Arrhythmic Mitral Valve Prolapse and Mitral Annular Disjunction: Clinical Features, Pathophysiology, Risk Stratification, and Management
Abstract
:1. Introduction
2. Arrhythmogenesis
3. Natural History
4. Risk Stratification for Sudden Cardiac Death
4.1. Demographic Characteristics
4.2. Electrocardiographic Abnormalities
4.3. Ventricular Ectopy
4.4. Mitral Valve Characteristics
4.5. Novel Echocardiographic Findings
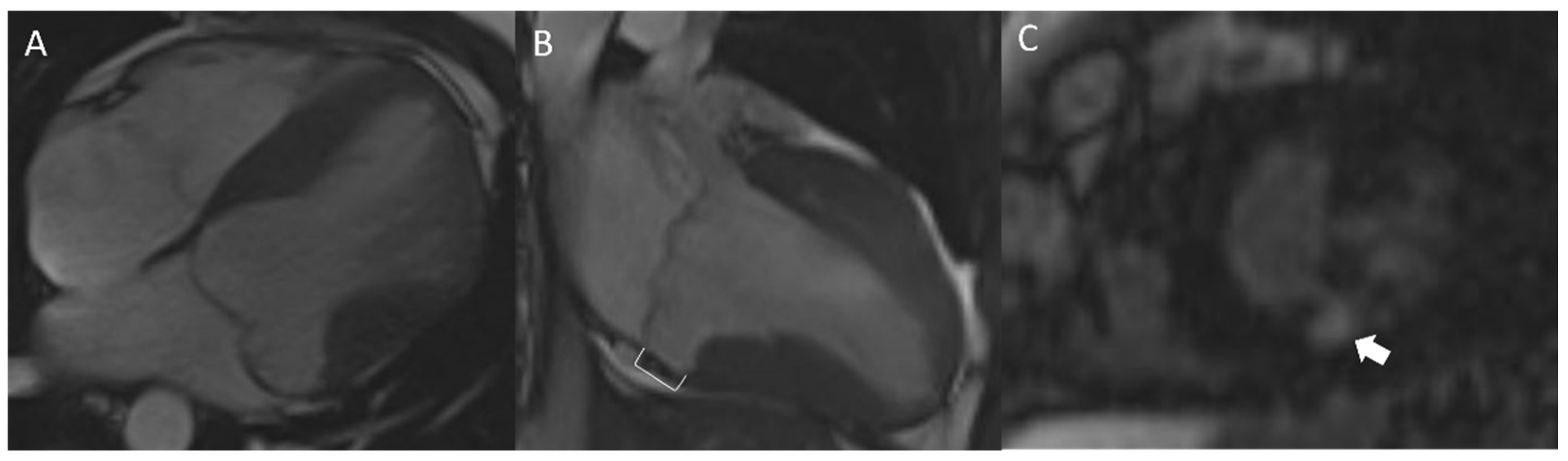
4.6. Cardiac Magnetic Resonance
4.7. Mitral Annular Disjunction and Annular Curling
4.8. Approach
5. Management
5.1. Electrophysiology Study
5.2. Anti-Arrhythmic Drug Therapy
5.3. Device Therapy
5.4. Catheter Ablation
5.5. Surgical Intervention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Criley, J.M.; Lewis, K.B.; Humphries, J.O.; Ross, R.S. Prolapse of the mitral valve: Clinical and cine-angiocardiographic findings. Heart 1966, 28, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Devereux, R.B.; Jones, E.C.; Roman, M.J.; Howard, B.V.; Fabsitz, R.R.; Liu, J.E.; Palmieri, V.; Welty, T.K.; Lee, E.T. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: The strong heart study. Am. J. Med. 2001, 111, 679–685. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Mahajan, R.; Elliott, A.D.; Haqqani, H.; Lau, D.H.; Vohra, J.K.; Morton, J.B.; Semsarian, C.; Marwick, T.; Kalman, J.M.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart 2019, 105, 144–151. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population. J. Am. Coll. Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, A.C.; Adams, D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin. Thorac. Cardiovasc. Surg. 2007, 19, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Van Wijngaarden, A.L.; Kruithof, B.P.T.; Vinella, T.; Barge-Schaapveld, D.Q.C.M.; Ajmone Marsan, N. Characterization of degenerative mitral valve disease: Differences between fibroelastic deficiency and Barlow’s disease. J. Cardiovasc. Dev. Dis. 2021, 8, 23. [Google Scholar] [CrossRef]
- Van Wijngaarden, A.L.; Hiemstra, Y.L.; Koopmann, T.T.; Ruivenkamp, C.A.L.; Aten, E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Barge-Schaapveld, D.Q.C.M.; Ajmone Marsan, N. Identification of known and unknown genes associated with mitral valve prolapse using an exome slice methodology. J. Med. Genet. 2020, 57, 843–850. [Google Scholar] [CrossRef]
- Toomer, K.A.; Yu, M.; Fulmer, D.; Guo, L.; Moore, K.S.; Moore, R.; Drayton, K.D.; Glover, J.; Peterson, N.; Ramos-Ortiz, S.; et al. Primary cilia defects causing mitral valve prolapse. Sci. Transl. Med. 2019, 11, eaax0290. [Google Scholar] [CrossRef] [Green Version]
- Bains, S.; Tester, D.J.; Asirvatham, S.J.; Noseworthy, P.A.; Ackerman, M.J.; Giudicessi, J.R. A Novel truncating variant in FLNC-encoded filamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileaflet mitral valve prolapse syndrome. Mayo Clin. Proc. 2019, 94, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Caretta, A.; Viani, G.M.; Schlossbauer, S.A.; Demertzis, S.; Ho, S.Y. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur. Hear. J. Cardiovasc. Imaging 2019, 20, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Pavon, A.G.; Ho, S.Y.; Maisano, F. Morphology of mitral annular disjunction in mitral valve prolapse. J. Am. Soc. Echocardiogr. 2021, 35, 176–186. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The mitral annulus disjunction arrhythmic syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Angelini, A.; Ho, S.Y.; Anderson, R.H.; Becker, A.E.; Davies, M.J. Disjunction of the mitral annulus in floppy mitral valve. N. Engl. J. Med. 1988, 318, 188–189. [Google Scholar]
- Bharati, S.; Granston, A.S.; Liebson, P.R.; Loeb, H.S.; Rosen, K.M.; Lev, M. The conduction system in mitral valve prolapse syndrome with sudden death. Am. Hear. J. 1981, 101, 667–670. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ. Cardiovasc. Imaging 2016, 8, e005030. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, B.W.; Schatz, R.A.; VonRamm, O.T.; Behar, V.S.; Kisslo, J.A. Mitral valve prolapse. Two-dimensional echocardiographic and angiographic correlation. Circulation 1976, 54, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.; Thamman, R.; Griffiths, T.; Oxley, C.; Khan, J.N.; Phan, T.; Patwala, A.; Heatlie, G.; Kwok, C.S. Mitral annular disjunction: A systematic review of the literature. Echocardiography 2019, 36, 1549–1558. [Google Scholar] [CrossRef]
- Lee, A.P.-W.; Jin, C.-N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional implication of mitral annular disjunction in mitral valve prolapse. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Van Wijngaarden, A.L.; de Riva, M.; Hiemstra, Y.L.; van der Bijl, P.; Fortuni, F.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Parameters associated with ventricular arrhythmias in mitral valve prolapse with significant regurgitation. Heart 2021, 107, 411–418. [Google Scholar] [CrossRef]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.-T.; Maalouf, J.; Thapa, P.; Asirvatham, S.; Michelena, H.I.; et al. The mitral annular disjunction of mitral valve prolapse. JACC Cardiovasc. Imaging 2021, 14, 2073–2087. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ali, S.G.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef]
- Putnam, A.J.; Kebed, K.; Mor-Avi, V.; Rashedi, N.; Sun, D.; Patel, B.; Balkhy, H.; Lang, R.M.; Patel, A.R. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int. J. Cardiovasc. Imaging 2020, 36, 1363–1370. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, M.J.; Bitkover, C.Y.; Omran, A.S.; David, T.E.; Ivanov, J.; Ali, M.J.; Woo, A.; Siu, S.C.; Rakowski, H. Mitral annular disjunction in advanced myxomatous mitral valve disease: Echocardiographic detection and surgical correction. J. Am. Soc. Echocardiogr. 2005, 18, 1014–1022. [Google Scholar] [CrossRef]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral-valve prolapse: Long-term follow-up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Delling, F.N.; Aung, S.; Vittinghoff, E.; Dave, S.; Lim, L.J.; Olgin, J.E.; Connolly, A.; Moffatt, E.; Tseng, Z.H. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countywide sudden deaths. JACC Clin. Electrophysiol. 2021, 7, 1025–1034. [Google Scholar] [CrossRef]
- Miller, M.A.; Adams, D.H.; Pandis, D.; Robson, P.M.; Pawale, A.; Pyzik, R.; Liao, S.L.; El-Eshmawi, A.; Boateng, P.; Garg, J.; et al. Hybrid positron emission tomography/magnetic resonance imaging in arrhythmic mitral valve prolapse. JAMA Cardiol. 2020, 5, 1000–1005. [Google Scholar] [CrossRef]
- Gornick, C.C.; Tobler, H.G.; Pritzker, M.C.; Tuna, I.C.; Almquist, A.; Benditt, D.G. Electrophysiologic effects of papillary muscle traction in the intact heart. Circulation 1986, 73, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ. Arrhythm. Electrophysiol. 2016, 9, e004005. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Hamilton-Craig, C.; Denman, R.; Haqqani, H.M. Catheter ablation of papillary muscle arrhythmias: Implications of mitral valve prolapse and systolic dysfunction. Pacing Clin. Electrophysiol. 2018, 41, 750–758. [Google Scholar] [CrossRef]
- Santoro, F.; Di Biase, L.; Hranitzky, P.; Sanchez, J.E.; Santangeli, P.; Perini, A.P.; Burkhardt, J.D.; Natale, A. Ventricular fibrillation triggered by PVCs from papillary muscles: Clinical features and ablation. J. Cardiovasc. Electrophysiol. 2014, 25, 1158–1164. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Avierinos, J.-F.; Gersh, B.J.; Melton, L.J.; Bailey, K.R.; Shub, C.; Nishimura, R.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.-T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and outcome of arrhythmic mitral valve prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Düren, D.R.; Becker, A.E.; Dunning, A.J. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: A prospective study. J. Am. Coll. Cardiol. 1988, 11, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Grigioni, F.; Enriquez-Sarano, M.; Ling, L.H.; Bailey, K.R.; Seward, J.B.; Tajik, A.; Frye, R.L. Sudden death in mitral regurgitation due to flail leaflet. J. Am. Coll. Cardiol. 1999, 34, 2078–2085. [Google Scholar] [CrossRef] [Green Version]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Narayanan, K.; Uy-Evanado, A.; Teodorescu, C.; Reinier, K.; Nichols, G.A.; Gunson, K.; Jui, J.; Chugh, S.S. Mitral valve prolapse and sudden cardiac arrest in the community. Hear Rhythm 2016, 13, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem diagnosis in sudden cardiac death victims: Macroscopic, microscopic and molecular findings. Cardiovasc. Res. 2001, 50, 290–300. [Google Scholar] [CrossRef]
- Han, H.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review. J. Am. Hear. Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef] [Green Version]
- Lobstein, H.P.; Horwitz, L.D.; Curry, G.C.; Mullins, C.B. Electrocardiographic abnormalities and coronary arteriograms in the mitral click-murmur syndrome. N. Engl. J. Med. 1973, 289, 127–131. [Google Scholar] [CrossRef]
- Jeresaty, R.M. Sudden death in the mitral valve prolapse-click syndrome. Am. J. Cardiol. 1976, 37, 317–318. [Google Scholar] [CrossRef]
- Chesler, E.; King, R.A.; Edwards, J.E. The myxomatous mitral valve and sudden death. Circulation 1983, 67, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, F.; Kakargias, F.; Panday, P.; Arcia Franchini, A.P.; Iskander, B.; Anwer, F.; Hamid, P. Arrhythmic mitral valve prolapse: Diagnostic parameters for high-risk patients: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2021, 44, 1746–1755. [Google Scholar] [CrossRef]
- Bekheit, S.G.; Ali, A.A.; Deglin, S.M.; Jain, A.C. Analysis of QT interval in patients with idiopathic mitral valve prolapse. Chest 1982, 81, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Kulan, K.; Komsuoğlu, B.; Tuncer, C.; Kulan, C. Significance of QT dispersion on ventricular arrhythmias in mitral valve prolapse. Int. J. Cardiol. 1996, 54, 251–257. [Google Scholar] [CrossRef]
- Zouridakis, E. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace 2001, 3, 292–298. [Google Scholar] [CrossRef]
- Tieleman, R.G.; Crijns, H.J.; Wiesfeld, A.C.; Posma, J.; Hamer, H.P.; Lie, K.I. Increased dispersion of refractoriness in the absence of QT prolongation in patients with mitral valve prolapse and ventricular arrhythmias. Heart 1995, 73, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Akcay, M.; Yuce, M.; Pala, S.; Akcakoyun, M.; Ergelen, M.; Kargin, R.; Emiroglu, Y.; Ozdemir, N.; Kaymaz, C.; Ozkan, M. Anterior mitral valve length is associated with ventricular tachycardia in patients with classical mitral valve prolapse: Predictors of ventricular tachycardia in mitral valve prolapse. Pacing Clin. Electrophysiol. 2010, 33, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.D.; Castelli, P.; Anderson, S. Mitral valve prolapse in the general population. 3. Dysrhythmias: The Framingham study. Am. J. Cardiol. 1982, 49, 997. [Google Scholar] [CrossRef]
- Enriquez, A.; Shirai, Y.; Huang, J.; Liang, J.; Briceño, D.; Hayashi, T.; Muser, D.; Fulton, B.; Han, Y.; Perez, A.; et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: Electrophysiologic substrate and catheter ablation outcomes. J. Cardiovasc. Electrophysiol. 2019, 30, 827–835. [Google Scholar] [CrossRef]
- Yuan, H.-T.; Yang, M.; Zhong, L.; Lee, Y.-H.; Vaidya, V.R.; Asirvatham, S.J.; Ackerman, M.J.; Pislaru, S.V.; Suri, R.M.; Slusser, J.P.; et al. Ventricular premature contraction associated with mitral valve prolapse. Int. J. Cardiol. 2016, 221, 1144–1149. [Google Scholar]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death: Dysrhythmias and death in BiMVP. J. Cardiovasc. Electrophysiol. 2016, 27, 463–468. [Google Scholar] [CrossRef]
- Farb, A.; Tang, A.L.; Atkinson, J.B.; McCarthy, W.F.; Virmani, R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am. J. Cardiol. 1992, 70, 234–239. [Google Scholar] [CrossRef]
- Turker, Y.; Ozaydin, M.; Acar, G.; Ozgul, M.; Hoscan, Y.; Varol, E.; Dogan, A.; Erdogan, D.; Yucel, H. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int. J. Cardiovasc. Imaging 2010, 26, 139–145. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Kwon, D.H.; Abou-Hassan, O.; Chetrit, M.; Harb, S.C.; Patel, D.; Kalahasti, V.; Popovic, Z.B.; Griffin, B.P.; Ayoub, C. Strain evaluation for mitral annular disjunction by echocardiography and magnetic resonance imaging: A case-control study. Int. J. Cardiol. 2021, 334, 154–156. [Google Scholar] [CrossRef]
- Muthukumar, L.; Rahman, F.; Jan, M.F.; Shaikh, A.; Kalvin, L.; Dhala, A.; Jahangir, A.; Tajik, A.J. The pickelhaube sign. JACC Cardiovasc. Imaging 2017, 10, 1078–1080. [Google Scholar] [CrossRef]
- Ignatowski, D.; Schweitzer, M.; Pesek, K.; Jain, R.; Muthukumar, L.; Khandheria, B.K.; Tajik, A.J. Pickelhaube spike, a high-risk marker for bileaflet myxomatous mitral valve prolapse: Sonographer’s quest for the highest spike. J. Am. Soc. Echocardiogr. 2020, 33, 639–640. [Google Scholar] [CrossRef] [Green Version]
- Tower-Rader, A.; Mohananey, D.; To, A.; Lever, H.M.; Popovic, Z.B.; Desai, M.Y. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1930–1942. [Google Scholar] [CrossRef]
- Gatti, M.; Palmisano, A.; Esposito, A.; Fiore, S.; Monti, C.B.; Andreis, A.; Pistelli, L.; Vergara, P.; Bergamasco, L.; Giustetto, C.; et al. Feature tracking myocardial strain analysis in patients with bileaflet mitral valve prolapse: Relationship with LGE and arrhythmias. Eur. Radiol. 2021, 31, 7273–7282. [Google Scholar] [CrossRef]
- Castillo-Sang, M.; Palmer, C.; Truong, V.T.; Young, M.; Wolking, S.; Alsaied, T.; Drake, D.; Mazur, W. Abnormal ventricular contractile pattern associated with late systolic mitral prolapse: A two-dimensional speckle tracking study. Int. J. Cardiovasc. Imaging 2020, 36, 2155–2164. [Google Scholar] [CrossRef]
- Muthukumar, L.; Jahangir, A.; Jan, M.F.; Galazka, P.; Umland, M.; Schweitzer, M.R.; Perez Moreno, A.C.; Singh, M.; Khandheria, B.K.; Tajik, A.J. Left Ventricular Global and Regional Deformation in Arrhythmic Myxomatous Bileaflet Mitral Valve Prolapse Syndrome. JACC Cardiovasc. Imaging 2020, 13, 1842–1844. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Hear. J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Huttin, O.; Pierre, S.; Venner, C.; Voilliot, D.; Sellal, J.-M.; Aliot, E.; Sadoul, N.; Juillière, Y.; Selton-Suty, C. Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: One more piece to the puzzle. Eur. Hear. J. Cardiovasc. Imaging 2017, 18, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Constant Dit Beaufils, A.-L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.-M.; et al. Replacement Myocardial Fibrosis in Patients With Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradella, S.; Grazzini, G.; Brandani, M.; Calistri, L.; Nardi, C.; Mori, F.; Miele, V.; Colagrande, S. Cardiac magnetic resonance in patients with mitral valve prolapse: Focus on late gadolinium enhancement and T1 mapping. Eur. Radiol. 2019, 29, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.-C.; Parsons, S.A.; Curl, C.L.; Teh, A.W.; Raaijmakers, A.J.A.; Koshy, A.N.; Leong, T.; Burrell, L.M.; O’Donnell, D.; Vohra, J.K.; et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm 2021, 18, 570–576. [Google Scholar] [CrossRef]
- Fulton, B.L.; Liang, J.J.; Enriquez, A.; Garcia, F.C.; Supple, G.E.; Riley, M.P.; Schaller, R.D.; Dixit, S.; Callans, D.J.; Marchlinski, F.E.; et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J. Cardiovasc. Electrophysiol. 2018, 29, 146–153. [Google Scholar] [CrossRef]
- Pavon, A.G.; Arangalage, D.; Pascale, P.; Hugelshofer, S.; Rutz, T.; Porretta, A.P.; Le Bloa, M.; Muller, O.; Pruvot, E.; Schwitter, J.; et al. Myocardial extracellular volume by T1 mapping: A new marker of arrhythmia in mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2021, 23, 102. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation 2018, 138, e91–e220. [Google Scholar]
- Marano, P.J.; Lim, L.J.; Sanchez, J.M.; Alvi, R.; Nah, G.; Badhwar, N.; Gerstenfeld, E.P.; Tseng, Z.H.; Marcus, G.M.; Delling, F.N. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J. Interv. Card. Electrophysiol. 2021, 61, 145–154. [Google Scholar] [CrossRef]
- Bumgarner, J.M.; Patel, D.; Kumar, A.; Clevenger, J.R.; Trulock, K.M.; Popović, Z.; Griffin, B.P.; Wazni, O.M.; Menon, V.; Desai, M.Y.; et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin. Electrophysiol. 2019, 42, 447–452. [Google Scholar] [CrossRef]
- Lin, A.N.; Shirai, Y.; Liang, J.J.; Chen, S.; Kochar, A.; Hyman, M.C.; Santangeli, P.; Schaller, R.D.; Frankel, D.S.; Arkles, J.S.; et al. Strategies for catheter ablation of left ventricular papillary muscle arrhythmias: An institutional experience. JACC Clin. Electrophysiol. 2020, 6, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Doppalapudi, H.; McElderry, H.T.; Okada, T.; Murakami, Y.; Inden, Y.; Yoshida, Y.; Yoshida, N.; Murohara, T.; Epstein, A.E.; et al. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: Relevance for catheter ablation. Circ. Arrhythm. Electrophysiol. 2010, 3, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Latchamsetty, R.; Bogun, F. Premature ventricular complex ablation in structural heart disease. Card. Electrophysiol. Clin. 2017, 9, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Ricapito, M.D.L.P.; Espinoza, J.; Belardi, D.; Albina, G.; Giniger, A.; Roux, J.-F.; Ayala-Paredes, F.; Scazzuso, F. Cryoablation for ventricular arrhythmias arising from the papillary muscles of the left ventricle guided by intracardiac echocardiography and image integration. JACC Clin. Electrophysiol. 2015, 1, 509–516. [Google Scholar] [CrossRef]
- Rivera, S.; Ricapito, M.d.l.P.; Tomas, L.; Parodi, J.; Bardera Molina, G.; Banega, R.; Bueti, P.; Orosco, A.; Reinoso, M.; Caro, M.; et al. Results of cryoenergy and radiofrequency-based catheter ablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration. Circ. Arrhythm. Electrophysiol. 2016, 9, e003874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.P.; Liang, J.J.; Pathak, R.K.; Zado, E.S.; Garcia, F.C.; Hutchinson, M.D.; Santangeli, P.; Schaller, R.D.; Frankel, D.S.; Marchlinski, F.E.; et al. Percutaneous cryoablation for papillary muscle ventricular arrhythmias after failed radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2018, 29, 1654–1663. [Google Scholar] [CrossRef]
- Kay, J.H.; Krohn, B.G.; Zubiate, P.; Hoffman, R.L. Surgical correction of severe mitral prolapse without mitral insufficiency but with pronounced cardiac arrhythmias. J. Thorac. Cardiovasc. Surg. 1979, 78, 259–268. [Google Scholar] [CrossRef]
- Pocock, W.A.; Barlow, J.B.; Marcus, R.H.; Barlow, C.W. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am. Heart J. 1991, 121, 199–202. [Google Scholar] [CrossRef]
- Reece, I.J.; Cooley, D.A.; Painvin, G.A.; Okereke, O.U.J.; Powers, P.L.; Pechacek, L.W.; Frazier, O.H. Surgical Treatment of Mitral Systolic Click Syndrome: Results in 37 Patients. Ann. Thorac. Surg. 1985, 39, 155–158. [Google Scholar] [CrossRef]
- Naksuk, N.; Syed, F.; Krittanawong, C.; Anderson, M.J.; Ebrille, E.; DeSimone, C.V.; Vaidya, V.R.; Ponamgi, S.P.; Suri, R.M.; Ackerman, M.J.; et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol. J. 2016, 16, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.R.; DeSimone, C.V.; Damle, N.; Naksuk, N.; Syed, F.F.; Ackerman, M.J.; Ponamgi, S.P.; Nkomo, V.T.; Suri, R.M.; Noseworthy, P.A.; et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J. Interv. Card. Electrophysiol. 2016, 46, 137–143. [Google Scholar] [CrossRef] [PubMed]
- El-Eshmawi, A.; Pandis, D.; Miller, M.A.; Boateng, P.; Dukkipati, S.R.; Reddy, V.Y.; Adams, D.H. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery. J. Am. Coll. Cardiol. 2020, 76, 3061–3062. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarti, A.K.; Bogun, F.; Liang, J.J. Arrhythmic Mitral Valve Prolapse and Mitral Annular Disjunction: Clinical Features, Pathophysiology, Risk Stratification, and Management. J. Cardiovasc. Dev. Dis. 2022, 9, 61. https://doi.org/10.3390/jcdd9020061
Chakrabarti AK, Bogun F, Liang JJ. Arrhythmic Mitral Valve Prolapse and Mitral Annular Disjunction: Clinical Features, Pathophysiology, Risk Stratification, and Management. Journal of Cardiovascular Development and Disease. 2022; 9(2):61. https://doi.org/10.3390/jcdd9020061
Chicago/Turabian StyleChakrabarti, Apurba K., Frank Bogun, and Jackson J. Liang. 2022. "Arrhythmic Mitral Valve Prolapse and Mitral Annular Disjunction: Clinical Features, Pathophysiology, Risk Stratification, and Management" Journal of Cardiovascular Development and Disease 9, no. 2: 61. https://doi.org/10.3390/jcdd9020061





