Advances in the Treatment Strategies in Hypertension: Present and Future
Abstract
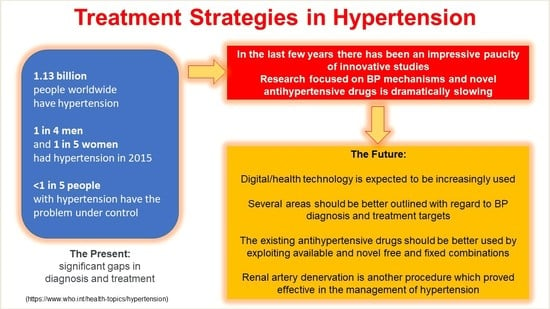
:1. Introduction
2. Digital/Health Technology for Diagnosis and Monitoring
- The system should be easily wearable, cheap, and non-intrusive. Systems included in normal smartwatches would be ideal;
- The system should be validated for accuracy at independent academic or hospital centers. It should allow continuous or almost-continuous BP detection over prolonged periods of time of months or even years;
- The system should be connectable to an easy-to-use protected digital repository, with software allowing easy BP retrieval over variable periods of time for calculation of appropriate statistical measures (BP averages, variability, etc.) and attached graphics;
- The system should be easily accessible to doctors, thereby enabling rapid check and response for patients and the suggestion of changes in drug treatment or other measures;
- Clinical research should urgently identify BP measures retrievable from the system which are more appropriate for the prediction of organ damage and, hopefully, prognosis. In other words, research should identify which BP measurements obtained by the system are more important for clinical decisions.
3. Definition of Hypertension and Establishment of Treatment Targets
- (a)
- (b)
4. Life-Style Measures
5. Chronotherapy
6. More Frequent Use of Mineral-Corticoid Receptor Antagonists
7. Endothelin Receptor Antagonists
8. Neprilysin Combined with Renin-Angiotensin System Inhibition
9. Angiotensin II Receptor Agonists
10. Sodium-Glucose Cotrasporter-2 Inhibitors
11. Renal Denervation
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Reboldi, G.; Angeli, F.; Trimarco, B.; Mancia, G.; Pogue, J.; Gao, P.; Sleight, P.; Teo, K.; Yusuf, S. Systolic and diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension 2015, 65, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboldi, G.; Angeli, F.; de Simone, G.; Staessen, J.A.; Verdecchia, P.; Cardio-Sis, I. Tight versus standard blood pressure control in patients with hypertension with and without cardiovascular disease. Hypertension 2014, 63, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertension, inflammation and atrial fibrillation. J. Hypertens. 2014, 32, 480–483. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Garofoli, M.; Reboldi, G. Aggressive blood pressure lowering is dangerous: The J-curve: Con side of the arguement. Hypertension 2014, 63, 37–40. [Google Scholar] [CrossRef] [Green Version]
- de Goma, E.M.; Knowles, J.W.; Angeli, F.; Budoff, M.J.; Rader, D.J. The evolution and refinement of traditional risk factors for cardiovascular disease. Cardiol. Rev. 2012, 20, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Verdecchia, P. “From apennines to andes”: Does body mass index affect the relationship between age and blood pressure? Hypertension 2012, 60, 6–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertension around the world: New insights from developing countries. J. Hypertens. 2013, 31, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Modernization and hypertension: Is the link changing? Hypertens. Res. 2013, 36, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Balatbat, C.A. Future of Hypertension. Hypertension 2019, 74, 450–457. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed] [Green Version]
- Hunter, P.G.; FChapman, A.; Dhaun, N. Hypertension: Current trends and future perspectives. Br. J. Clin. Pharmacol. 2021, 87, 3721–3736. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R.C.; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Nordon, G.; Radinsky, K.; Shalev, V. Machine learning of big data in gaining insight into successful treatment of hypertension. Pharmacol. Res. Perspect. 2018, 6, e00396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhurantakam, S.; Babu, K.J.; Rayappan, J.B.B.; Krishnan, U.M. Nanotechnology-based electrochemical detection strategies for hypertension markers. Biosens. Bioelectron. 2018, 116, 67–80. [Google Scholar] [CrossRef]
- Park, S.H.; Zhang, Y.; Rogers, J.A.; Gallon, L. Recent advances of biosensors for hypertension and nephrology. Curr. Opin. Nephrol. Hypertens. 2019, 28, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. Authors/Task Force, 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [PubMed] [Green Version]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; de Palma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Gentile, G.; Pinzagli, M.G.; Aita, A.; Verdecchia, P. European and US guidelines for arterial hypertension: Similarities and differences. Eur. J. Intern. Med. 2019, 63, 3–8. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Cavallini, C.; Reboldi, G. Keep Blood Pressure Low, but Not Too Much. Circ. Res. 2018, 123, 1205–1207. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F. The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure: The weapons are ready. Rev. Esp. Cardiol. 2003, 56, 843–847. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Verdecchia, P.; Reboldi, G.; Angeli, F. The 2020 International Society of Hypertension global hypertension practice guidelines-key messages and clinical considerations. Eur. J. Intern. Med. 2020, 82, 1–6. [Google Scholar] [CrossRef]
- Tsioufis, C.; Andrikou, I.; Thomopoulos, C.; Syrseloudis, D.; Stergiou, G.; Stefanadis, C. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. J. Hum. Hypertens. 2011, 25, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Verdecchia, P.; Porcellati, C.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Battistelli, M.; Guerrieri, M.; Gatteschi, C.; Zampi, I.; Santucci, A.; et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994, 24, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.Y.; Melgarejo, J.D.; Thijs, L.; Zhang, Z.Y.; Boggia, J.; Wei, F.F.; Hansen, T.W.; Asayama, K.; Ohkubo, T.; Jeppesen, J.; et al. Association of Office and Ambulatory Blood Pressure With Mortality and Cardiovascular Outcomes. JAMA 2019, 322, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Calvo, C.; Lopez, J.E.; Mojon, A.; Fontao, M.J.; Soler, R.; Fernandez, J.R. Effects of time of day of treatment on ambulatory blood pressure pattern of patients with resistant hypertension. Hypertension 2005, 46, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermida, R.C.; Ayala, D.E.; Fontao, M.J.; Mojon, A.; Alonso, I.; Fernandez, J.R. Administration-time-dependent effects of spirapril on ambulatory blood pressure in uncomplicated essential hypertension. Chronobiol. Int. 2010, 27, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.; Poulter, N.; Macdonald, T.; Ford, I.; Mackenzie, I.; Findlay, E.; Williams, B.; Brown, M.; Lang, C.; Webb, D. Chronotherapy in hypertension: The devil is in the details. Eur. Heart J. 2020, 41, 1606–1607. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.; Kjeldsen, S.E.; Burnier, M.; Narkiewicz, K.; Oparil, S.; Mancia, G. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project. Blood Press. 2020, 29, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.; Anderson, A.; Jones, E. The effect on 24 h blood pressure control of an angiotensin converting enzyme inhibitor (perindopril) administered in the morning or at night. J. Hypertens. 1997, 15, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Greene, T.; Phillips, R.A.; Agodoa, L.Y.; Bakris, G.L.; Charleston, J.; Contreras, G.; Gabbai, F.; Hiremath, L.; Jamerson, K.; et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension 2013, 61, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Rorie, D.A.; Rogers, A.; Mackenzie, I.S.; Ford, I.; Webb, D.J.; Willams, B.; Brown, M.; Poulter, N.; Findlay, E.; Saywood, W.; et al. Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: The Treatment In Morning versus Evening (TIME) study. BMJ Open 2016, 6, e010313. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, N.M. Chlorthalidone versus hydrochlorothiazide: A tale of tortoises and a hare. Hypertension 2011, 58, 994–995. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, T.W. Chlorthalidone: Don’t call it “thiazide-like” anymore. Hypertension 2010, 56, 335–337. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; ASinha, D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef]
- Williams, B.; MacDonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; MacDonald, T.M.; Morant, S.V.; Webb, D.J.; Sever, P.; McInnes, G.T.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Padmanabhan, S.; et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: The PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018, 6, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Struthers, A.; Krum, H.; Williams, G.H. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin. Cardiol. 2008, 31, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.S.; Wu, M.H.; Masson, S.C.; Tsang, M.P.; Stabler, S.N.; Kinkade, A.; Tung, A.; Tejani, A.M. Eplerenone for hypertension. Cochrane Database Syst. Rev. 2017, 2, CD008996. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular endothelin in hypertension. Vascul. Pharmacol. 2005, 43, 19–29. [Google Scholar] [CrossRef]
- Angeli, F.; Verdecchia, P.; Reboldi, G. Aprocitentan, A Dual Endothelin Receptor Antagonist Under Development for the Treatment of Resistant Hypertension. Cardiol. Ther. 2021, 10, 397–406. [Google Scholar] [CrossRef]
- Barton, M. Aging and endothelin: Determinants of disease. Life Sci. 2014, 118, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Bondurand, N.; Dufour, S.; Pingault, V. News from the endothelin-3/EDNRB signaling pathway: Role during enteric nervous system development and involvement in neural crest-associated disorders. Dev. Biol. 2018, 444 (Suppl. 1), S156–S169. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, W.; Lattmann, T.; Amann, K.; Szibor, M.; Morawietz, H.; Munter, K.; Muller, S.P.; Shaw, S.; Barton, M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: Implications for atherosclerosis. Biochem. Biophys. Res. Commun. 2001, 280, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, K.; Saleh, L.; Lankhorst, S.; Smilde, J.E.; van Ingen, M.M.; Garrelds, I.M.; Friesema, E.C.; Russcher, H.; van den Meiracker, A.H.; Visser, W.; et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 2015, 65, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, P.; Danaietash, P.; Flamion, B.; Menard, J.; Bellet, M. Randomized Dose-Response Study of the New Dual Endothelin Receptor Antagonist Aprocitentan in Hypertension. Hypertension 2020, 75, 956–965. [Google Scholar] [CrossRef]
- Trensz, F.; Bortolamiol, C.; Kramberg, M.; Wanner, D.; Hadana, H.; Rey, M.; Strasser, D.S.; Delahaye, S.; Hess, P.; Vezzali, E.; et al. Pharmacological Characterization of Aprocitentan, a Dual Endothelin Receptor Antagonist, Alone and in Combination with Blockers of the Renin Angiotensin System, in Two Models of Experimental Hypertension. J. Pharmacol. Exp. Ther. 2019, 368, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- Malek, V.; Gaikwad, A.B. Neprilysin inhibitors: A new hope to halt the diabetic cardiovascular and renal complications? Biomed. Pharmacother. 2017, 90, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Vardeny, O. The Role of Neprilysin Inhibitors in Cardiovascular Disease. Curr. Heart Fail. Rep. 2015, 12, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Maillard, M.; Burnier, M. Recent clinical trials with omapatrilat: New developments. Curr. Hypertens. Rep. 2003, 5, 346–352. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruilope, L.M.; Dukat, A.; Bohm, M.; Lacourciere, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Reboldi, G.; Gentile, G.; Angeli, F.; Verdecchia, P. Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: Update after recent clinical trials. Vasc. Health Risk Manag. 2009, 5, 411–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumners, C.; Peluso, A.A.; Haugaard, A.H.; Bertelsen, J.B.; Steckelings, U.M. Anti-fibrotic mechanisms of angiotensin AT2 -receptor stimulation. Acta Physiol. 2019, 227, e13280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Padia, S.H.; Carey, R.M. AT(2) receptor activation induces natriuresis and lowers blood pressure. Circ. Res. 2014, 115, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoia, C.; Ebrahimian, T.; He, Y.; Gratton, J.P.; Schiffrin, E.L.; Touyz, R.M. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J. Hypertens. 2006, 24, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Ghatage, T.; Goyal, S.G.; Dhar, A.; Bhat, A. Novel therapeutics for the treatment of hypertension and its associated complications: Peptide- and nonpeptide-based strategies. Hypertens. Res. 2021, 44, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Gentile, G.; Angeli, F.; Reboldi, G. Beyond blood pressure: Evidence for cardiovascular, cerebrovascular, and renal protective effects of renin-angiotensin system blockers. Ther. Adv. Cardiovasc. Dis. 2012, 6, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diab. Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Tahrani, A.A.; Bailey, C.J.; del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef]
- Grant, P.J.; Cosentino, F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur. Heart J. 2019, 40, 3215–3217. [Google Scholar] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.-P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Cannon, C.P.; Tikkanen, I.; Zeller, C.; Ley, L.; Woerle, H.J.; Broedl, U.C.; Johansen, O.E. Impact of Empagliflozin on Blood Pressure in Patients With Type 2 Diabetes Mellitus and Hypertension by Background Antihypertensive Medication. Hypertension 2016, 68, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Baker, W.L.; Buckley, L.F.; Kelly, M.S.; Bucheit, J.D.; Parod, E.D.; Brown, R.; Carbone, S.; Abbate, A.; Dixon, D.L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on 24-Hour Ambulatory Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e005686. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Gao, H.K.; Kengne, A.P. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials With 22 528 Patients. J. Am. Heart Assoc. 2017, 6, e004007. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Di Bona, G.F. Neural control of renal function in health and disease. Clin. Auton. Res. 1994, 4, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, G.F.; Kopp, U.C. Neural control of renal function. Physiol. Rev. 1997, 77, 75–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Denton, K.M. Renal Denervation. Hypertension 2018, 72, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, G.F.; Esler, M. Translational medicine: The antihypertensive effect of renal denervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R245–R253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symplicity HTN-2 Investigators; Esler, M.D.; Krum, H.; Sobotka, P.A.; Schlaich, M.P.; Schmieder, R.E.; Bohm, M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet 2010, 376, 1903–1909. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Bohm, M.; Kario, K.; Kandzari, D.E.; Mahfoud, F.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Konstantinidis, D.; Choi, J.W.; et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): A multicentre, randomised, sham-controlled trial. Lancet 2020, 395, 1444–1451. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Bohm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Sobotka, P.A.; Krum, H.; Lambert, E.; Esler, M.D. Renal sympathetic-nerve ablation for uncontrolled hypertension. N. Engl. J. Med. 2009, 361, 932–934. [Google Scholar] [CrossRef]
- Azizi, M.; Pereira, H.; Bobrie, G.; Gosse, P.; Renal Denervation for Hypertension (DENERHTN) Investigators. Renal denervation for resistant hypertension-Authors’ reply. Lancet 2015, 386, 1240. [Google Scholar] [CrossRef]
- Mahfoud, F.; Bohm, M.; Schmieder, R.; Narkiewicz, K.; Ewen, S.; Ruilope, L.; Schlaich, M.; Williams, B.; Fahy, M.; Mancia, G. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur. Heart J. 2019, 40, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Fengler, K.; Rommel, K.P.; Blazek, S.; Besler, C.; Hartung, P.; von Roeder, M.; Petzold, M.; Winkler, S.; Hollriegel, R.; Desch, S.; et al. A Three-Arm Randomized Trial of Different Renal Denervation Devices and Techniques in Patients With Resistant Hypertension (RADIOSOUND-HTN). Circulation 2019, 139, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Azizi, M.; Ewen, S.; Pathak, A.; Ukena, C.; Blankestijn, P.J.; Bohm, M.; Burnier, M.; Chatellier, G.; Zaleski, I.D.; et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur. Heart J. 2020, 41, 1588–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, R.M.; Taddei, S.; Borghi, C.; Colivicchi, F.; Desideri, G.; Grassi, G.; Mazza, A.; Muiesan, M.L.; Parati, G.; Pontremoli, R.; et al. Italian Society of Arterial Hypertension (SIIA) Position Paper on the Role of Renal Denervation in the Management of the Difficult-to-Treat Hypertensive Patient. High Blood Press. Cardiovasc. Prev. 2020, 27, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, H.; Cohen, J.B. Renal Denervation for the Treatment of Hypertension: Unnerving or Underappreciated? Clin. J. Am. Soc. Nephrol. 2021, 16, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Luscher, T.F.; Andersson, B.; Baumgartner, I.; Cifkova, R.; Dimario, C.; Doevendans, P.; Fagard, R.; Fajadet, J.; Komajda, M.; et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur. Heart J. 2013, 34, 2149–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacaksiz, A.; Uyarel, H.; Jafarov, P.; Kucukbuzcu, S. Iatrogenic renal artery stenosis after renal sympathetic denervation. Int. J. Cardiol. 2014, 172, e389–e390. [Google Scholar] [CrossRef]
- Celik, I.E.; Acar, B.; Kurtul, A.; Murat, S.N. De novo renal artery stenosis after renal sympathetic denervation. J. Clin. Hypertens. 2015, 17, 242–243. [Google Scholar] [CrossRef]
- Diego-Nieto, A.; Cruz-Gonzalez, I.; Martin-Moreiras, J.; Rama-Merchan, J.C.; Rodriguez-Collado, J.; Sanchez-Fernandez, P.L. Severe Renal Artery Stenosis After Renal Sympathetic Denervation. JACC Cardiovasc. Interv. 2015, 8, e193–e194. [Google Scholar] [CrossRef] [Green Version]
- Kaltenbach, B.; Id, D.; Franke, J.C.; Sievert, H.; Hennersdorf, M.; Maier, J.; Bertog, S.C. Renal artery stenosis after renal sympathetic denervation. J. Am. Coll. Cardiol. 2012, 60, 2694–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y. What is the true incidence of renal artery stenosis after sympathetic denervation? Front. Physiol. 2014, 5, 311. [Google Scholar] [CrossRef] [Green Version]
- Vonend, O.; Antoch, G.; Rump, L.C.; Blondin, D. Secondary rise in blood pressure after renal denervation. Lancet 2012, 380, 778. [Google Scholar] [CrossRef]
- Mahfoud, F.; Luscher, T.F. Renal denervation: Symply trapped by complexity? Eur. Heart J. 2015, 36, 199–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhudia, R.P. Treatment of the hypertensive patient in 2030. J. Hum. Hypertens. 2021, 35, 818–820. [Google Scholar] [CrossRef]





|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdecchia, P.; Cavallini, C.; Angeli, F. Advances in the Treatment Strategies in Hypertension: Present and Future. J. Cardiovasc. Dev. Dis. 2022, 9, 72. https://doi.org/10.3390/jcdd9030072
Verdecchia P, Cavallini C, Angeli F. Advances in the Treatment Strategies in Hypertension: Present and Future. Journal of Cardiovascular Development and Disease. 2022; 9(3):72. https://doi.org/10.3390/jcdd9030072
Chicago/Turabian StyleVerdecchia, Paolo, Claudio Cavallini, and Fabio Angeli. 2022. "Advances in the Treatment Strategies in Hypertension: Present and Future" Journal of Cardiovascular Development and Disease 9, no. 3: 72. https://doi.org/10.3390/jcdd9030072