Genetic Profile and Clinical Characteristics of Brugada Syndrome in the Chinese Population
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. ECG Measurements
2.3. Genetic Testing
2.4. Variants Screening
2.5. Sanger Sequencing
2.6. Waterfall Plot and Needle Plot
2.7. Protein 3D Structure Prediction
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics
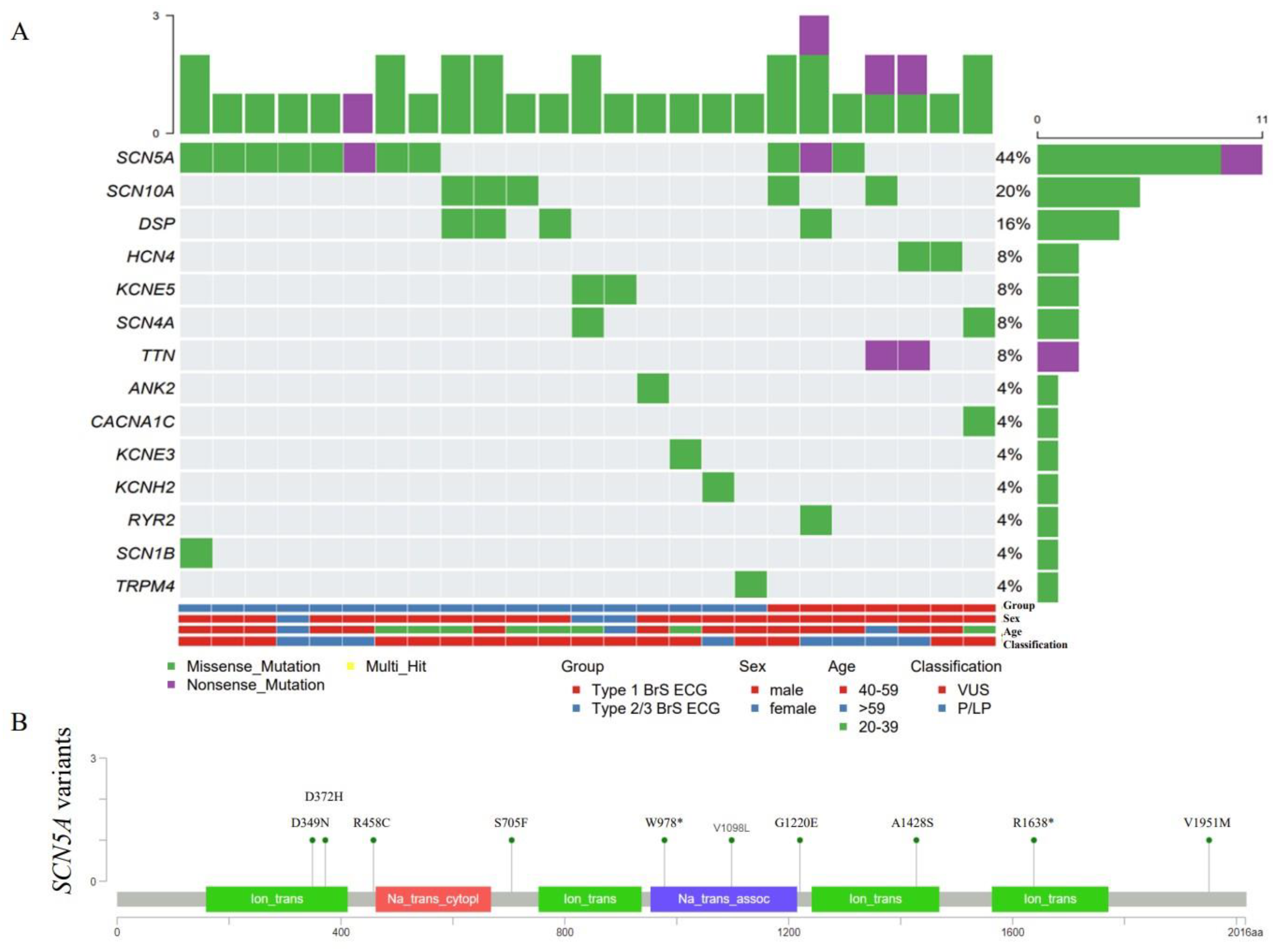
3.2. Genetic Testing and Screening Variants
3.3. Genetic Characteristics Analysis
3.4. Clinical and Genetic Features of Four Probands Carrying Novel P/LP Mutations
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.-E.; Huikuri, H.; et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013, 15, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Vutthikraivit, W.; Rattanawong, P.; Putthapiban, P.; Sukhumthammarat, W.; Vathesatogkit, P.; Ngarmukos, T.; Thakkinstian, A. Worldwide Prevalence of Brugada Syndrome: A Systematic Review and Meta-Analysis. Acta Cardiol. Sin. 2018, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Shimizu, W.; Yang, P.; Koopmann, T.T.; Tanck, M.W.T.; Miyamoto, Y.; Kamakura, S.; Roden, D.M.; Wilde, A.A.M. Common sodium channel promoter haplotype in asian subjects underlies variability in cardiac conduction. Circulation 2006, 113, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Perez Riera, A.R.; et al. Brugada syndrome: Report of the second consensus conference. Heart Rhythm 2005, 2, 429–440. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Yan, G.-X.; Ackerman, M.J.; Borggrefe, M.; Corrado, D.; Guo, J.; Gussak, I.; Hasdemir, C.; Horie, M.; Huikuri, H.; et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Europace 2017, 19, 665–694. [Google Scholar] [CrossRef]
- Probst, V.; Veltmann, C.; Eckardt, L.; Meregalli, P.G.; Gaita, F.; Tan, H.L.; Babuty, D.; Sacher, F.; Giustetto, C.; Schulze-Bahr, E.; et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation 2010, 121, 635–643. [Google Scholar] [CrossRef]
- Campuzano, O.; Sarquella-Brugada, G.; Cesar, S.; Arbelo, E.; Brugada, J.; Brugada, R. Update on Genetic Basis of Brugada Syndrome: Monogenic, Polygenic or Oligogenic? Int. J. Mol. Sci. 2020, 21, 7155. [Google Scholar] [CrossRef]
- Campuzano, O.; Sarquella-Brugada, G.; Fernandez-Falgueras, A.; Cesar, S.; Coll, M.; Mates, J.; Arbelo, E.; Perez-Serra, A.; Del Olmo, B.; Jordá, P.; et al. Genetic interpretation and clinical translation of minor genes related to Brugada syndrome. Hum. Mutat. 2019, 40, 749–764. [Google Scholar] [CrossRef]
- Le Scouarnec, S.; Karakachoff, M.; Gourraud, J.-B.; Lindenbaum, P.; Bonnaud, S.; Portero, V.; Duboscq-Bidot, L.; Daumy, X.; Simonet, F.; Teusan, R.; et al. Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Hum. Mol. Genet. 2015, 24, 2757–2763. [Google Scholar] [CrossRef]
- Kapplinger, J.D.; Tester, D.J.; Alders, M.; Benito, B.; Berthet, M.; Brugada, J.; Brugada, P.; Fressart, V.; Guerchicoff, A.; Harris-Kerr, C.; et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010, 7, 33–46. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Kim, R.; Udupa, S.; Costain, G.; Jobling, R.; Liston, E.; Jamal, S.M.; Szybowska, M.; Morel, C.F.; Bowdin, S.; et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation 2018, 138, 1195–1205. [Google Scholar] [CrossRef]
- Mok, N.S.; Priori, S.G.; Napolitano, C.; Chan, K.K.; Bloise, R.; Chan, H.W.; Fung, W.H.; Chan, Y.S.; Chan, W.K.; Lam, C.; et al. Clinical profile and genetic basis of Brugada syndrome in the Chinese population. Hong Kong Med. J. 2004, 10, 32–37. [Google Scholar]
- Tse, G.; Lee, S.; Liu, T.; Yuen, H.C.; Wong, I.C.K.; Mak, C.; Mok, N.S.; Wong, W.T. Identification of Novel SCN5A Single Nucleotide Variants in Brugada Syndrome: A Territory-Wide Study From Hong Kong. Front. Physiol. 2020, 11, 574590. [Google Scholar] [CrossRef]
- Juang, J.-M.J.; Tsai, C.-T.; Lin, L.-Y.; Liu, Y.-B.; Yu, C.-C.; Hwang, J.-J.; Chen, J.-J.; Chiu, F.-C.; Chen, W.-J.; Tseng, C.-D.; et al. Unique clinical characteristics and SCN5A mutations in patients with Brugada syndrome in Taiwan. J. Formos Med. Assoc. 2015, 114, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Alings, M.; Wilde, A. “Brugada” syndrome: Clinical data and suggested pathophysiological mechanism. Circulation 1999, 99, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Sieira, J.; Brugada, P. The definition of the Brugada syndrome. Eur. Heart J. 2017, 38, 3029–3034. [Google Scholar] [CrossRef]
- Polovina, M.M.; Vukicevic, M.; Banko, B.; Lip, G.Y.H.; Potpara, T.S. Brugada syndrome: A general cardiologist’s perspective. Eur. J. Intern. Med. 2017, 44, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wu, D.; Sun, Y.; Hu, D.; Dai, J.; Chen, Y.; Wang, D. Whole-exome sequencing reveals genetic risks of early-onset sporadic dilated cardiomyopathy in the Chinese Han population. Sci. China Life Sci. 2022, 65, 770–780. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Zhao, J.; Chen, C.; Wang, H.; Ding, H.; Wang, D.W.; Wang, D.W. Rapid molecular genetic diagnosis of hypertrophic cardiomyopathy by semiconductor sequencing. J. Transl. Med. 2014, 12, 173. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Juang, J.-M.J.; Chen, C.-Y.J.; Chen, Y.-H.; Wu, I.C.; Hsu, C.-C.; Chen, L.-N.; Tang, F.-C.; Wang, C.-C.; Juan, C.-C.; Chiu, H.-C.; et al. Prevalence and prognosis of Brugada electrocardiogram patterns in an elderly Han Chinese population: A nation-wide community-based study (HALST cohort). Europace 2015, 17 (Suppl. S2), ii54–ii62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tian, C.; Li, X.; Yang, X.; Wang, X.; Yang, Y.; Liu, N.; Kusano, K.F.; Barajas-Martinez, H.; Hu, D.; et al. Gender Differences in Prognosis and Risk Stratification of Brugada Syndrome: A Pooled Analysis of 4140 Patients From 24 Clinical Trials. Front. Physiol. 2018, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Sarkozy, A.; Mont, L.; Henkens, S.; Berruezo, A.; Tamborero, D.; Arzamendi, D.; Berne, P.; Brugada, R.; Brugada, P.; et al. Gender differences in clinical manifestations of Brugada syndrome. J. Am. Coll. Cardiol. 2008, 52, 1567–1573. [Google Scholar] [CrossRef]
- Castro Hevia, J.; Dorantes Sanchez, M.; Martinez Lopez, F.; Castañeda Chirino, O.; Falcon Rodriguez, R.; Puga Bravo, M.; de Zayas Galguera, J.; Antzelevitch, C. Multiple serial ECGs aid with the diagnosis and prognosis of Brugada syndrome. Int. J. Cardiol. 2019, 277, 130–135. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Gasparini, M.; Pappone, C.; Della Bella, P.; Giordano, U.; Bloise, R.; Giustetto, C.; De Nardis, R.; Grillo, M.; et al. Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation 2002, 105, 1342–1347. [Google Scholar] [CrossRef]
- Yamagata, K.; Horie, M.; Aiba, T.; Ogawa, S.; Aizawa, Y.; Ohe, T.; Yamagishi, M.; Makita, N.; Sakurada, H.; Tanaka, T.; et al. Genotype-Phenotype Correlation of Mutation for the Clinical and Electrocardiographic Characteristics of Probands With Brugada Syndrome: A Japanese Multicenter Registry. Circulation 2017, 135, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Lee, S.; Li, A.; Chang, D.; Li, G.; Zhou, J.; Liu, T.; Zhang, Q. Automated Electrocardiogram Analysis Identifies Novel Predictors of Ventricular Arrhythmias in Brugada Syndrome. Front. Cardiovasc. Med. 2020, 7, 618254. [Google Scholar] [CrossRef]
- Meregalli, P.G.; Wilde, A.A.M.; Tan, H.L. Pathophysiological mechanisms of Brugada syndrome: Depolarization disorder, repolarization disorder, or more? Cardiovasc. Res. 2005, 67, 367–378. [Google Scholar] [CrossRef]
- Ohkubo, K.; Watanabe, I.; Okumura, Y.; Ashino, S.; Kofune, M.; Nagashima, K.; Kofune, T.; Nakai, T.; Kunimoto, S.; Kasamaki, Y.; et al. Prolonged QRS duration in lead V2 and risk of life-threatening ventricular Arrhythmia in patients with Brugada syndrome. Int. Heart J. 2011, 52, 98–102. [Google Scholar] [CrossRef]
- Rattanawong, P.; Kewcharoen, J.; Techorueangwiwat, C.; Kanitsoraphan, C.; Mekritthikrai, R.; Prasitlumkum, N.; Puttapiban, P.; Mekraksakit, P.; Vutthikraivit, W.; Sorajja, D. Wide QRS complex and the risk of major arrhythmic events in Brugada syndrome patients: A systematic review and meta-analysis. J. Arrhythm 2020, 36, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, M.V.; Anaclerio, M.; Iacoviello, M.; Forleo, C.; Guida, P.; Troccoli, R.; Massari, F.; Mastropasqua, F.; Sorrentino, S.; Manghisi, A.; et al. QT-interval prolongation in right precordial leads: An additional electrocardiographic hallmark of Brugada syndrome. J. Am. Coll. Cardiol. 2003, 42, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Zhou, J.; Lee, S.; Liu, T.; Bazoukis, G.; Mililis, P.; Wong, I.C.K.; Chen, C.; Xia, Y.; Kamakura, T.; et al. Incorporating Latent Variables Using Nonnegative Matrix Factorization Improves Risk Stratification in Brugada Syndrome. J. Am. Heart Assoc. 2020, 9, e012714. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nagase, S.; Morita, H.; Wada, T.; Tanaka, M.; Murakami, M.; Watanabe, A.; Nishii, N.; Nakamura, K.; Kusano, K.F.; et al. Impact of premature activation of the right ventricle with programmed stimulation in Brugada syndrome. J. Cardiovasc. Electrophysiol. 2018, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jeevaratnam, K.; Zhang, Y.; Guzadhur, L.; Duehmke, R.M.; Lei, M.; Grace, A.A.; Huang, C.L.H. Differences in sino-atrial and atrio-ventricular function with age and sex attributable to the Scn5a+/− mutation in a murine cardiac model. Acta Physiol. 2010, 200, 23–33. [Google Scholar] [CrossRef]
- Papadatos, G.A.; Wallerstein, P.M.R.; Head, C.E.G.; Ratcliff, R.; Brady, P.A.; Benndorf, K.; Saumarez, R.C.; Trezise, A.E.O.; Huang, C.L.H.; Vandenberg, J.I.; et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc. Natl. Acad. Sci. USA 2002, 99, 6210–6215. [Google Scholar] [CrossRef]
- Santos, L.F.; Rodrigues, B.; Moreira, D.; Correia, E.; Nunes, L.; Costa, A.; Elvas, L.; Pereira, T.; Machado, J.C.; Castedo, S.; et al. Criteria to predict carriers of a novel SCN5A mutation in a large Portuguese family affected by the Brugada syndrome. Europace 2012, 14, 882–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Malderen, S.C.H.; Kerkhove, D.; Theuns, D.A.M.J.; Weytjens, C.; Droogmans, S.; Tanaka, K.; Daneels, D.; Van Dooren, S.; Meuwissen, M.; Bonduelle, M.; et al. Prolonged right ventricular ejection delay identifies high risk patients and gender differences in Brugada syndrome. Int. J. Cardiol. 2015, 191, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kors, J.A.; de Bruyne, M.C.; Hoes, A.W.; van Herpen, G.; Hofman, A.; van Bemmel, J.H.; Grobbee, D.E. T axis as an indicator of risk of cardiac events in elderly people. Lancet 1998, 352, 601–605. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Nelson, J.C.; Kronmal, R.A.; Zhang, Z.M.; Robbins, J.; Gottdiener, J.S.; Furberg, C.D.; Manolio, T.; Fried, L. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study). Am. J. Cardiol. 2001, 88, 118–123. [Google Scholar] [CrossRef]
- Pinto, M.M.; Brant, L.C.C.; Padilha-da-Silva, J.L.; Foppa, M.; Lotufo, P.A.; Mill, J.G.; Vasconcelo-Silva, P.R.; Almeida, M.d.C.C.; Barreto, S.M.; Ribeiro, A.L.P. Electrocardiographic Findings in Brazilian Adults without Heart Disease: ELSA-Brasil. Arq. Bras. Cardiol. 2017, 109, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Cerrone, M.; Morley, G.; Vasquez, C.; Fowler, S.; Liu, N.; Bernstein, S.A.; Liu, F.-Y.; Zhang, J.; Rogers, C.S.; et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J. Clin. Investig. 2015, 125, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, M.; Ohno, S.; Makiyama, T.; Horie, M. Novel SCN10A variants associated with Brugada syndrome. Europace 2016, 18, 905–911. [Google Scholar] [CrossRef]
- Hu, D.; Barajas-Martínez, H.; Pfeiffer, R.; Dezi, F.; Pfeiffer, J.; Buch, T.; Betzenhauser, M.J.; Belardinelli, L.; Kahlig, K.M.; Rajamani, S.; et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J. Am. Coll. Cardiol. 2014, 64, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.-M.J.; Horie, M. Genetics of Brugada syndrome. J. Arrhythm 2016, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Abrudan, J.L.; Dulik, M.C.; Sasson, A.; Brunton, J.; Jayaraman, V.; Dugan, N.; Haley, D.; Rajagopalan, R.; Biswas, S.; et al. Utility and limitations of exome sequencing as a genetic diagnostic tool for conditions associated with pediatric sudden cardiac arrest/sudden cardiac death. Hum. Genom. 2015, 9, 15. [Google Scholar] [CrossRef] [PubMed]


| Feature | Overall (n = 79) | Type 1 BrS ECG (n = 26) | Type 2/3 BrS ECG (n = 53) | p-Value |
|---|---|---|---|---|
| Male (n, %) | 69 (87.34%) | 25 (96.15%) | 44 (83.02%) | 0.197 |
| Age at diagnosis (years) | 43 (36–54) | 47 (32–52) | 42 (36–54) | 0.697 |
| Spontaneous Type 1 ECG (n, %) | 26 (32.91%) | 26 | 0 | — |
| Symptomatic patients (n, %) | 41 (51.90%) | 17 (65.38%) | 24 (45.28%) | 0.093 |
| Documented VT/VF (n, %) | 7 (8.86%) | 2 (7.69%) | 5 (9.43%) | 1.000 |
| Syncope (n, %) | 28 (35.44%) | 10 (38.46%) | 18 (33.96%) | 0.694 |
| Family history of SCD (n, %) | 5 (6.33%) | 2 (7.69%) | 3 (5.66%) | 1.000 |
| ICD (n, %) | 11 (13.92%) | 5 (19.23%) | 6 (11.32%) | 0.543 |
| Heart rate (bpm) | 70 (62–76) | 71 (65–75) | 70 (62–77) | 0.688 |
| P-wave duration (ms) | 100 (86–104) | 100 (80–104) | 98 (87–107) | 0.810 |
| QRS duration (ms) | 100 (90–109) | 109 (94–115) | 98 (89–106) | 0.004 * |
| T-wave duration (ms) | 162 (120–200) | 180 (150–200) | 160 (110–200) | 0.172 |
| PR interval (ms) | 162 (142–180) | 166 (144–189) | 160 (136–177) | 0.244 |
| QT interval (ms) | 388 (363–407) | 389 (368–402) | 388 (360–414) | 0.770 |
| QTc interval (ms) | 411 (390–432) | 418 (405–438) | 409 (386–426) | 0.058 |
| P-wave axis (deg) | 57 (41–67) | 49 (40–66) | 61 (40–71) | 0.612 |
| R-wave axis (deg) | 48 (21–64) | 48 (32–62) | 49 (19–69) | 0.972 |
| QRS axis (deg) | 47 (15–69) | 53 (14–77) | 44 (16–69) | 0.342 |
| T-wave axis (deg) | 50 (29–66) | 48 (28–66) | 52 (32–68) | 0.437 |
| R-wave Amplitude in lead V1 (mV) | 0.17 (0.10–0.32) | 0.25 (0.05–0.36) | 0.15 (0.10–0.32) | 0.758 |
| R-wave Amplitude in lead V5 (mV) | 1.46 (1.13–1.80) | 1.43 (1.13–1.78) | 1.46 (1.11–1.81) | 0.946 |
| S-wave Amplitude in lead V1 (mV) | 0.66 (0.39–1.06) | 0.60 (0.35–1.00) | 0.66 (0.42–1.09) | 0.555 |
| S-wave Amplitude in lead V5 (mV) | 0.30 (0.10–0.50) | 0.30 (0.10–0.66) | 0.30 (0.11–0.48) | 0.500 |
| RV5 + SV1 (mV) | 2.18 (1.60–2.65) | 2.20 (1.59–2.62) | 2.15 (1.65–2.67) | 0.594 |
| RV1 + SV5 (mV) | 0.55 (0.38–0.80) | 0.58 (0.42–0.86) | 0.50 (0.37–0.80) | 0.431 |
| Age at Diagnosis | Sex | Symptom | Type of BrS ECG | Amino Acid Change | rs Number | Nucleotide Change | Variant Type | Exon | Location | ACMG |
|---|---|---|---|---|---|---|---|---|---|---|
| 43 | M | (+) | 1 | p.S705F | rs199473148 | c.2114C > T | Missense | 14 | DI-DII | VUS |
| 54; 53 | M; M | (+); (−) | 2; 2 | p.V1951M | rs41315493 | c.5851G > A | Missense | 28 | C-terminus | VUS |
| 66 | F | (−) | 2 | p.A1428S | rs200034939 | c.4282G > T | Missense | 24 | DIII-S5/S6 | LP |
| 47 | M | (+) | 1 | p.D349N | rs779687673 | c.1045G > A | Missense | 9 | DI-S5/S6 | VUS |
| 40 | M | (−) | 1 | p.G1220E | . | c.3659G > A | Missense | 20 | DIII-S1 | LP |
| 59 | M | (−) | 3 | p.W978X | . | c.2933G > A | Nonsense | 17 | DII-DIII | P |
| 30; 34 | M; M | (−); (−) | 2; 2 | p.V1098L | rs199473191 | c.3292G > T | Missense | 18 | DII-DIII | VUS |
| 34 | M | (−) | 2 | p.R458C | rs752130196 | c.1372C > T | Missense | 11 | DI-DII | VUS |
| 48 | M | (+) | 1 | p.D372H | . | c.1114G > C | Missense | 9 | DI-S5/S6 (Pore) | LP |
| 41 | M | (+) | 1 | p.R1638X | rs761505217 | c.4912C > T | Nonsense | 28 | DIV-S4/S5 | P |
| Feature | P/LP (n = 8) | VUS (n = 17) | Negative (n = 34) | p-Value |
|---|---|---|---|---|
| Male (n, %) | 7 (87.50%) | 15 (88.24%) | 28 (82.35%) | 0.880 |
| Age at diagnosis (years) | 49 (42–64) | 40 (31–49) | 44 (36–55) | 0.091 |
| Spontaneous Type 1 ECG (n, %) | 4 (50.00%) | 3 (17.65%) | 12 (35.29%) | 0.237 |
| Symptomatic patients (n, %) | 3 (37.50%) | 8 (47.06%) | 22 (64.71%) | 0.243 |
| Documented VT/VF (n, %) | 0 (0.00%) | 1 (5.88%) | 2 (5.88%) | 1.000 |
| Syncope (n, %) | 2 (25.00%) | 4 (23.53%) | 15 (44.12%) | 0.319 |
| ICD (n, %) | 0 (0.00%) | 3 (17.65%) | 2 (5.88%) | 0.395 |
| Heart rate (bpm) | 74 (73–81) | 70 (62–74) | 69 (61–76) | 0.097 |
| P-wave duration (ms) | 113 (98–119) | 98 (88–103) | 98 (86–104) | 0.145 |
| QRS duration (ms) | 105 (97–123) | 96 (90–111) | 100 (88–108) | 0.074 |
| T-wave duration (ms) | 180 (105–200) | 180 (150–210) | 159 (100–196) | 0.200 |
| PR interval (ms) | 173 (161–203) | 164 (154–186) | 153 (128–175) | 0.107 |
| QT interval (ms) | 385 (370–407) | 390 (366–417) | 385 (360–400) | 0.862 |
| QTc interval (ms) | 432 (415–436) | 394 (384–434) | 411 (396–420) | 0.050 |
| P-wave axis (deg) | 49 (41–70) | 61 (48–69) | 59 (39–66) | 0.727 |
| R-wave axis (deg) | 24 (-15–79) | 42 (21–63) | 49 (25–63) | 0.403 |
| QRS axis (deg) | 24 (-19–98) | 34 (12–73) | 48 (30–64) | 0.640 |
| T-wave axis (deg) | 41 (4–72) | 53 (35–81) | 55 (29–66) | 0.371 |
| R-wave Amplitude in lead V1 (mV) | 0.23 (0.08–0.34) | 0.12 (0.08–0.26) | 0.15 (0.09–0.36) | 0.516 |
| R-wave Amplitude in lead V5 (mV) | 1.22 (1.02–1.49) | 1.34 (1.04–1.79) | 1.47 (0.99–1.85) | 0.510 |
| S-wave Amplitude in lead V1 (mV) | 0.58 (0.17–0.82) | 0.72 (0.48–1.05) | 0.87 (0.39–1.13) | 0.689 |
| S-wave Amplitude in lead V5 (mV) | 0.37 (0.26–0.91) | 0.30 (0.17–0.57) | 0.28 (0.10–0.46) | 0.220 |
| RV5 + SV1 (mV) | 1.72 (1.19–2.22) | 2.15 (1.67–2.93) | 2.32 (1.68–2.90) | 0.445 |
| RV1 + SV5 (mV) | 0.72 (0.48–1.06) | 0.58 (0.30–0.81) | 0.50 (0.37–0.75) | 0.336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-L.; Chen, Y.-H.; Sun, Y.; Huang, M.; Wei, H.-R.; Liu, H.; Xu, K.; Song, X.-L.; Chen, P.; Tan, L.; et al. Genetic Profile and Clinical Characteristics of Brugada Syndrome in the Chinese Population. J. Cardiovasc. Dev. Dis. 2022, 9, 369. https://doi.org/10.3390/jcdd9110369
Wang L-L, Chen Y-H, Sun Y, Huang M, Wei H-R, Liu H, Xu K, Song X-L, Chen P, Tan L, et al. Genetic Profile and Clinical Characteristics of Brugada Syndrome in the Chinese Population. Journal of Cardiovascular Development and Disease. 2022; 9(11):369. https://doi.org/10.3390/jcdd9110369
Chicago/Turabian StyleWang, Lin-Lin, Yang-Hui Chen, Yang Sun, Man Huang, Hao-Ran Wei, Hao Liu, Ke Xu, Xiu-Li Song, Peng Chen, Lun Tan, and et al. 2022. "Genetic Profile and Clinical Characteristics of Brugada Syndrome in the Chinese Population" Journal of Cardiovascular Development and Disease 9, no. 11: 369. https://doi.org/10.3390/jcdd9110369
APA StyleWang, L.-L., Chen, Y.-H., Sun, Y., Huang, M., Wei, H.-R., Liu, H., Xu, K., Song, X.-L., Chen, P., Tan, L., Huang, J., Li, Z.-Z., Li, R., Yu, T., Ma, F., Ding, H., Wang, Y., Wang, D.-W., Wang, H., & Zhao, C.-X. (2022). Genetic Profile and Clinical Characteristics of Brugada Syndrome in the Chinese Population. Journal of Cardiovascular Development and Disease, 9(11), 369. https://doi.org/10.3390/jcdd9110369






