Initial Experience to Follow Lung Fluid Levels during Hemodialysis: A Possibility of Remote Dielectric Sensing-Guided Hemodialysis
Abstract
:1. Introduction
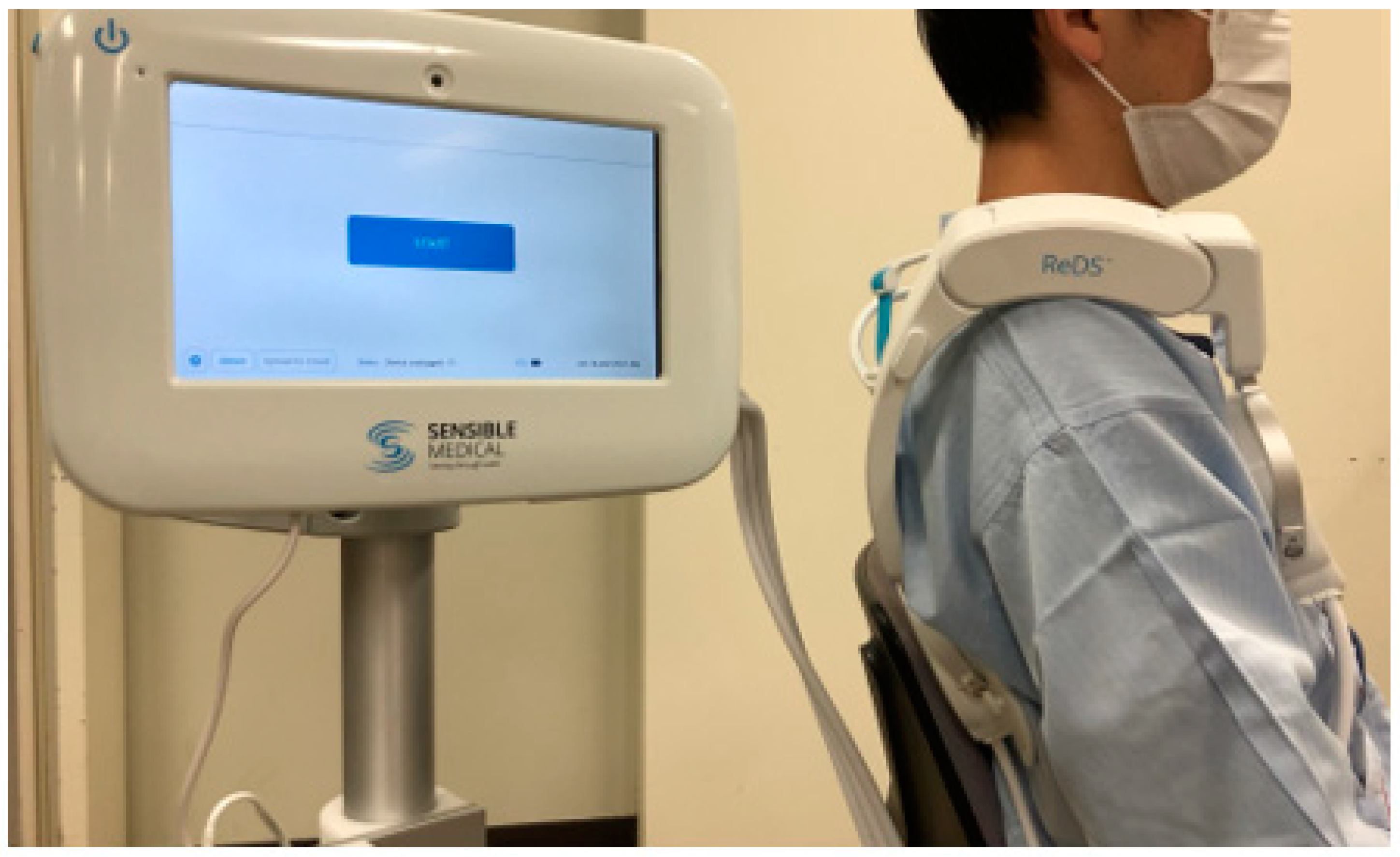
2. Case Report
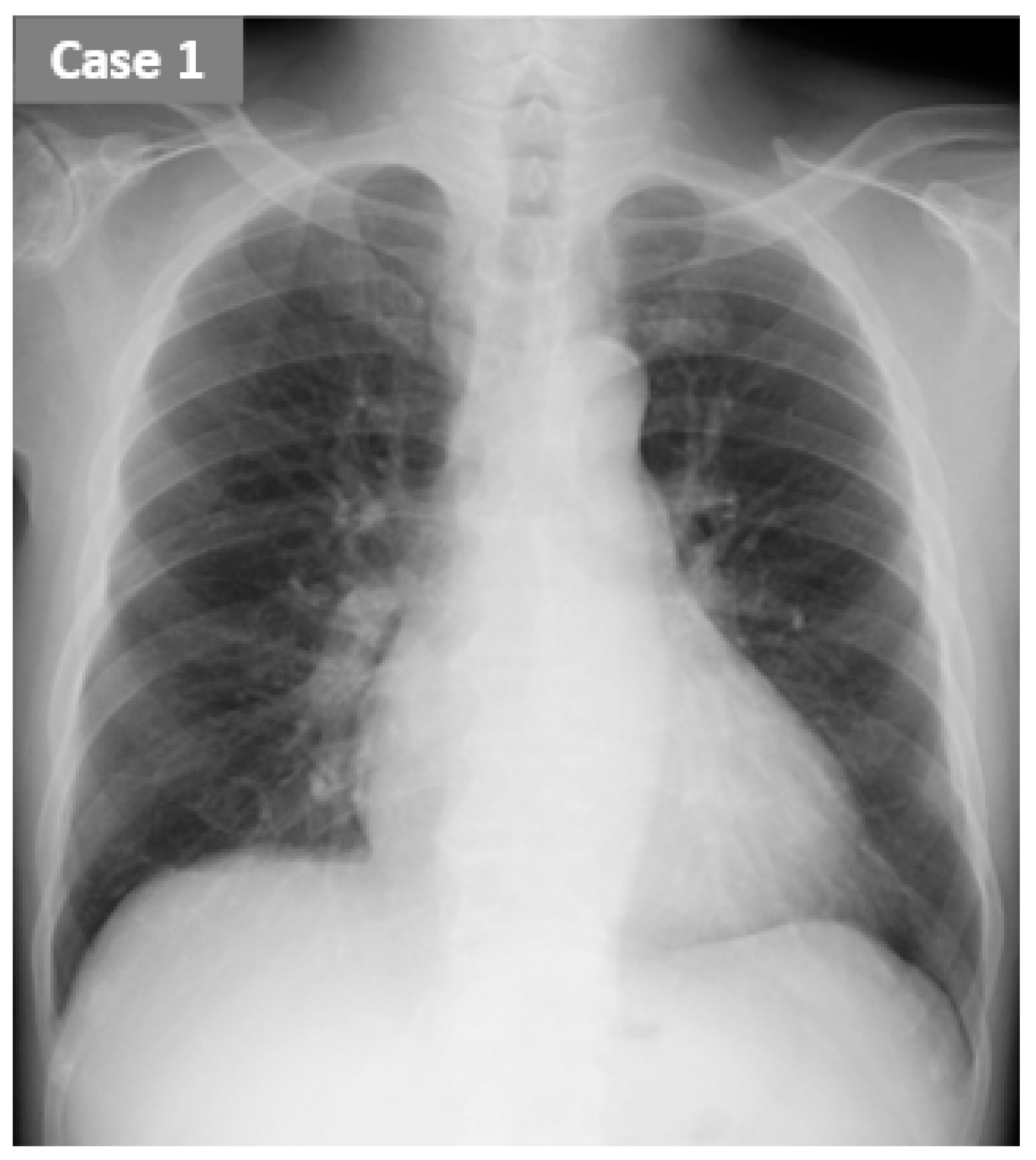
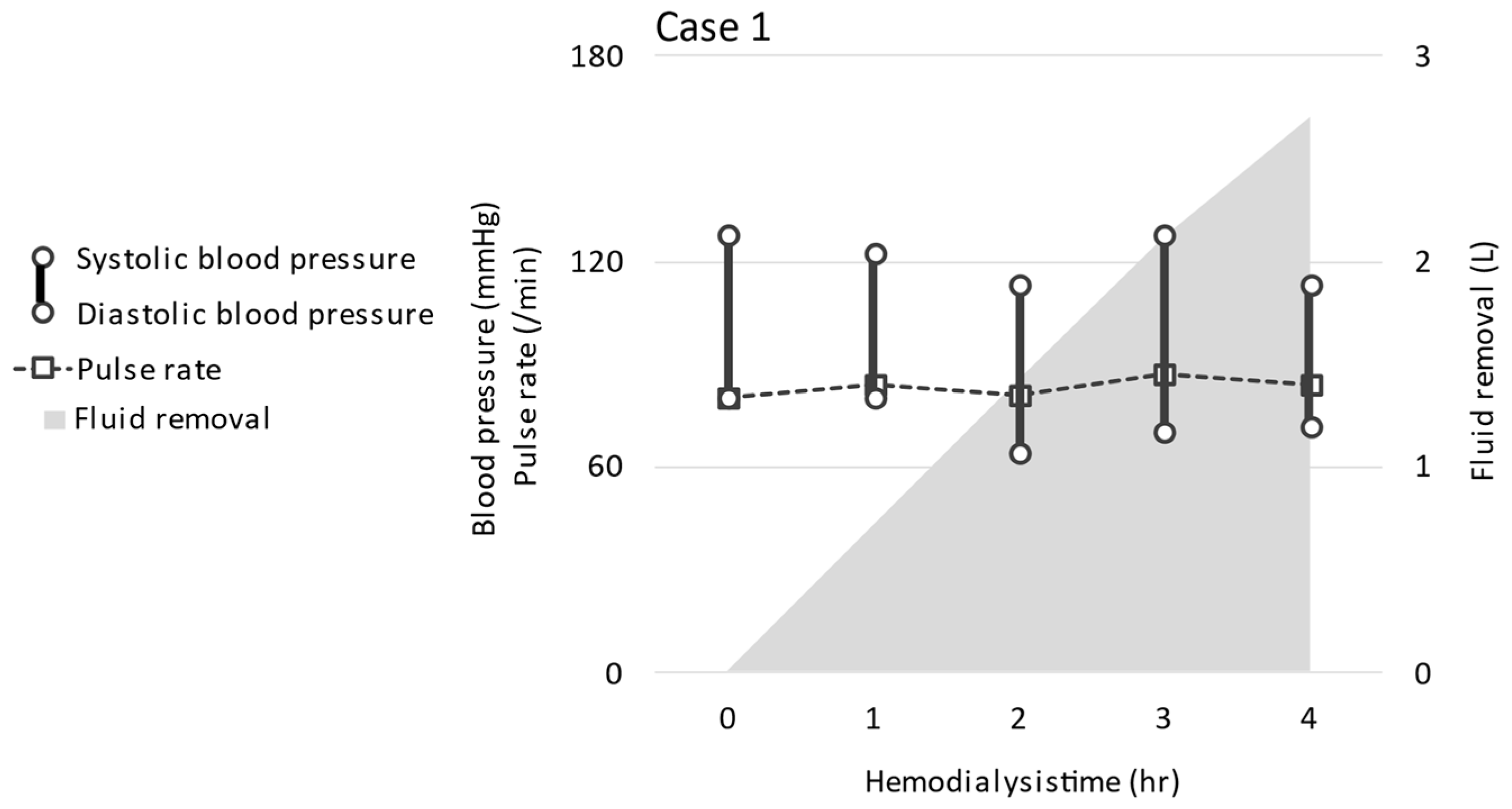
2.1. CASE 1 (ReDS Change from 32 to 30%)
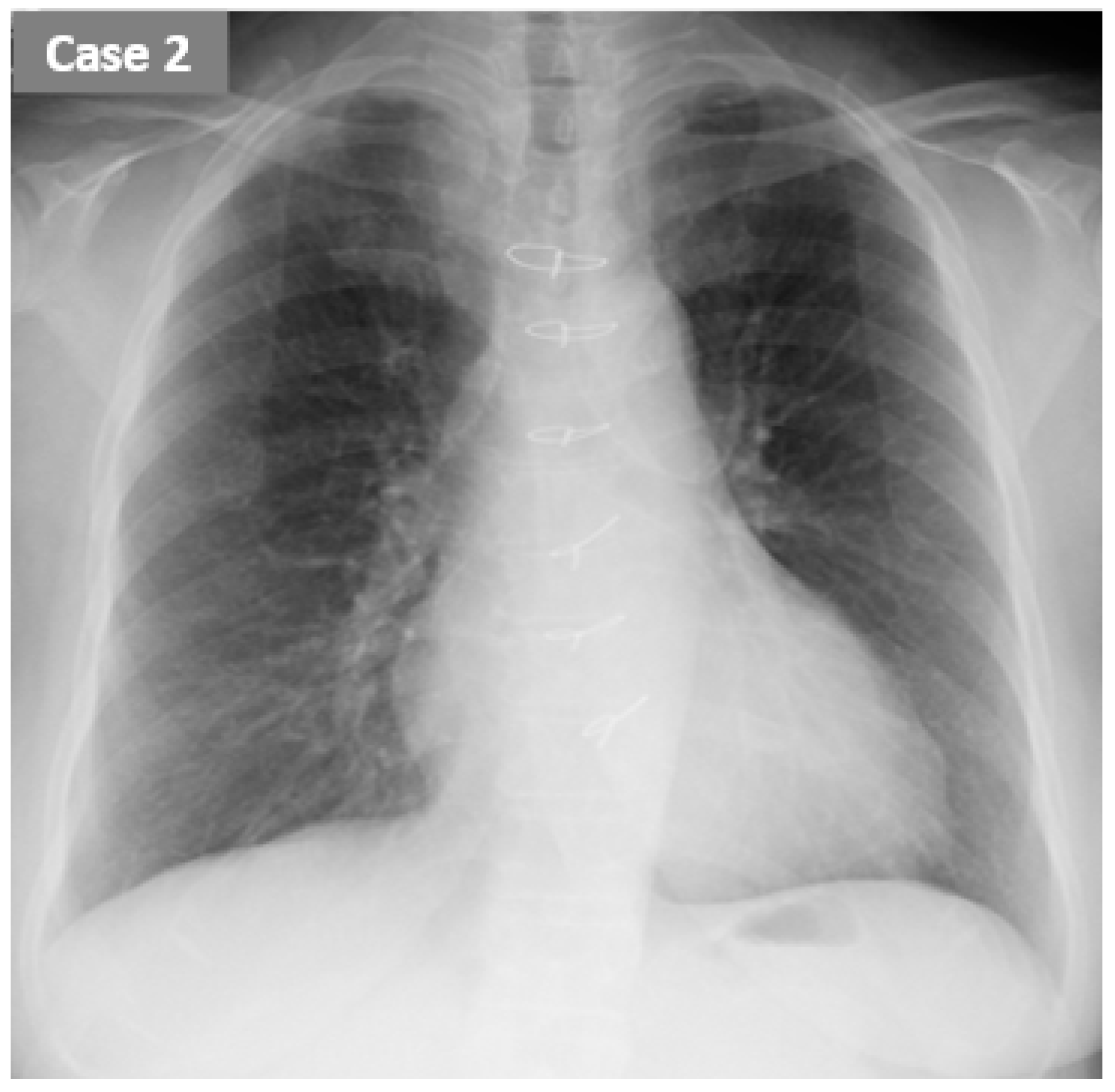
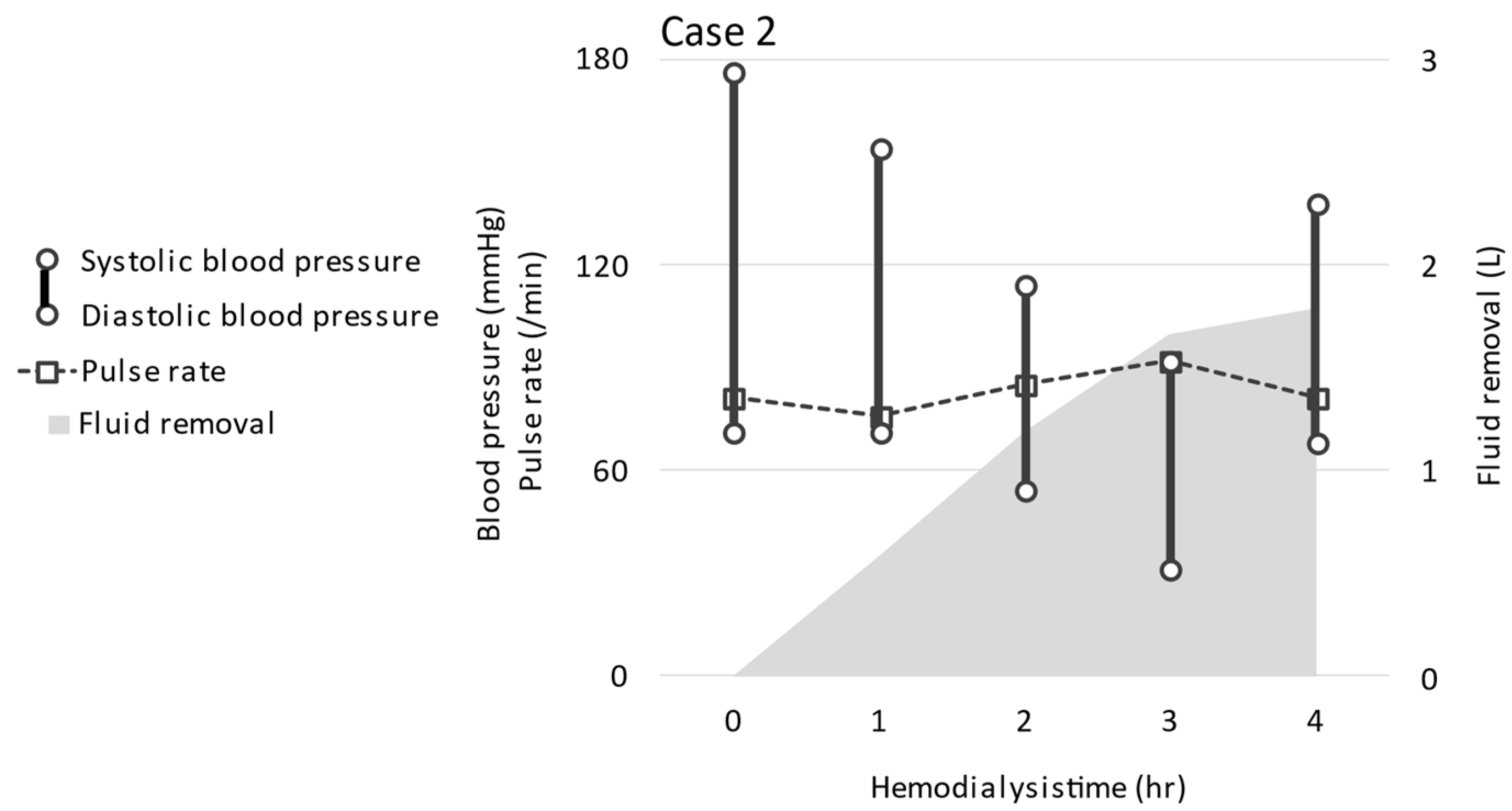
2.2. CASE 2 (ReDS Change from 37 to 27%)
3. Discussion
3.1. Lung Fluid Assessment
3.2. ReDS Measurement and Optimal Hemodialysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flythe, J.E.; Chang, T.I.; Gallagher, M.P.; Lindley, E.; Madero, M.; Sarafidis, P.A.; Unruh, M.L.; Wang, A.Y.-M.; Weiner, D.E.; Cheung, M.; et al. Blood pressure and volume management in dialysis: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 861–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assimon, M.M.; Wenger, J.B.; Wang, L.; Flythe, J.E. Ultrafiltration Rate and Mortality in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2016, 68, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, J.; Jefferies, H.J.; Selby, N.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.; Crowley, L. Dying to Feel Better: The Central Role of Dialysis-Induced Tissue Hypoxia. Clin. J. Am. Soc. Nephrol. 2016, 11, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Rappaport, D.; Zafrir, B.; Abraham, W.T. A novel approach to monitoring pulmonary congestion in heart failure: Initial animal and clinical experiences using remote dielectric sensing technology. Congest. Heart Fail. 2013, 19, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Ben-Gal, T.; Weinstein, J.M.; Schliamser, J.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int. J. Cardiol. 2017, 240, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lala, A.; Barghash, M.H.; Giustino, G.; Alvarez-Garcia, J.; Konje, S.; Parikh, A.; Ullman, J.; Keith, B.; Donehey, J.; Mitter, S.S.; et al. Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Fail. 2020, 8, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Azzam, Z.S.; Gaspar, T.; Faranesh-Abboud, S.; Andria, N.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Validation of remote dielectric sensing (ReDS) technology for quantification of lung fluid status: Comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int. J. Cardiol. 2016, 221, 841–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, T.; Gonoi, W.; Hori, M.; Ueno, Y.; Narang, N.; Onoda, H.; Tanaka, S.; Nakamura, M.; Kataoka, N.; Ushijima, R.; et al. Validation of Noninvasive Remote Dielectric Sensing System to Quantify Lung Fluid Levels. J. Clin. Med. 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Steuer, R.R.; Leypoldt, J.K.; Cheung, A.K.; Harris, D.H.; Conis, J.M. Hematocrit as an indicator of blood volume and a predictor of intradialytic morbid events. ASAIO J. 1994, 40, M691–M696. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Matsushita, Y.; Inoue, T.; Morii, H.; Ishibashi, M.; Yamaji, T. Plasma levels of atrial natriuretic peptide in patients with chronic renal failure. J. Clin. Endocrinol. Metab. 1986, 63, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Goei, D.; Schouten, O.; Boersma, E.; Welten, G.M.; Dunkelgrun, M.; Lindemans, J.; van Gestel, Y.R.; Hoeks, S.E.; Bax, J.J.; Poldermans, D. Influence of renal function on the usefulness of N-terminal pro-B-type natriuretic peptide as a prognostic cardiac risk marker in patients undergoing noncardiac vascular surgery. Am. J. Cardiol. 2008, 101, 122–126. [Google Scholar] [CrossRef]
- Rodriguez, H.J.; Domenici, R.; Diroll, A.; Goykhman, I. Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int. 2005, 68, 854–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Case 1 | Case 2 | ||
|---|---|---|---|
| Total protein | (g/dL) | 7.4 | 7.3 |
| Albumin | (g/dL) | 4.1 | 3.7 |
| Urea nitrogen | (mg/dL) | 76.4 | 74.7 |
| Creatinine | (mg/dL) | 11.53 | 8.35 |
| Uric acid | (mg/dL) | 4.3 | 7.5 |
| Sodium | (mEq/L) | 138 | 130 |
| Potassium | (mEq/L) | 5.2 | 6.1 |
| Chloride | (mEq/L) | 103 | 64 |
| Calcium | (mg/dL) | 8.8 | 8.1 |
| Phosphorus | (mg/dL) | 6.3 | 7.4 |
| Glucose | (mg/dL) | 99 | 185 |
| C-reactive protein | (mg/dL) | 0.31 | 0.67 |
| White blood cells | (/μL) | 6220 | 10,810 |
| Red blood cells | (104/μL) | 386 | 343 |
| Hemoglobin | (g/dL) | 11.9 | 10 |
| Hematocrit | (%) | 35.6 | 30.6 |
| Platelets | (104/μL) | 18.3 | 25.4 |
| Glycoalbumin | (%) | 15.7 | 26.6 |
| Intact parathyroid hormone | (pg/mL) | 125 | 233 |
| β2-microglobulin | (μg/L) | 40.9 | |
| Kt/V for urea | 1.51 | 1.45 |
| Case 1 | Case 2 | ||||
|---|---|---|---|---|---|
| Pre-HD | Post-HD | Pre-HD | Post-HD | ||
| Body weight | (kg) | 52.5 | 49.8 | 57.3 | 55.8 |
| ReDS value | (%) | 32 | 30 | 37 | 27 |
| Systolic blood pressure | (mmHg) | 118 | 113 | 179 | 143 |
| Diastolic blood pressure | (mmHg) | 74 | 72 | 92 | 66 |
| Pulse rate | (/min) | 87 | 84 | 75 | 81 |
| Inspiratory IVC diameter | (mm) | 7 | 2 | 6 | 3 |
| Expiratory IVC diameter | (mm) | 9 | 0 | 7 | 4 |
| Brain natriuretic peptide | (pg/mL) | 47.3 | - | 189.7 | - |
| Human atrial natriuretic peptide | (pg/mL) | - | 19.4 | - | 51.9 |
| Albumin | (g/dL) | 3.9 | 4.7 | 3.9 | 4.2 |
| Hematocrit | (%) | 34.5 | 40.9 | 32.4 | 32.7 |
| Intradialytic plasma volume decrease | (%) | 15.6 | 0.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujioka, H.; Imamura, T.; Koike, T.; Kinugawa, K. Initial Experience to Follow Lung Fluid Levels during Hemodialysis: A Possibility of Remote Dielectric Sensing-Guided Hemodialysis. J. Cardiovasc. Dev. Dis. 2022, 9, 57. https://doi.org/10.3390/jcdd9020057
Fujioka H, Imamura T, Koike T, Kinugawa K. Initial Experience to Follow Lung Fluid Levels during Hemodialysis: A Possibility of Remote Dielectric Sensing-Guided Hemodialysis. Journal of Cardiovascular Development and Disease. 2022; 9(2):57. https://doi.org/10.3390/jcdd9020057
Chicago/Turabian StyleFujioka, Hayato, Teruhiko Imamura, Tsutomu Koike, and Koichiro Kinugawa. 2022. "Initial Experience to Follow Lung Fluid Levels during Hemodialysis: A Possibility of Remote Dielectric Sensing-Guided Hemodialysis" Journal of Cardiovascular Development and Disease 9, no. 2: 57. https://doi.org/10.3390/jcdd9020057






