagai, K.; Ogita, H.; Hirata, A.; Asai, M.; Makino, N.; Kioka, H.; Kusuoka, H.; et al. Comparison of the prognostic value of cardiac MIBG imaging and heart rate variability in patients with chronic heart failure. A prospective study. J. Am. Coll. Cardiol. 2003, 41, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Somsen, G.A.; van Vlies, B.; de Milliano, P.A.; Borm, J.J.; van Royen, E.A.; Endert, E.; Lie, K.I. Increased myocardial [123-I] metaiodobenzylguanidine uptake after enalapril treatment in patients with chronic heart failure. Heart 1996, 76, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, S.; Hayashida, K.; Hirose, Y. Use of MIBG myocardial imaging to predict the effectiveness of betablocker therapy in patients with dilated cardiomyopathy. Eur. J. Nucl. Med. 1997, 24, 523–529. [Google Scholar]
- Kasama, S.; Toyama, T.; Kumakura, H.; Takayama, Y.; Ichikawa, S.; Suzuki, T.; Kurabayashi, M. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved LVEF. J. Am. Coll. Cardiol. 2005, 45, 661–667. [Google Scholar] [CrossRef]
- Gerson, M.C.; Craft, L.L.; McGuire, N.; Suresh, D.P.; Abraham, W.T.; Wagoner, L.E. Carvedilol improves left ventricular function in heart failure patients with idiopathic dilated cardiomyopathy and a wide range of sympathetic nervous system function as measured by MIBG. J. Nucl. Cardiol. 2002, 9, 608–615. [Google Scholar] [CrossRef]
- Suwa, M.; Otake, Y.; Moriguchi, A.; Ito, T.; Hirota, Y.; Kawamura, K.; Adachi, I.; Narabayashi, I. Iodine-123 metaiodobenzylguanidine myocardial scintigraphy for prediction of response to beta-blocker therapy in patients with dilated cardiomyopathy. Am. Heart J. 1997, 133, 353–358. [Google Scholar] [CrossRef]
- Hasking, G.J.; Esler, M.D.; Jennings, G.L.; Dewar, E.; Lambert, G. Norepinephrine spillover to plasma during steady-state supine bicycle exercise. Comparison of patients with congestive heart failure and normal subjects. Circulation 1988, 78, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Maehara, K.; Yaoita, H.; Otani, H.; Hirosaka, A.; Saito, T.; Onuki, N.; Komatsu, N.; Ishihata, T.; Maruyama, Y. Correlation between cardiac norepinephrine overflow during exercise and cardiac123I-MIB Guptake in patients with chronic heart failure. J. Nucl. Med. 2003, 44, 1618–1624. [Google Scholar] [PubMed]
- Agostini, D.; Lecluse, E.; Belin, A.; Babatasi, G.; Amar, M.H.; Grollier, G.; Potier, J.C.; Bouvard, G. Impact of exercise rehabilitation on cardiac neuronal function in heart failure: An iodine-123metaiodobenzylguanidine scintigraphy study. Eur. J. Nucl. Med. 1998, 25, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Valborgland, T.; Isaksen, K.; Munk, P.S.; Grabowski, Z.P.; Larsen, A.I. Impact of an exercise training program on cardiac neuronal function in heart failure patients on optimal medical therapy: A randomized Iodine-123 metaiodobenzylguanidine scintigraphy study. J. Nucl. Cardiol. 2018, 25, 1164–1171. [Google Scholar] [CrossRef]
- Tamaki, S.; Yamada, T.; Okuyama, Y.; Morita, T.; Sanada, S.; Tsukamoto, Y.; Masuda, M.; Okuda, K.; Iwasaki, Y.; Yasui, T.; et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: Results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J. Am. Coll. Cardiol. 2009, 53, 426–435. [Google Scholar]
- Bonfiglioli, R.; Nanni, C.; Martignani, C.; Zanoni, L.; La Donna, R.; Diemberger, I.; Boriani, G.; Pettinato, C.; Sambuceti, G.; Fanti, S.; et al. 11C-mHED for PET / CT: Principles of Synthesis, Methodology and First Clinical Applications. Curr. Radiopharm 2014, 7, 79–83. [Google Scholar] [CrossRef]
- Noordzij, W.; Elvan, A.; Demirel, F.; Lager, P.L.; Tio, R.A.; Slart, R.H. Sympathetic denervation in patients with ischemic cardiomyopathy and risk on ventricular tachy-arrhythmias. A pilot study. Q J. Nucl. Med. Mol. Imaging 2018, 62, 429–435. [Google Scholar] [CrossRef]
- Zelt, J.G.E.; Mielniczuk, L.M.; Orlandi, C.; Robinson, S.; Hadizad, T.; Walter, O.; Garrard, L.; Beanlands, R.S.B.; deKamp, R.A. PET imaging of sympathetic innervation with [18F]Flurobenguane vs [11C]mHED in a patient with ischemic cardiomyopathy. J. Nucl. Cardiol. 2020, 27, 702. [Google Scholar] [CrossRef] [Green Version]
- Werner, R.A.; Rischpler, C.; Onthank, D.; Lapa, C.; Robinson, S.; Samnick, S.; Javadi, M.; Schwaiger, M.; Nekolla, S.G.; Higuchi, T. Retention Kinetics of the 18F-Labeled Sympathetic Nerve PET Tracer LMI1195: Comparison with 11C-Hydroxyephedrine and 123I-MIBG. J. Nucl. Med. 2015, 56, 1429–1433. [Google Scholar] [CrossRef] [Green Version]
- Pandit-Taskar, N.; Zanzonico, P.; Staton, K.D.; Carrasquillo, J.A.; Reidy-Lagunes, D.; Lyashchenko, S.; Burnazi, E.; Zhang, H.; Lewis, J.S.; Blasberg, R.; et al. Biodistribution and Dosimetry of 18 F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies. J. Nucl. Med. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckberg, D.L. Sympathovagal balance: A critical appraisal. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Montano, N.; Mela, G.S. Sympathovagal balance: A reappraisal. Circulation 1998, 98, 2640–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, M.; Montano, N.; Porta, A.; Malliani, A.; Abboud, F.M.; Birkett, C.; Somers, V.K. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 1997, 95, 1441–1448. [Google Scholar] [CrossRef]
- Iellamo, F.; Legramante, J.M.; Massaro, M.; Galante, A.; Pigozzi, F.; Nardozi, C.; Santilli, V. Spontaneous baroreflex modulation of heart rate and heart rate variability during orthostatic stress in tetraplegics and healthy subjects. J. Hypertens 2001, 19, 2231–2240. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iellamo, F.; Perrone, M.A.; Cimini, A.; Caminiti, G.; Chiaravalloti, A.; Parisi, A.; Schillaci, O. Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Training Effects. J. Cardiovasc. Dev. Dis. 2022, 9, 181. https://doi.org/10.3390/jcdd9060181
Iellamo F, Perrone MA, Cimini A, Caminiti G, Chiaravalloti A, Parisi A, Schillaci O. Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Training Effects. Journal of Cardiovascular Development and Disease. 2022; 9(6):181. https://doi.org/10.3390/jcdd9060181
Chicago/Turabian StyleIellamo, Ferdinando, Marco Alfonso Perrone, Andrea Cimini, Giuseppe Caminiti, Agostino Chiaravalloti, Attilio Parisi, and Orazio Schillaci. 2022. "Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Training Effects" Journal of Cardiovascular Development and Disease 9, no. 6: 181. https://doi.org/10.3390/jcdd9060181







