A Single-Cell Atlas of the Atherosclerotic Plaque in the Femoral Artery and the Heterogeneity in Macrophage Subtypes between Carotid and Femoral Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
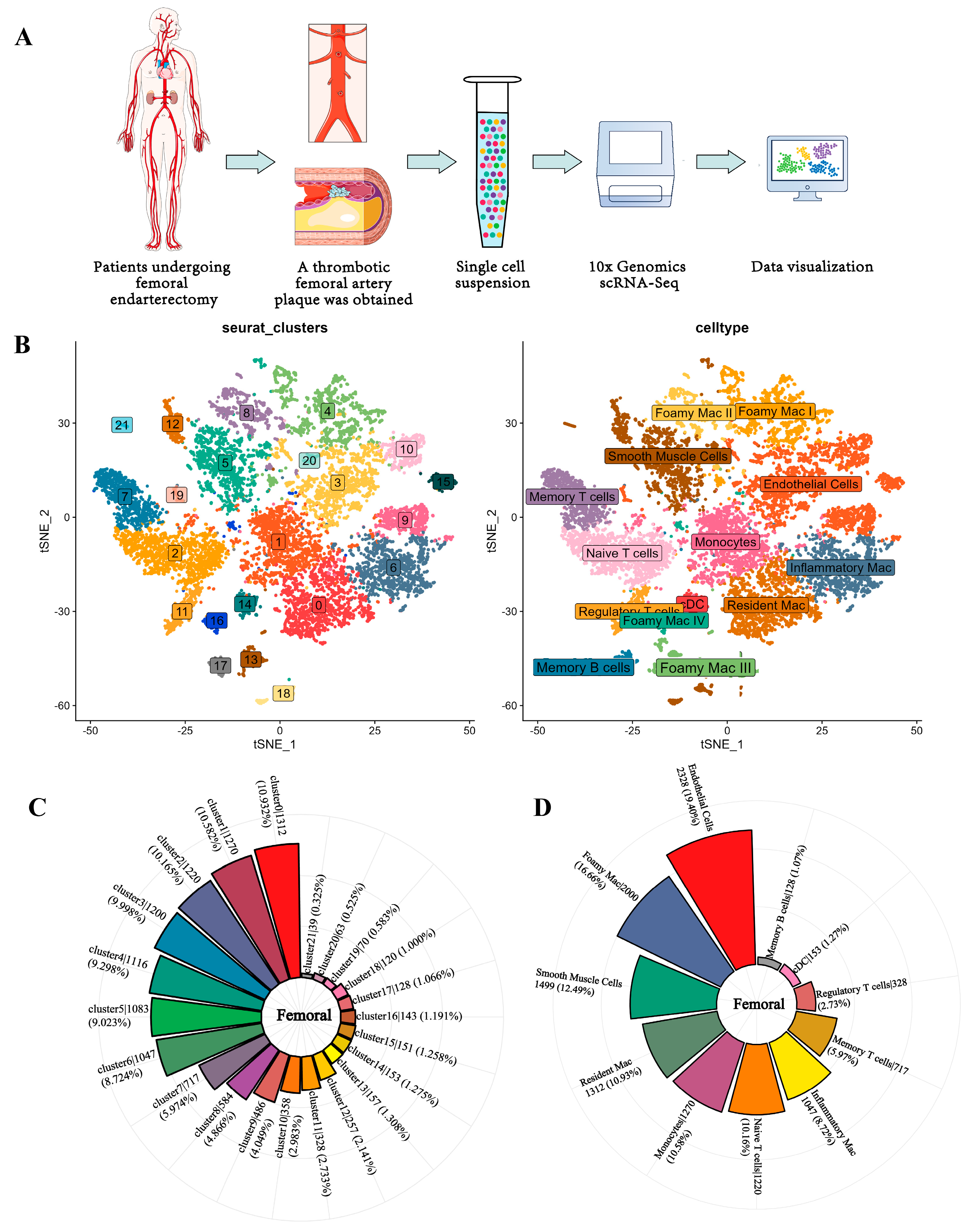
2.1. Sample Collection
2.2. Plaque Processing and Quality Testing
2.3. Construction and Sequencing of Single-Cell Libraries
2.4. Bioinformatics Analysis Process of the Single-Cell Libraries
2.4.1. Sequencing Data Quality Control and Quantification Based on CellRanger
2.4.2. Advanced Analysis of Single-Cell Data Based on Seurat
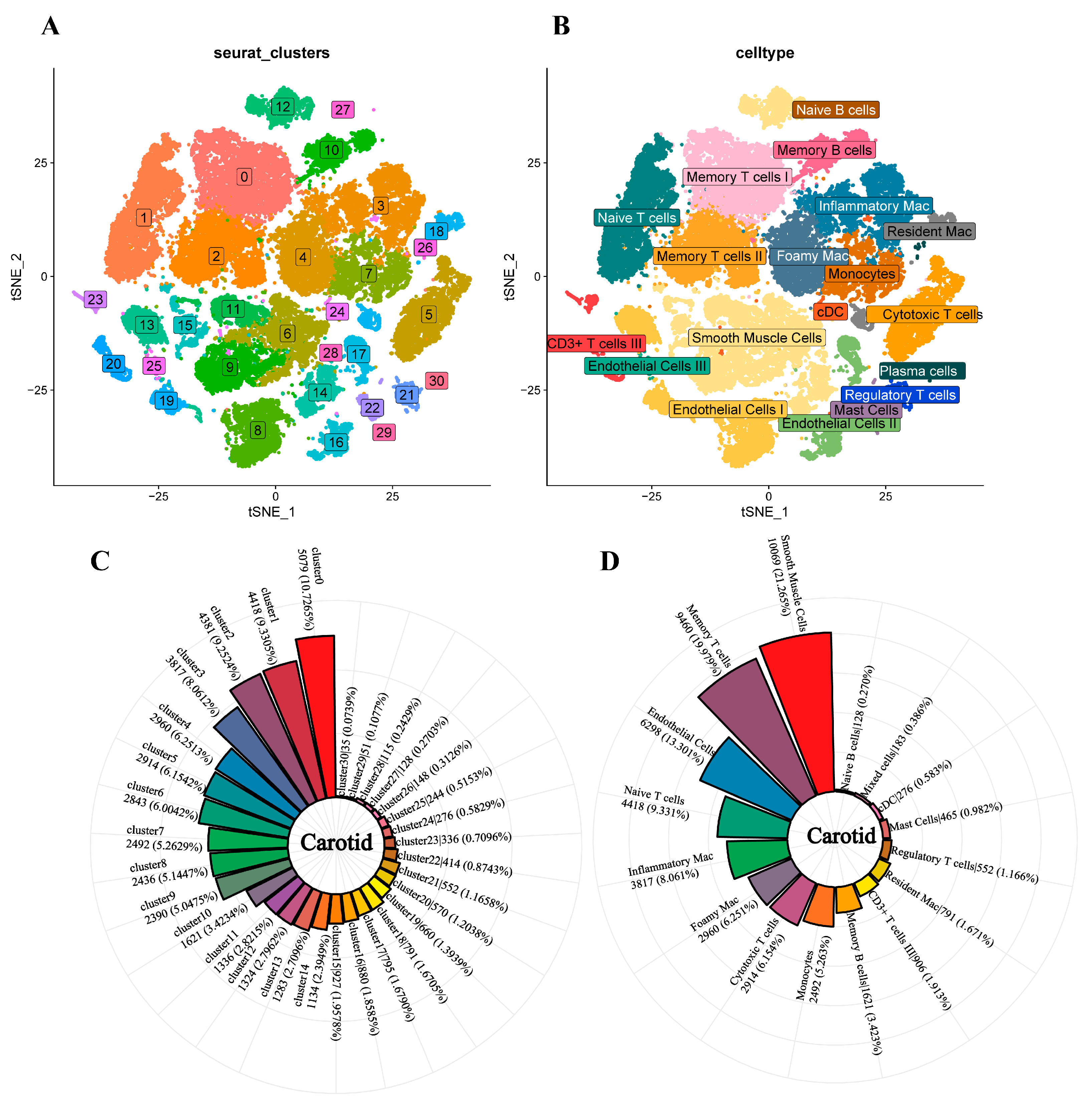
2.5. Drawing of Pie Chart and Histogram
2.6. Data Sets of Carotid Atherosclerosis
2.7. Data Acquisition and Preprocessing
2.8. Gene Set Pathway Enrichment Analysis
2.9. Immunofluorescence Staining
3. Results
3.1. Single-Cell Analysis of Femoral Atherosclerosis
3.2. Single-Cell Analysis of Carotid Atherosclerosis
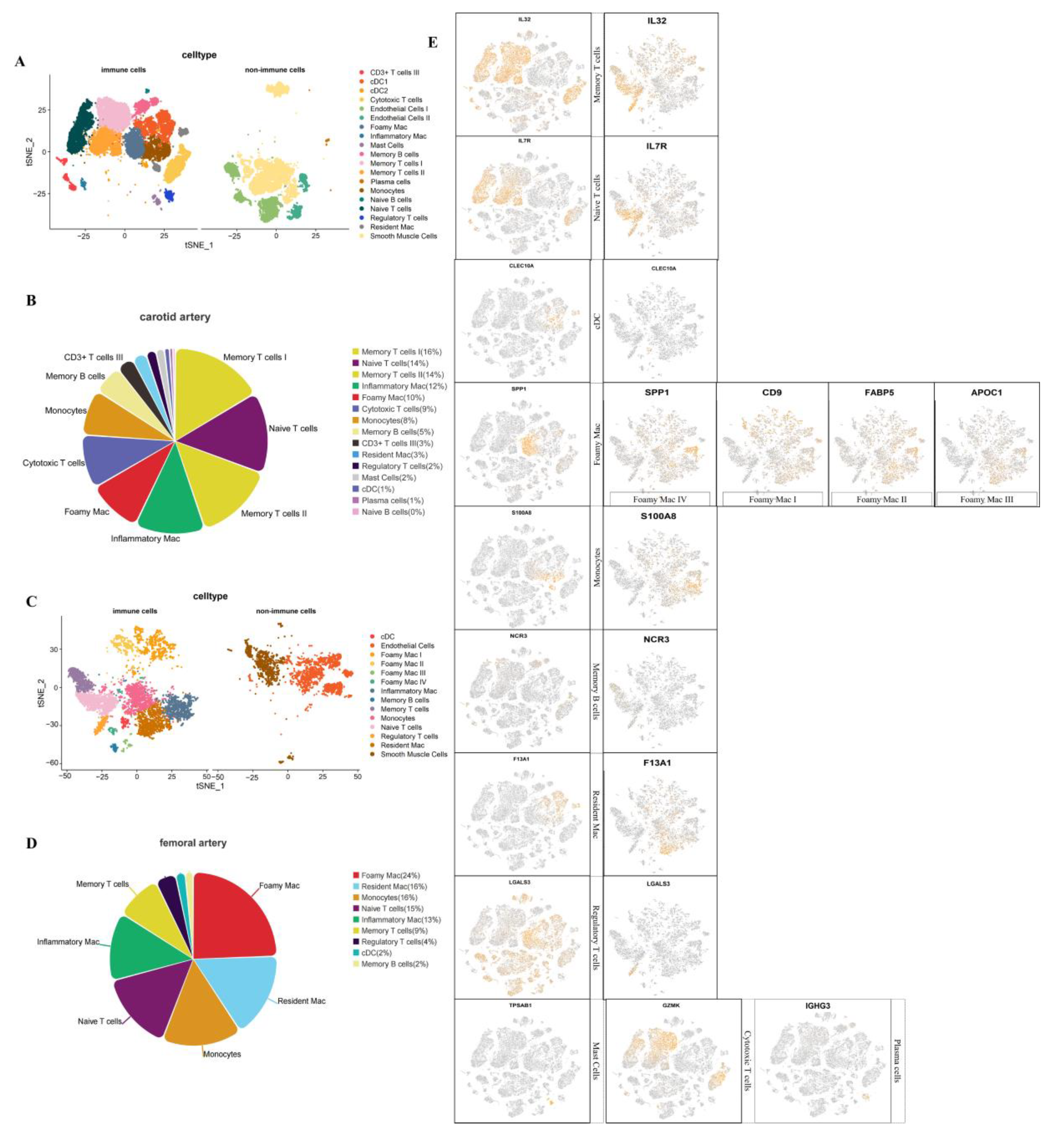
3.3. Analysis of Immune Cells of Carotid and Femoral Atherosclerosis
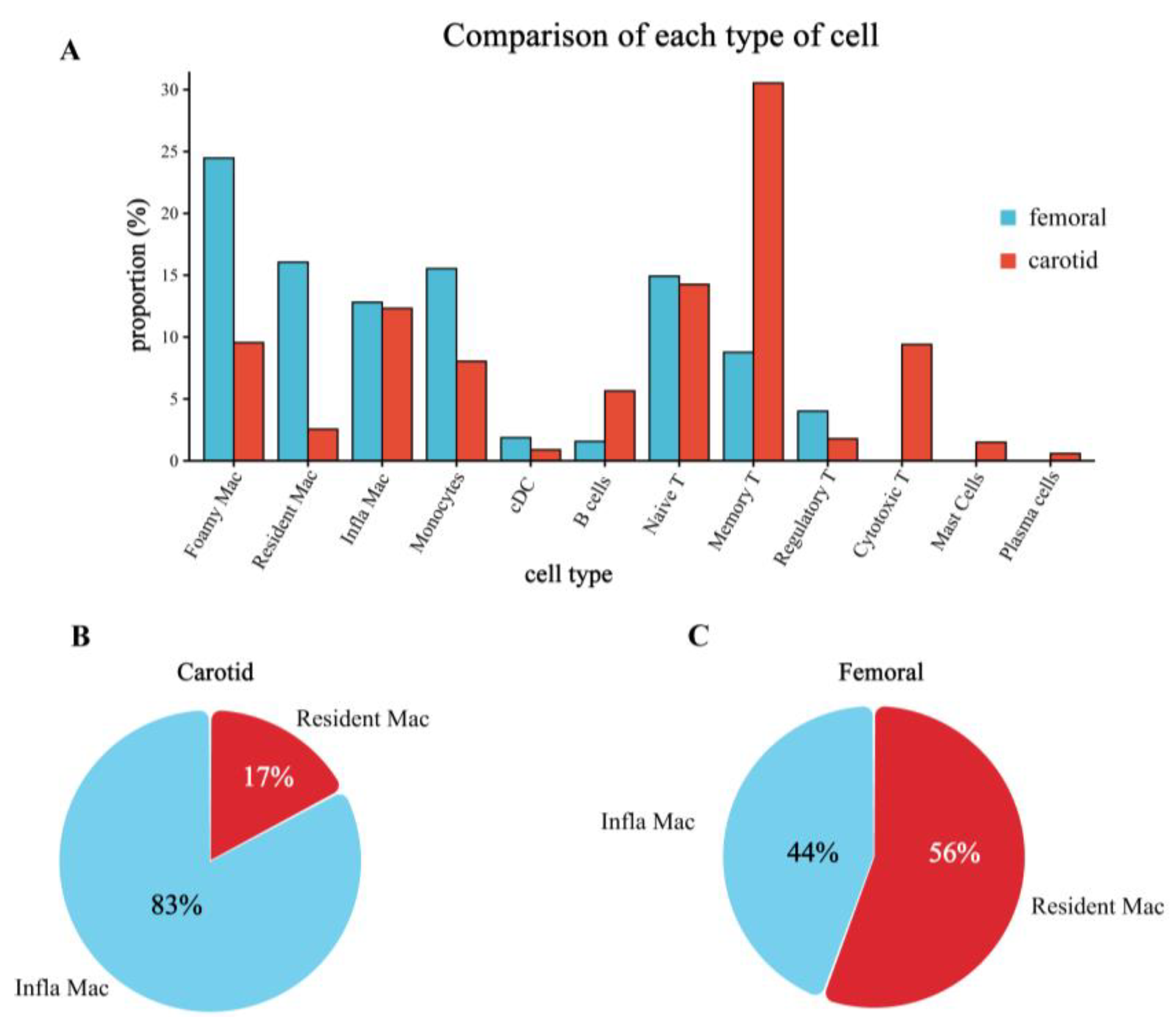
3.4. Differences in the Proportion of Immune Cell Infiltration between Femoral and Carotid Plaques
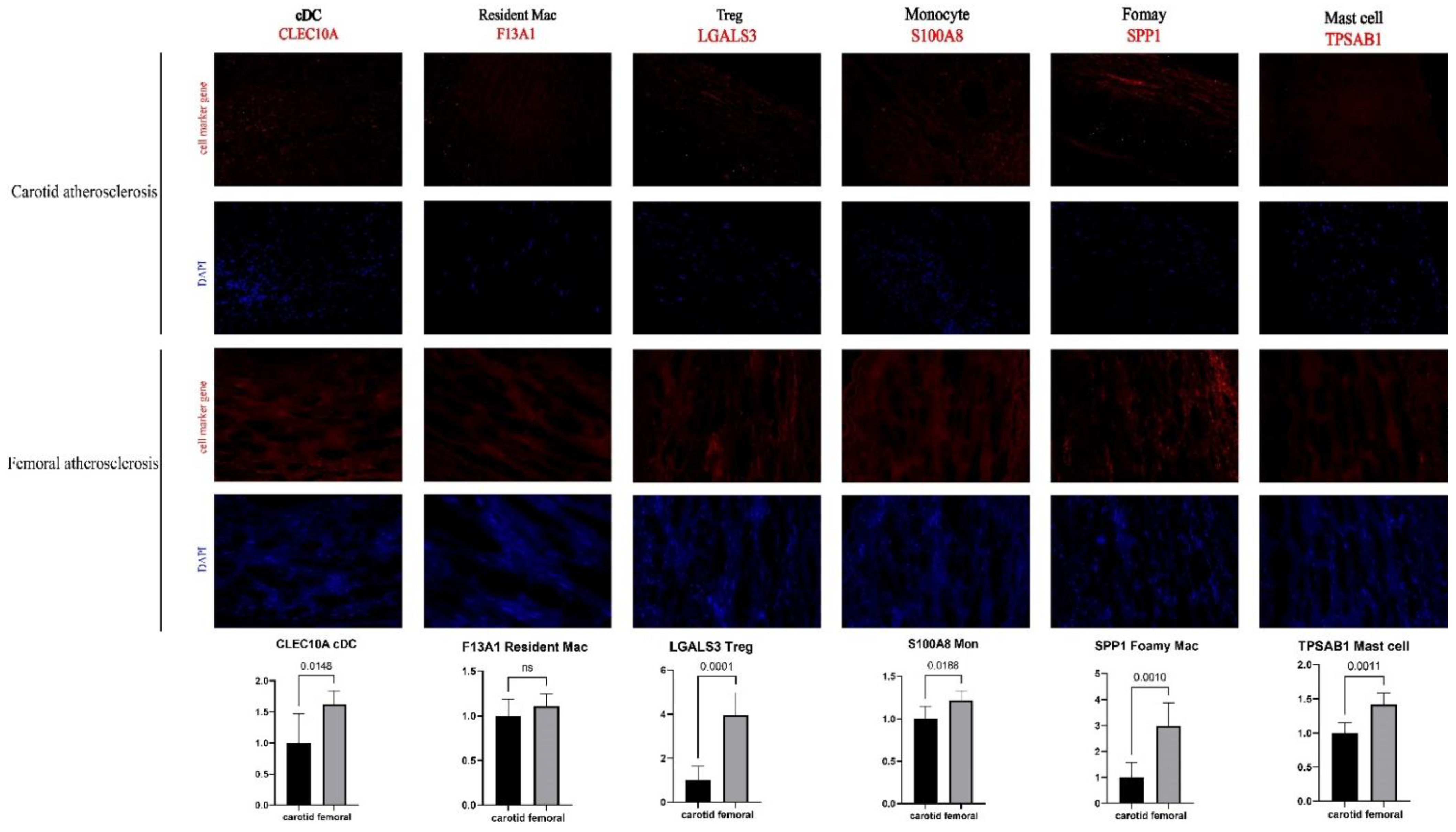
3.5. Successful Validation of the Difference in Cell Proportion between Carotid and Femoral Arteries
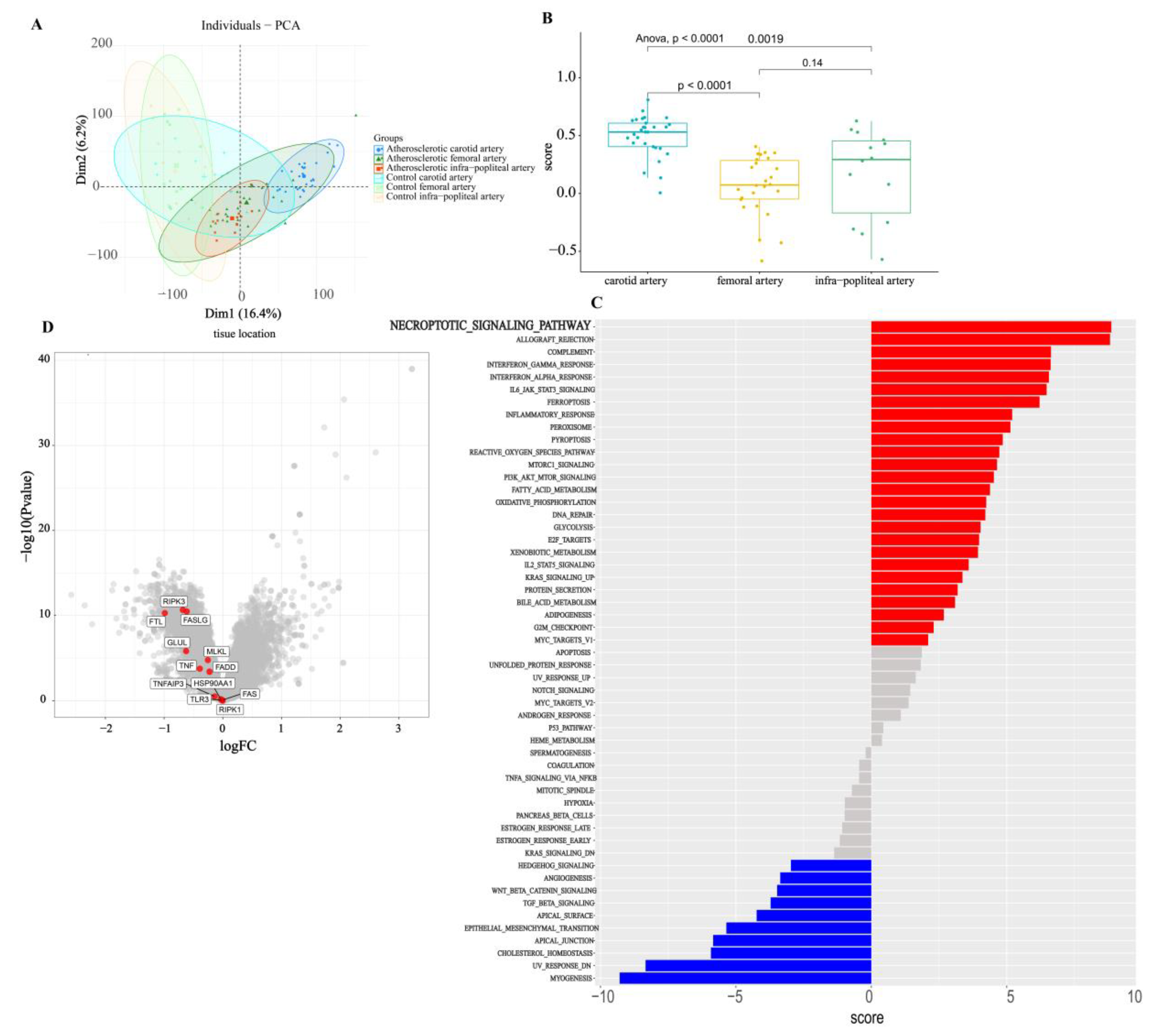
3.6. Bulk Analysis of Two Types of Atherosclerosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360, j5842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santovito, D.; Egea, V.; Weber, C. Small but smart: MicroRNAs orchestrate atherosclerosis development and progression. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 2075–2086. [Google Scholar] [CrossRef]
- Schober, A.; Nazari-Jahantigh, M.; Weber, C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 361–374, Erratum in Nat. Rev. Cardiol. 2016, 13, 120. [Google Scholar] [CrossRef]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Subramanian, M.; Sansbury, B.E.; Lin, C.-S.; Spite, M.; Fredman, G.; Tabas, I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, 6526–6531. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.-F.; et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.-H. Cytotoxic and Proinflammatory CD8 + T Lymphocytes Promote Development of Vulnerable Atherosclerotic Plaques in ApoE-Deficient Mice. Circulation 2013, 127, 1028–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seijkens, T.T.P.; Poels, K.; Meiler, S.; van Tiel, C.M.; Kusters, P.J.H.; Reiche, M.; Atzler, D.; Winkels, H.; Tjwa, M.; Poelman, H.; et al. Deficiency of the T cell regulator Casitas B-cell lymphoma-B aggravates atherosclerosis by inducing CD8+ T cell-mediated macrophage death. Eur. Heart J. 2019, 40, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Cochain, C.; Koch, M.; Chaudhari, S.M.; Busch, M.; Pelisek, J.; Boon, L.; Zernecke, A. CD8+ T Cells Regulate Monopoiesis and Circulating Ly6C-high Monocyte Levels in Atherosclerosis in Mice. Circ. Res. 2015, 117, 244–253. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Schwartzenberg, S.; Medvedovsky, D.; Jonas, M.; Charach, G.; Afek, A.; Shamiss, A. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis 2012, 222, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 2006, 27, 2530–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Liu, C. Guidelines for the treatment of arteriosclerotic obliterans of the lower extremities. Chin. J. Pract. Surg. 2008, 28, 923–924. [Google Scholar]
- Chen, Z.; Yang, Y. Guidelines for the diagnosis and treatment of carotid stenosis. Chin. J. Vasc. Surg. 2017, 9, 169–175. [Google Scholar]
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, W.; An, J.; Liang, M.; Li, Y.; Zhang, F.; Tong, Q.; Huang, K. Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2. Nat. Commun. 2019, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Salisbury, D.; Sallam, T. Long Noncoding RNAs in Atherosclerosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2380–2390. [Google Scholar] [CrossRef]
- Kwee, B.J.; Seo, B.R.; Najibi, A.J.; Li, A.W.; Shih, T.-Y.; White, D.; Mooney, D.J. Treating ischemia via recruitment of antigen-specific T cells. Sci. Adv. 2019, 5, eaav6313. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Brittendena, J.; Lahirib, R.; Brownb, P.A.J.; Thiesa, F.; Wilsona, H.M. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 491–497. [Google Scholar] [CrossRef]
- Ortega, E.; Gilabert, R.; Nuñez, I.; Cofán, M.; Sala-Vila, A.; de Groot, E.; Ros, E. White blood cell count is associated with carotid and femoral atherosclerosis. Atherosclerosis 2012, 221, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.; Heymann, M.-F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-cell exhaustion: Characteristics, causes and conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Witztum, J.L.; Lichtman, A.H. The Influence of Innate and Adaptive Immune Responses on Atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 73–102. [Google Scholar] [CrossRef] [Green Version]
- Lichtman, A.H. T Cell Costimulatory and Coinhibitory Pathways in Vascular Inflammatory Diseases. Front. Physiol. 2012, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.-A.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef]
- Vallejo, J.; Saigusa, R.; Gulati, R.; Armstrong Suthahar, S.S.; Suryawanshi, V.; Alimadadi, A.; Durant, C.P.; Ghosheh, Y.; Roy, P.; Ehinger, E.; et al. Combined protein and transcript single-cell RNA sequencing in human pe ripheral blood mononuclear cells. BMC Biol. 2022, 20, 193. [Google Scholar]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baardman, J.; Verberk, S.G.S.; van der Velden, S.; Gijbels, M.J.J.; van Roomen, C.P.P.A.; Sluimer, J.C.; Broos, J.Y.; Griffith, G.R.; Prange, K.H.M.; van Weeghel, M.; et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat. Commun. 2020, 11, 6296. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zheng, L.; Qiao, M.; Zhao, T.; Zhang, R.; Dong, H. A Single-Cell Atlas of the Atherosclerotic Plaque in the Femoral Artery and the Heterogeneity in Macrophage Subtypes between Carotid and Femoral Atherosclerosis. J. Cardiovasc. Dev. Dis. 2022, 9, 465. https://doi.org/10.3390/jcdd9120465
Wang P, Zheng L, Qiao M, Zhao T, Zhang R, Dong H. A Single-Cell Atlas of the Atherosclerotic Plaque in the Femoral Artery and the Heterogeneity in Macrophage Subtypes between Carotid and Femoral Atherosclerosis. Journal of Cardiovascular Development and Disease. 2022; 9(12):465. https://doi.org/10.3390/jcdd9120465
Chicago/Turabian StyleWang, Ping, Lin Zheng, Maolin Qiao, Tianliang Zhao, Ruijing Zhang, and Honglin Dong. 2022. "A Single-Cell Atlas of the Atherosclerotic Plaque in the Femoral Artery and the Heterogeneity in Macrophage Subtypes between Carotid and Femoral Atherosclerosis" Journal of Cardiovascular Development and Disease 9, no. 12: 465. https://doi.org/10.3390/jcdd9120465



